Abstract
Low cerebral blood flow (CBF) states have been demonstrated in children early after traumatic brain injury (TBI), and have been correlated with poorer outcomes. Cerebral perfusion pressure (CPP) support following severe TBI is commonly implemented to correct cerebral hypoperfusion, but the efficacy of various vasopressors has not been determined. Sixteen 4-week-old female swine underwent nonimpact inertial brain injury in the sagittal plane. Intraparenchymal monitors were placed to measure intracranial pressure (ICP), CBF, brain tissue oxygen tension (PbtO2), and cerebral microdialysis 30 min to 6 h post-injury. One hour after injury, animals were randomized to receive either phenylephrine (PE) or norepinephrine (NE) infusions titrated to a CPP >70 mm Hg for 5 h. Animals were euthanized 6 h post-TBI, and brains were fixed and stained to assess regions of cell and axonal injury. After initiation of CPP augmentation with NE or PE infusions, there were no differences in ICP between the groups or over time. Animals receiving NE had higher PbtO2 than those receiving PE (29.6±10.2 vs. 19.6±6.4 torr at 6 h post-injury, p<0.05). CBF increased similarly in both the NE and PE groups. CPP support with PE resulted in a greater reduction in metabolic crisis than with NE (lactate/pyruvate ratio 16.7±2.4 vs. 42.7±10.2 at 6 h post-injury, p<0.05). Augmentation of CPP to 70 mm Hg with PE resulted in significantly smaller cell injury volumes at 6 h post-injury than CPP support with NE (0.4% vs. 1.4%, p<0.05). Despite similar increases in CBF, CPP support with NE resulted in greater brain tissue oxygenation and hypoxic-ischemic injury than CPP support with PE. Future clinical studies comparing the effectiveness of various vasopressors for CPP support are warranted.
Key words: : CBF, CPP, NE, PE, TBI
Introduction
Low cerebral blood flow (CBF) states have been demonstrated in children early after traumatic brain injury (TBI) and have been correlated with poorer outcomes.1,2 Cerebral perfusion pressure (CPP) support following severe TBI is commonly implemented to correct cerebral hypoperfusion and prevent or minimize secondary brain injury.3 The most recent Brain Trauma Foundation guidelines recommended a minimum CPP threshold of 40 mm Hg with no recommendations on how to achieve this threshold or on what is an optimal upper limit for age- specific CPP thresholds.4
Vasopressors are typically employed as a treatment strategy to increase CPP after TBI by raising mean arterial pressure (MAP). Currently, there are no recommendations for the choice of vasopressor, and there are only limited studies comparing the effectiveness of commonly used agents for CPP support in TBI.5–9 Adult studies comparing vasopressor efficacy have been limited to single center retrospective and small prospective studies.6–9 None of these studies observed significant differences in CBF velocity, metabolism, or oxygenation. In a single center retrospective study of pediatric patients with severe TBI, phenylephrine (PE) followed by norepinephrine (NE) were found to be the most common vasopressors utilized, and post-hoc analysis did not reveal any significant differences in cerebral hemodynamic responses between the two vasopressors.5
We have developed a critical care model of pediatric closed head injury that utilizes a large animal model to enable full clinical modalities used in neurointensive care, as well as a recently employed clinically relevant anesthetic plan to improve the translational potential.10,11 We have previously reported that early CPP support to 70 mm Hg with PE resulted in a greater reduction in metabolic crisis and cell injury volumes than targeting a CPP of 40 mm Hg.12 The goal of this study was to compare the efficacy of PE with NE to target a CPP of 70 mm Hg early after nonimpact inertial brain injury. To determine differences in the injured brain's response to PE and NE, we measured cerebrovascular physiology including CBF, brain tissue oxygen tension, and cellular metabolism (via microdialysis) and assessed short-term neuropathology.
Methods
Animal preparation
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Female, 4-week-old piglets (8–10 kg), were pre-medicated with intramuscular injection of ketamine (20 mg/kg) and xylazine (2 mg/kg) followed by 4% isoflurane and 100% FiO2 via snout mask, until abolishment of a reflexive withdrawal to a pinch stimulus. Endotracheal intubation was followed by a decrease in FiO2 to 21–30%, and maintenance anesthesia was provided using 2% isoflurane. Femoral artery and venous catheters were placed for continuous MAP monitoring, arterial blood gas sampling, and intravenous (IV) fluid administration. Vital signs, including heart rate, respiratory rate, MAP zeroed to the right atrium, arterial oxygen saturation (SaO2), end tidal CO2, and rectal temperature, were recorded every 10 min for the duration of the experiment. A 20 mL/kg normal saline (NS) bolus was administered for replacement of pre-anesthetic dehydration secondary to required fasting, followed by an NS infusion at 4 mL/kg/h. Mechanical ventilation was titrated with goals of normoxia (PaO2 >90 mmHg) and normocarbia (PaCO2 38–45 mm Hg). A core body temperature goal of 37–38.5°C to ensure accurate thermal diffusion CBF measurements was accomplished with conductive warming/cooling blanket system (Gaymar, Orchard Park, NY). Piglets were then given a bolus of 1 mg/kg midazolam and 50 μg/kg fentanyl and started on midazolam and fentanyl infusions (1 mg/kg/h and 100 μg/kg/h, respectively), and isoflurane was discontinued. Pre- and post-injury arterial blood gas samples were obtained hourly (Nova Biomedical, Waltham, MA) until euthanasia 6 h after injury.
Nonimpact rotational brain injury
Animals (n=16) experienced rapid sagittal head rotations without impact (angle rotation 90 degrees over 10–12 msec) as described previously.13–15 Angular velocity was measured using an angular rate sensor (ATA, Albuquerque, NM) attached to the linkage sidearm. After injury, the animal was removed from the bite plate and placed in the prone position.
Neuromonitoring
After injury, four burr holes were prepared for placement of fiberoptic intracranial pressure monitor, CBF thermal diffusion probe, brain tissue oxygen monitor, and microdialysis probe. The fiberoptic intracranial pressure probe (Integra, Plainsboro, NJ), was inserted 0.5 cm posterior to the coronal suture and 0.5 cm to the left of the sagittal suture and secured with a single lumen bolt. The Licox catheter system (Integra, Plainsboro, NJ) was placed to measure brain tissue oxygenation (PbtO2) 1 cm anterior to the intracranial pressure probe. The brain tissue oxygen probe was inserted to a depth of 1.5 cm, secured with bone wax, and allowed to equilibrate for 30 min before values were recorded. A hyperoxia test was performed to confirm proper catheter placement. Data for intracranial pressure and PbtO2 were recorded every 15 min until euthanasia.
A microdialysis probe (PAS 12, 4 mm length, CMA, North Chelmsford, MA) was placed on the opposite side of the skull from the PbtO2 catheter into subcortical white matter. Immediately after insertion, the probe was infused with sterile 0.9% NaCl at a rate of 1 μL/min. Dialysate samples were collected every 30 min until euthanasia, and stored at −80°C and analyzed for lactate and pyruvate using a CMA600 analyzer (CMA, North Chelmsford, MA).
CBF of the right frontal lobe subcortical white matter was measured continuously with a thermal diffusion intraparenchymal probe (Hemedex, Cambridge, MA) inserted 1 cm anterior to the microdialysis probe.16,17
Cerebral perfusion modulation
One hour after injury, animals were randomized to receive either PE (n=8) or NE (n=8) infusions titrated to a CPP >70 mm Hg for 5 h until euthanasia.
Histology
At 6 hours post-injury, animals were euthanized via an overdose of pentobarbital. Brains were perfusion fixed, and two 6 μm sections were cut from every 3 mm block. Sections were stained with Hematoxylin and Eosin (H&E), or with the immunohistochemical marker for axonal injury beta-amyloid precursor protein (β-APP) (Chemicron 22C11 used at dilution of 1:5000) and counterstained with Meyer's hematoxylin. Areas of cell injury on H&E staining were identified by changes in staining intensity, and characterized by cell shrinkage and cytoplasmic eosinophilia, so-called “red cell change.”18 Regions of β−APP immunoreactivity and cell injury were noted by a blinded neuropathologist. and the locations of white matter damage and cell injury were traced in each slice using our previously described procedure to determine total area.10 Total and injured areas were multiplied by section thickness to determine total and injured brain volumes.
Statistical analysis
Physiological, arterial blood gas, and neuromonitoring parameter (intracranial pressure [ICP], PbtO2, microdialysis, CBF) data were analyzed across groups and time using repeated measures ANOVA tests, and Tukey–Kramer tests were used for post-hoc analysis with significance defined as p<0.05. Neuropathology comparisons between groups were analyzed using Student's t test.
Results
Injury and physiological data
Angular peak velocity and acceleration were similar between the two groups (Table 1). Physiological and arterial blood gas data for NE and PE are shown in Table 2 and Table 3 respectively. Heart rate was significantly higher in the NE group after vasopressor initiation for CPP modulation. Higher blood glucose and lactate levels 5 h after initiation of vasopressor support occurred in animals receiving NE (Table 3). It is of note that we observed a baseline metabolic alkalosis in all animals consistent with previous reports.19
Table 1.
Injury, Weight, and Vasopressor Dose Table
| Parameter | Phenylephrine | Norepinephrine |
|---|---|---|
| Weight (kg) | 8.4±1.4 | 8.6±0.8 |
| Velocity (rad/sec) | 142±3.1 | 142.2±1.7 |
| Acceleration (rad/sec2) | 62,603±10,369 | 55,911±6,919 |
| Maximum vasopressor dosage (μg/kg/min) | 7.9±5.2 | 0.9±0.7 |
Table 2.
Physiological Data Pre-Injury, 1 h, 3 h, and 6 h Post-Injury
| Parameter | Phenylephrine | Norepinephrine |
|---|---|---|
| Heart rate (beats/min) | ||
| Baseline | 117±17 | 122±14 |
| 1 h | 92±14 | 102±26 |
| 3 h | 66±14 | 147±55* |
| 6 h | 81±25 | 127±16* |
| MAP (mm Hg) | ||
| Baseline | 70±9 | 76±7 |
| 1 h | 73±10 | 71±5 |
| 3 h | 87±5 | 86±12 |
| 6 h | 90±9 | 88±15 |
| Temperature (°C) | ||
| Baseline | 36.4±0.2 | 36.5±0.5 |
| 1 h | 36.5±0.5 | 36.6±0.6 |
| 3 h | 36.9±0.4 | 37.3±0.6 |
| 6 h | 37.2±0.5 | 37.5±0.5 |
Denotes statistically significant difference, p<0.05 with Tukey–Kramer post-hoc analysis,±standard deviation.
MAP, mean arterial pressure.
Table 3.
Arterial Blood Gas Values
| Parameter | Phenylephrine | Norepinephrine |
|---|---|---|
| pH | ||
| Baseline | 7.54±0.04 | 7.55±0.03 |
| 1 h | 7.54±0.04 | 7.54±0.05 |
| 3 h | 7.54±0.04 | 7.54±0.04 |
| 6 h | 7.55±0.03 | 7.52±0.04 |
| PaCO2 (torr) | ||
| Baseline | 43.5±2.0 | 42.2±3.6 |
| 1 h | 43.7±4.9 | 40.5±2.8 |
| 3 h | 40.5±0.5 | 41.3±4.4 |
| 6 h | 37.8±2.5 | 40.6±4.7 |
| PaO2 (torr) | ||
| Baseline | 115.1±19.9 | 119.1±20.7 |
| 1 h | 123.6±15.6 | 127.9±11.5 |
| 3 h | 125.1±23.1 | 121.7±22.1 |
| 6 h | 115.2±13.0 | 123.8±18.4 |
| Lactate (mmol/L) | ||
| Baseline | 1.5±0.1 | 1.2±0.3 |
| 1 h | 0.9±0.2 | 0.9±0.2 |
| 3 h | 1.0±0.2 | 1.7±0.2 |
| 6 h | 0.7±0.1 | 1.5±0.4* |
| Sodium (mmol/L) | ||
| Baseline | 141.2±2.5 | 140.8±2.9 |
| 1 h | 139.6±2.3 | 140.1±2.1 |
| 3 h | 138.0±2.1 | 140.1±4.3 |
| 6 h | 138.1±1.8 | 137.8±2.2 |
| Glucose (mg/dL) | ||
| Baseline | 73±8 | 75±10 |
| 1 h | 130±20 | 126±22 |
| 3 h | 131±25 | 175±31 |
| 6 h | 88±20 | 175±28* |
Arterial blood gas data pre-injury, 1 h, 3 h, and 6 h post-injury.
Denotes statistically significant difference, p<0.05±standard deviation.
Intracranial monitoring
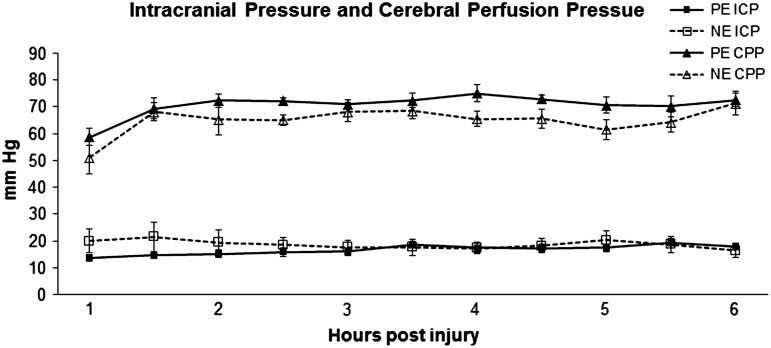
After initiation of CPP modulation with NE or PE infusion, there was no statistically significant difference in CPP between the groups (Fig. 1). ICP was unchanged after the initiation of vasopressor infusions for CPP support (Fig. 1).
FIG. 1.
Intracranial pressure (ICP) and cerebral perfusion pressure (CPP) over time. PE, phenylephrine; NE, norepinephrine.
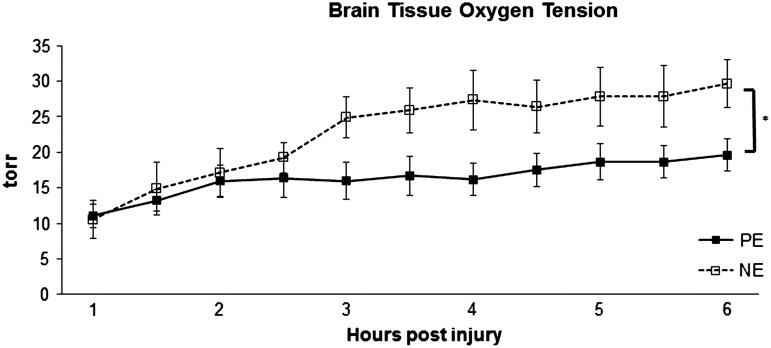
In order to assess brain tissue's response to CPP modulation, we measured PbtO2, microdialysate lactate pyruvate ratios (LPR), and CBF. PbtO2 was higher for NE than for PE 2 h after the initiation of CPP support (Fig. 2). PbtO2 responded to CPP modulation in both groups, and was significantly higher during the post-injury interval of 3–6 h compared with initial values measured within 1 h of injury in both groups.
FIG. 2.
Brain tissue oxygen tension over time. PE, phenylephrine; NE, norepinephrine. *p<0.05 for drug effect on two way ANOVA analysis.
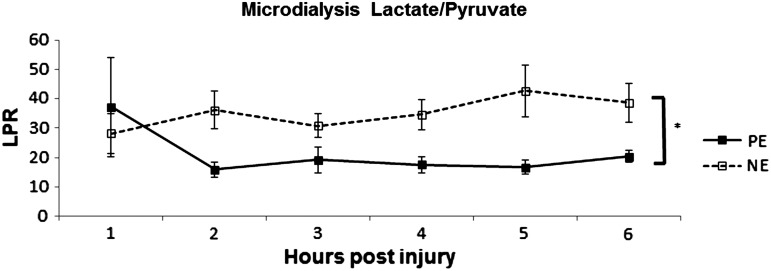
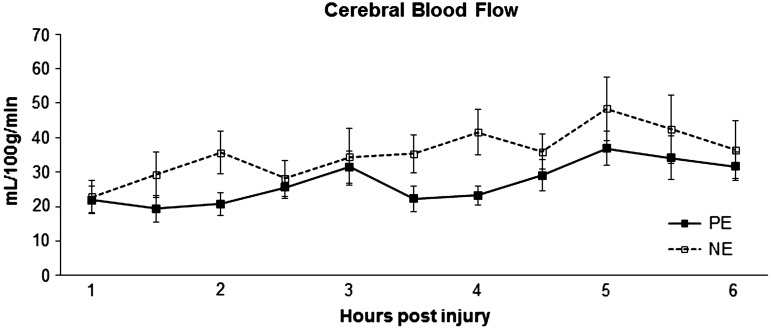
After initiation of CPP support, PE had significantly lower LPR than NE (p<0.05) (Fig. 3). There was no correlation between microdialysate and serum lactate levels. Repeated measures ANOVA analysis of CBF revealed only a significant time effect (Fig. 4) (p<0.05) with CBF increasing in both groups over time.
FIG. 3.
Lactate pyruvate ratio (LPR) over time. PE, phenylephrine. NE, norepinephrine. *p<0.05.
FIG. 4.
Cerebral blood flow over time measured via thermal diffusion. PE, phenylephrine; NE, norepinephrine.
Neuropathology
Neuropathology revealed regions of injury predominantly in the frontal and parietal lobes. One animal was excluded from traumatic axonal injury analysis because of global ischemic injury with widespread associated ischemic white matter injury, which prevented accurate assessment of traumatic axonal injury. Traumatic axonal injury volumes at 6 h post- injury as determined by β-APP immunohistochemistry were not different between the two groups (Fig. 5). Cell injury volumes determined by H&E were significantly larger in NE than in PE (1.4% vs. 0.4%, p<0.05). Regions of cell injury were predominantly observed in cortical regions of the frontal lobes.
FIG. 5.
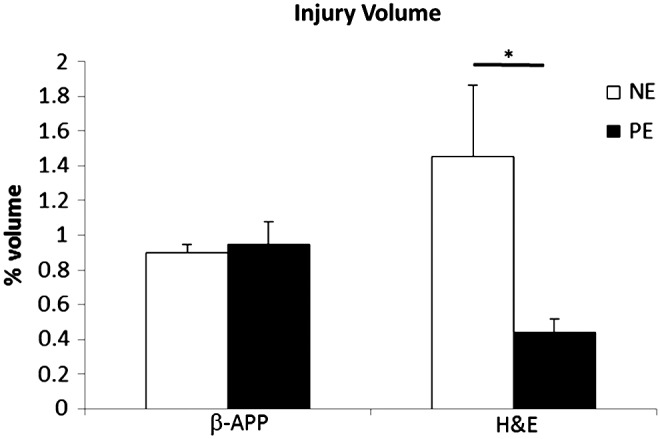
Traumatic axonal injury volumes determined by beta-amyloid precursor protein (β-APP) immunohistochemistry and cell injury volumes determined by hematoxylin and eosin (H&E) at 6 h post-injury. PE, phenylephrine; NE, norepinephrine. *p<0.05.
Discussion
We have previously demonstrated a neuroprotective effect of early CPP augmentation following TBI with PE to a CPP of 70 mm Hg in a clinically relevant large animal model of pediatric neurocritical care.12 However, several other vasopressors are commonly utilized clinically to augment CPP, and the conclusions in our previous study were limited by the use of volatile anesthetics during the post-injury period. In the present study, utilizing an opioid-benzodiazepine-based anesthetic protocol, we compared early CPP augmentation with PE or NE, and identified differences in physiologic responses, brain tissue oxygen, cellular metabolism, and acute neuropathology.
Physiologic effects of utilizing a clinically relevant anesthetic protocol
In our previous study, animals were anesthetized with a combination of inhaled isoflurane and intravenous fentanyl infusions for the duration of the study. Volatile anesthetics have well-known effects on cerebral autoregulation and neuroprotection, and are not typically utilized in the intensive care management of the child with a severe brain injury, a limitation in our previous study.20–24 Furthermore, uninjured immature swine anesthetized with an opioid-benzodiazepine-based anesthetic protocol have a preserved autoregulation response to vasopressor challenges compared with animals anesthetized with a volatile anesthetic-based protocol.11 This is illustrated by the differences in CBF measured in our current experiments compared with previous studies that utilized a volatile anesthetic-based protocol.11 CPP augmentation to 70 mm Hg with PE resulted in significantly higher CBF values in swine receiving a volatile anesthetic-based protocol than in our current studies (Table 4). The translatability of our findings to the clinical setting in the intensive care unit (ICU) is strengthened by the use of an opioid-benzodiazepine-based anesthetic protocol.
Table 4.
Cerebral Blood Flow at 6 h Post-Injury
| Group | Cerebral blood flow (mL/100g/min) | Cerebral perfusion pressure (mm Hg) |
|---|---|---|
| Sham | 35±10 | 71±11 |
| Sham with isoflurane | 60±9 | 54±2 |
| Injury+norepinephrine | 37±12 | 71±7 |
| Injury+phenylephrine | 32±10 | 73±7 |
| Injury+phenylephrine with isoflurane | 53±8 | 71±4 |
Differences in physiologic effects of CPP augmentation with PE and NE
We expected differences in physiologic responses of the animals to PE and NE infusions based on the α-agonist-only activity of PE and the α and β-agonist activity of NE. Not surprisingly, animals receiving PE infusions developed a relative reflexive bradycardia during CPP augmentation, whereas animals receiving NE did not. Similar findings were found in a retrospective cohort of adult TBI patients receiving vasopressor infusions.6 We did not observe any evidence for systemic tissue hypoperfusion in either group. Although arterial blood lactate levels were statistically higher at 6 h post-injury in the NE group (Table 3), this was not clinically significant. Previous studies in adults have reported an increase in adult respiratory distress syndrome (ARDS) with aggressive CPP support, and pulmonary edema has been reported in adult swine receiving phenylephrine infusions for CPP support.25,26 We did not observe any changes in PaO2 or FiO2 requirements during PE or NE infusions. Norepinephrine is a known an inhibitor of insulin release from pancreatic β-cells, whereas phenylephrine has been reported to have little or no influence on blood glucose levels.27,28 Not surprisingly, we did observe higher blood glucose levels in animals receiving NE infusions than in animals receiving PE infusions.
CPP support with PE or NE improves CBF following nonimpact brain injury
Human studies of direct repeated measurements of CBF over time during cerebral resuscitation are limited.29 Our clinically relevant large animal model provides a unique opportunity to monitor changes in CBF, and, more accurately models early cerebrovascular dynamics in the severely brain-injured child. Increasing CPP with either PE or NE increased CBF, with slightly larger increases observed with NE. No increases in ICP were observed during CPP support during this early post-injury time interval. It should be noted that CPP support to 70 mm Hg with either vasopressor resulted in CBF reaching levels previously observed in uninjured animals receiving the same anesthetic protocol (Table 4).11
NE infusions for CPP support improved PbtO2, but worsened brain cellular metabolic crisis
Increases in PbtO2 following initiation of CPP support was observed in both groups, but animals receiving NE infusions had statistically significantly higher PbtO2. These differences in PbtO2 can be at least partly attributed to higher increases in CBF in animals receiving NE infusions. Increases in regional oxygenation and CBF have also been found in adult severe TBI patients receiving NE to drive CPP to >70 mm Hg.30 CPP augmentation with NE did not reduce microdialysate LPR similar to findings in adult patients with TBI.30 However, vasopressor support with PE did result in a reduction in LPR, similar to our previous results.12 Interpretation of these varied responses to two different vasopressors is complex, but discussion of the limitations of the multimodal monitoring methods utilized and the relevant literature provides some insight. There is no clear consensus on the physiological interpretation of PbtO2.31 Most likely, PbtO2 reflects the accumulation of oxygen in the brain extracellular space, which is directly influenced by three factors: oxygen delivery, oxygen diffusion characteristics of the region of interest, and oxygen utilization by the brain tissue. If we assume that infusions of PE and NE have similar effects on diffusion characteristics of the region of interest, differences in PbtO2 can be attributed to oxygen delivery and/or oxygen utilization. Animals receiving NE had higher CBF, although not statistically significant compared with PE. With regard to oxygen utilization, brain mitochondrial dysfunction has been previously reported in swine undergoing nonimpact inertial brain injury.32 Furthermore, in vitro exposure of neurons to NE, epinephrine, or dopamine resulted in induction of mitochondrial dysfunction and exacerbation of oxidative stress.33 Despite similar CBF between NE and PE, we speculate that reductions in mitochondrial function with NE may be responsible for less oxygen utilization (higher PbtO2) and more hypoxic-ischemic injury (Fig. 5) than PE. Similar studies with α-only agonists such as PE have not been published, to our knowledge. Further investigation is warranted on the effects of various vasopressors on brain mitochondrial dysfunction following TBI.
Early CPP augmentation with PE results in greater reduction in cell injurythan with NE
In our model, we evaluated the efficacy of CPP support with PE compared with NE before severe intracranial hypertension. At 6 h post-injury, there were no differences in the amount of axonal injury detected by β-APP immunohistochemistry, similar to our previous investigations. CPP support early after TBI may not alter axonal injury, or the early time point for evaluation may preclude detecting differences between the groups.34,35 Both groups demonstrated a reduction in cell injury compared with previous investigations with no CPP support, or only to a level of 40 mm Hg. However, animals receiving PE had less cell injury than those receiving NE. One possible explanation is the proposed differences in the vasopressors' effect on mitochondrial dysfunction, as described.
Limitations
There are several limitations to our experimental design, which must be considered when attempting to translate our findings to the ICU. First, our model of nonimpact inertial brain injury does not encompass the full range of the pathophysiology of pediatric TBI. Specifically, the pathophysiologic responses of pericontusional tissue may differ from what we observed in our diffuse brain injury model. The newly funded comparative effectiveness trial in pediatric TBI will, we hope, provide further insight into the efficacy of various vasopressors for CPP support.36 Second, our time point for histological examination was relatively acute, and differences in the response of white matter injury to the effects of our intervention may not be apparent at such an earlier post-injury time point.34,35 Third, animals in our study did not achieve severe intracranial hypertension, because of the short post-injury interval. It is unclear if cerebrovascular responses to NE and PE would change in the presence of severe intracranial hypertension. Finally, we only utilized female swine in our studies, and sex-dependent differences in the cerebrovascular responses to various vasopressors in swine following TBI have been reported.37–39
Conclusion
Utilizing a clinically relevant opioid-benzodiazepine-based anesthetic protocol, early CPP support following TBI with NE or PE resulted in differences in brain tissue oxygen, cellular metabolism, and acute neuropathology. Early CPP augmentation after TBI with NE resulted in greater increase in brain tissue oxygen tension than augmentation with PE, despite similar increases in CBF. However, animals receiving PE were found to have greater reduction in metabolic crisis and cell injury. Future clinical studies comparing the effectiveness of various vasopressors for CPP support are warranted.
Acknowledgments
This work was supported by National Institutes of Health grants K08-NS064051 (S.H.F.) R01-NS39679 (S.S.M.), and U01-NS069545 (S.S.M. and T.J.K.)
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adelson P.D., Clyde B., Kochanek P.M., Wisniewski S.R., Marion D.W., and Yonas H. (1997). Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr. Neurosurg. 26, 200–207 [DOI] [PubMed] [Google Scholar]
- 2.Adelson P.D., Srinivas R., Chang Y., Bell M., and Kochanek P.M. (2011). Cerebrovascular response in children following severe traumatic brain injury. Childs Nerv. Syst. 27, 1465–1476 [DOI] [PubMed] [Google Scholar]
- 3.Diringer M.N., and Axelrod Y. (2007). Hemodynamic manipulation in the neuro-intensive care unit: cerebral perfusion pressure therapy in head injury and hemodynamic augmentation for cerebral vasospasm. Curr. Opin. Crit. Care 13, 156–162 [DOI] [PubMed] [Google Scholar]
- 4.Kochanek P.M., Carney N., Adelson P.D., Ashwal S., Bell M.J., Bratton S., Carson S., Chesnut R.M., Ghajar J., Goldstein B., Grant G.A., Kissoon N., Peterson K., Selden N.R., Tasker R.C., Tong K.A., Vavilala M.S., Wainwright M.S., Warden C.R., American Academy of Pediatrics–Section on Neurological, S., American Association of Neurological Surgeons/Congress of Neurological, S., Child Neurology, S., European Society of, P., Neonatal Intensive, C., Neurocritical Care, S., Pediatric Neurocritical Care Research, G., Society of Critical Care, M., Paediatric Intensive Care Society, U.K., Society for Neuroscience in, A., Critical, C., World Federation of Pediatric, I. and Critical Care, S. (2012). Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents—second edition. Pediatr. Crit. Care. Med. 13, Suppl. 1, S1–82 [DOI] [PubMed] [Google Scholar]
- 5.Di Gennaro J.L., Mack C.D., Malakouti A., Zimmerman J.J., Armstead W., and Vavilala M.S. (2010). Use and effect of vasopressors after pediatric traumatic brain injury. Dev. Neurosci. 32, 420–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sookplung P., Siriussawakul A., Malakouti A., Sharma D., Wang J., Souter M.J., Chesnut R.M., and Vavilala M.S. (2011). Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit. Care 15, 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ract C., and Vigue B. (2001). Comparison of the cerebral effects of dopamine and norepinephrine in severely head-injured patients. Intensive Care Med. 27, 101–106 [DOI] [PubMed] [Google Scholar]
- 8.Steiner L.A., Johnston A.J., Czosnyka M., Chatfield D.A., Salvador R., Coles J.P., Gupta A.K., Pickard J.D., and Menon D.K. (2004). Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head-injured patients. Crit. Care Med. 32, 1049–1054 [DOI] [PubMed] [Google Scholar]
- 9.Johnston A.J., Steiner L.A., Chatfield D.A., Coles J.P., Hutchinson P.J., Al-Rawi P.G., Menon D.K., and Gupta A.K. (2004). Effect of cerebral perfusion pressure augmentation with dopamine and norepinephrine on global and focal brain oxygenation after traumatic brain injury. Intensive Care Med. 30, 791–797 [DOI] [PubMed] [Google Scholar]
- 10.Friess S.H., Ralston J., Eucker S.A., Helfaer M.A., Smith C., and Margulies S.S. (2011). Neurocritical care monitoring correlates with neuropathology in a swine model of pediatric traumatic brain injury. Neurosurgery 69, 1139–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruins B., Kilbaugh T.J., Margulies S.S., and Friess S.H. (2013). The anesthetic effects on vasopressor modulation of cerebral blood flow in an immature swine model. Anesth. Analg. 116, 838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friess S.H., Smith C., Kilbaugh T.J., Frangos S.G., Ralston J., Helfaer M.A., and Margulies S.S. (2012). Early cerebral perfusion pressure augmentation with phenylephrine after traumatic brain injury may be neuroprotective in a pediatric swine model. Crit. Care Med. 40, 2400–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friess S.H., Ichord R.N., Owens K., Ralston J., Rizol R., Overall K.L., Smith C., Helfaer M.A., and Margulies S.S. (2007). Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp. Neurol. 204, 234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghupathi R., and Margulies S.S. (2002). Traumatic axonal injury after closed head injury in the neonatal pig. J. Neurotrauma 19, 843–853 [DOI] [PubMed] [Google Scholar]
- 15.Raghupathi R., Mehr M.F., Helfaer M.A., and Margulies S.S. (2004). Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J. Neurotrauma 21, 307–316 [DOI] [PubMed] [Google Scholar]
- 16.Vajkoczy P., Roth H., Horn P., Lucke T., Thome C., Hubner U., Martin G.T., Zappletal C., Klar E., Schilling L., and Schmiedek P. (2000). Continuous monitoring of regional cerebral blood flow: experimental and clinical validation of a novel thermal diffusion microprobe. J. Neurosurg. 93, 265–274 [DOI] [PubMed] [Google Scholar]
- 17.Clausen T., Scharf A., Menzel M., Soukup J., Holz C., Rieger A., Hanisch F., Brath E., Nemeth N., Miko I., Vajkoczy P., Radke J., and Henze D. (2004). Influence of moderate and profound hyperventilation on cerebral blood flow, oxygenation and metabolism. Brain Res. 1019, 113–123 [DOI] [PubMed] [Google Scholar]
- 18.Eucker S.A., Smith C., Ralston J., Friess S.H., and Margulies S.S. (2011). Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Exp. Neurol. 227, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannon J.P., Bossone C.A., and Wade C.E. (1990). Normal physiological values for conscious pigs used in biomedical research. Lab. Anim. Sci. 40, 293–298 [PubMed] [Google Scholar]
- 20.Cucchiara R.F., Theye R.A., and Michenfelder J.D. (1974). The effects of isoflurane on canine cerebral metabolism and blood flow. Anesthesiology 40, 571–574 [DOI] [PubMed] [Google Scholar]
- 21.Todd M.M., and Weeks J. (1996). Comparative effects of propofol, pentobarbital, and isoflurane on cerebral blood flow and blood volume. J. Neurosurg. Anesthesiol. 8, 296–303 [DOI] [PubMed] [Google Scholar]
- 22.Smith J.H., Karsli C., Lagace A., Luginbuehl I., Barlow R., and Bissonnette B. (2005). Cerebral blood flow velocity increases when propofol is changed to desflurane, but not when isoflurane is changed to desflurane in children. Acta Anaesthesiol. Scand. 49, 23–27 [DOI] [PubMed] [Google Scholar]
- 23.Kochs E., Hoffman W.E., Werner C., Albrecht R.F., and Schulte am Esch J. (1993). Cerebral blood flow velocity in relation to cerebral blood flow, cerebral metabolic rate for oxygen, and electroencephalogram analysis during isoflurane anesthesia in dogs. Anesth. Analg. 76, 1222–1226 [DOI] [PubMed] [Google Scholar]
- 24.Statler K.D., Alexander H., Vagni V., Holubkov R., Dixon C.E., Clark R.S., Jenkins L.W., and Kochanek P.M. (2006). Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Res. 1076, 216–224 [DOI] [PubMed] [Google Scholar]
- 25.Contant C.F., Valadka A.B., Gopinath S.P., Hannay H.J., and Robertson C.S. (2001). Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J. Neurosurg. 95, 560–568 [DOI] [PubMed] [Google Scholar]
- 26.Malhotra A.K., Schweitzer J.B., Fox J.L., Fabian T.C., and Proctor K.G. (2003). Cerebral perfusion pressure directed therapy following traumatic brain injury and hypotension in swine. J. Neurotrauma 20, 827–839 [DOI] [PubMed] [Google Scholar]
- 27.Straub S.G., and Sharp G.W. (2012). Evolving insights regarding mechanisms for the inhibition of insulin release by norepinephrine and heterotrimeric G proteins. Am. J. Physiol. Cell Physiol. 302, C1687–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imura H., Kato Y., Ikeda M., Morimoto M., and Yawata M. (1971). Effect of adrenergic-blocking or -stimulating agents on plasma growth hormone, immunoreactive insulin, and blood free fatty acid levels in man. J. Clin. Invest. 50, 1069–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenthal G., Sanchez–Mejia R.O., Phan N., Hemphill J.C., 3rd, Martin C., and Manley G.T. (2011). Incorporating a parenchymal thermal diffusion cerebral blood flow probe in bedside assessment of cerebral autoregulation and vasoreactivity in patients with severe traumatic brain injury. J. Neurosurg. 114, 62–70 [DOI] [PubMed] [Google Scholar]
- 30.Johnston A.J., Steiner L.A., Coles J.P., Chatfield D.A., Fryer T.D., Smielewski P., Hutchinson P.J., O'Connell M.T., Al-Rawi P.G., Aigbirihio F.I., Clark J.C., Pickard J.D., Gupta A.K., and Menon D.K. (2005). Effect of cerebral perfusion pressure augmentation on regional oxygenation and metabolism after head injury. Crit. Care Med. 33, 189–195 [DOI] [PubMed] [Google Scholar]
- 31.Rohlwink U.K., and Figaji A.A. (2010). Methods of monitoring brain oxygenation. Childs Nerv. Syst. 26, 453–464 [DOI] [PubMed] [Google Scholar]
- 32.Kilbaugh T.J., Bhandare S., Lorom D.H., Saraswati M., Robertson C.L., and Margulies S.S. (2011). Cyclosporin A preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J. Neurotrauma 28, 763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu W., Luo H., Parthasarathy S. and Mattson M.P. (1998). Catecholamines potentiate amyloid beta-peptide neurotoxicity: involvement of oxidative stress, mitochondrial dysfunction, and perturbed calcium homeostasis. Neurobiol. Dis. 5, 229–243 [DOI] [PubMed] [Google Scholar]
- 34.Weeks D., Sullivan S., Kilbaugh T., Smith C., and Margulies S.S. (2014). Influences of developmental age on the resolution of diffuse traumatic intracranial hemorrhage and axonal injury. J. Neurotrauma 31, 206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellewell S.C., Yan E.B., Agyapomaa D.A., Bye N., and Morganti–Kossmann M.C. (2010). Post-traumatic hypoxia exacerbates brain tissue damage: analysis of axonal injury and glial responses. J. Neurotrauma 27, 1997–2010 [DOI] [PubMed] [Google Scholar]
- 36.Bell M.J., Adelson P.D., Hutchison J.S., Kochanek P.M., Tasker R.C., Vavilala M.S., Beers S.R., Fabio A., Kelsey S.F., Wisniewski S.R., and Multiple Medical Therapies for Pediatric Traumatic Brain Injury Workgroup (2013). Differences in medical therapy goals for children with severe traumatic brain injury-an international study. Pediatr. Crit. Care Med. 14, 811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstead W.M., Kiessling J.W., Kofke W.A., and Vavilala M.S. (2010). Impaired cerebral blood flow autoregulation during posttraumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by extracellular signal-related kinase mitogen-activated protein kinase upregulation. Crit. Care Med. 38, 1868–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstead W.M., Kiessling J.W., Kofke W.A., and Vavilala M.S. (2010). SNP improves cerebral hemodynamics during normotension but fails to prevent sex dependent impaired cerebral autoregulation during hypotension after brain injury. Brain Res. 1330, 142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstead W.M., Kiessling J.W., Riley J., Kofke W.A., and Vavilala M.S. (2011). Phenylephrine infusion prevents impairment of ATP and calcium sensitive k channel mediated cerebrovasodilation after brain injury in female but aggravates impairment in male piglets through modulation of ERK MAPK upregulation. J. Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]