Abstract
Cardiac tissue engineering constructs are a promising therapeutic treatment for myocardial infarction, which is one of the leading causes of death. In order to further advance the development and regeneration of engineered cardiac tissues using biomaterial platforms, it is important to have a complete overview of the effects that substrates have on cardiomyocyte (CM) morphology and function. This article summarizes recent studies that investigate the effect of mechanical cues on the CM differentiation, maturation, and growth. In these studies, CMs derived from embryos, neonates, and mesenchymal stem cells were seeded on different substrates of various elastic modulus. Measuring the contractile function by force production, work output, and calcium handling, it was seen that cell behavior on substrates was optimized when the substrate stiffness mimicked that of the native tissue. The contractile function reflected changes in the sarcomeric protein confirmation and organization that promoted the contractile ability. The analysis of the literature also revealed that, in addition to matrix stiffness, mechanical stimulation, such as stretching the substrate during cell seeding, also played an important role during cell maturation and tissue development.
Introduction
Myocardial infarction is one of the leading causes of death worldwide, accounting approximately for 30% of the total mortality, yearly.1 The effectiveness of existing mitigation treatments is low, and heart transplantation is restricted to the limited number of donors.2 Hence, cardiac tissue engineering constructs (cardiac patches) offer a promising novel cell-based therapeutic treatment for myocardial infarction.3 Approximately one third of the cells in the adult myocardium are cardiomyocytes (CMs), occupying 80–90% of the total myocardium volume, and have a low capacity to proliferate.4 The remaining two-thirds are non-myocytes, such as endothelial cells and fibroblasts, which are capable of proliferating. Thus, for the development, success, and implementation of the cardiac patch, a source of CMs is required. Furthermore, it is necessary to understand the influence of the cells' extracellular environment and, in particular, that of the patch (matrix) stiffness on the cell morphology and function.5,6 This has a crucial effect on the design of the biomaterial to be used for cardiac patch applications.
In this review, focus is on the effect that mechanical cues have on the functional maturation and morphology of embryonic and neonatal CMs. Topics that will be discussed are the effect of substrate elasticity on force production, work output, calcium handling, and sarcomeric protein conformation and organization, as well as the CM response to cyclic mechanical strains.
Microenvironmental Effect on CMs
CMs are mechanically anisotropic7–10 and their main characteristics are their rhythmic beating11 and their inability to proliferate in the postembryonic stage.4 A limited number of dead CMs are replaced with new ones12 through a progenitor pool, and in order for the new CMs to maintain the cardiac function and mechanical demands (i.e., adapt to tissue stresses due to pressure or volume overload) they grow in size by elongating and becoming thicker.13
This hypertrophy is achieved by increasing the contractile protein content, which results in an increase in size as new myofilaments are added in parallel or in series. Although one would expect biochemical factors to affect the contractile protein content, mechanical loading also plays a significant role in this protein's content. Furthermore, mechanical loads also affect the cytoskeletal or sarcomeric organization in order to regulate the cell shape and alignment.14,15 There are various forms of mechanical loadings that develop in cardiac patches and affect cell maturation and development, and these are due to the microenvironmental cues, including cell shape, extracellular matrix (ECM), biomolecules, neighboring cells, substrate stiffness, applied strain, and topology. The CM development is best on substrates of comparable stiffness to the native tissue16 (Table 1 summarizes the elasticity of different types of heart tissue). However, the influence of all mechanical factors on the CMs maturation should be understood in order to aid in the development of cardiac cell therapies.17–20 Particularly, the effect of the intrinsic load (cell contraction) on CMs is affected by the extrinsic dynamics and the surrounding microenvironment (Fig. 1).
Table 1.
Elastic Modulus Values of Different Myocardial Tissues
FIG. 1.
Micromechanical loading in cardiomyocytes (CMs). (a) The intrinsic load as a result of the contractile activity, (b) an extrinsic load may be applied to stimulate a preload. (c) The microenvironment influences the after-load of tension that is generated in cells in vitro; it can be controlled by surface chemistry, stiffness, topography, and 3D architecture of scaffolds.
Specialized proteins such as costameres, adherens junctions, and titin embedded in the cytoskeleton detect those mechanical inputs, by remodeling and adapting to the extracellular boundaries and extrinsic mechanical loads. The cytoskeleton is responsible for transmitting the mechanical inputs to mediate other functions, for example, signaling, excitability, impulse propagation, contractility, and gene expression. CM contractile forces are produced through the sliding of α-actinin and myosin filaments in sarcomeres, as triggered by action potentials and intracellular calcium signals.
Effect of Matrix Elasticity on Calcium Dynamics and Contractility
Several in vitro studies have investigated the effect of mechanical cues (substrate stiffness or applied strain) on the CM structure, function, as well as beating forces and velocities, demonstrating the effect on the expression and organization of the fiber assembly of contractile proteins.5,6,16,21,22
A main reason that substrate mechanics affect the CMs function is the fact that CMs are characterized by their rhythmic beating,11 as previously mentioned. During contraction, the calcium transient size and duration regulates the contractile force. Most of the calcium in adult CMs comes from the sarcoplasmic reticulum (SR), while calcium release is controlled by the sarcoplasmic/endoplasmic reticular calcium ATPase 2a (SERCA2a) and the ryanodine receptor (RyR2). SERCA2a controls the sarcoplasmic storage size and the release rate of the calcium ions by pumping calcium into sarcoplasmic stores. Moreover, RyR2 controls the release of calcium from the SR.23 SERCA2a is mainly regulated by phospholamban (PLN), which when phosphorylated is self-suppressed.24
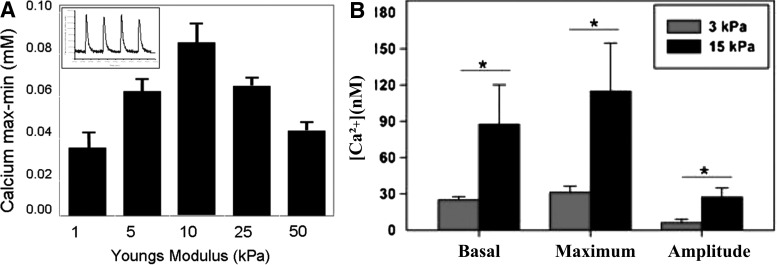
Studies on neonatal rat ventricular myocytes (NRVMs) (days 1–30) have demonstrated an increase in both RyR225 and SERCA2a26,27 during maturation; however, the PLN after embryo day 12 does not change significantly.25 Thus, in those CMs, changes occur in the regulation and amount of the calcium storage,28 leading to alteration in the calcium transient shape and size during cell contraction.29 A study investigated NRVMs that were grown on collagen-coated polyacrylamide (PA) gel substrates of different elastic moduli values ranging from 1 to 50 kPa.6 The substrate with a 10 kPa modulus (around that of native myocardium5) exhibited a maximum contractile force and amplitude of calcium dynamics. This revealed that the magnitude of the calcium transient, the amount of SR calcium and SERCA2a are correlated with the magnitude of force generation in NRVMs. Thus, calcium dynamics as a mechanism of contractile force generation is affected by the substrate stiffness as shown in Fig. 2A.
FIG. 2.
(A) The calcium transients of different matrices was measured as shown in the inset; it is seen that the magnitude of calcium transients on 10 kPa gels was significantly greater than transients on 1 and 50 kPa gels *p<0.05 (reproduced with permission from Liu et al.34). (B) The intracellular calcium increases with substrate stiffness. The basal levels, maximum calcium concentration, and the amplitude of the rise in calcium for a twitch contraction increase with substrate stiffness. *p<0.05 (reproduced with permission from Moorman et al.26).
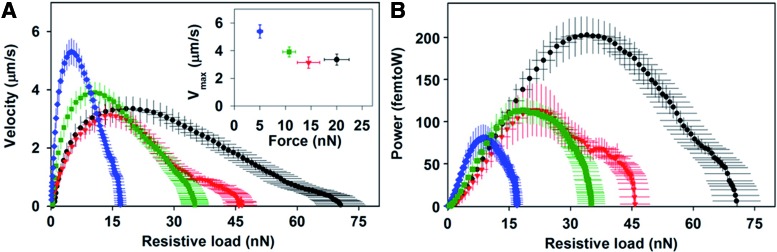
Moreover, another study which seeded NRVM on fibronectin-coated micropillar arrays of different elastic moduli values ranging from 3 to 20 kPa showed that NRVMs cultured on 15 kPa substrates had a higher calcium activity compared with the 3 kPa substrates as seen in Figure 2B.22 Furthermore, as the substrate modulus increased, the twitch (contraction) force, work, and power generated by a single cell increased, while the maximum velocity decreased (Fig. 3).22 It was concluded that the increase in the calcium levels along with changes in the sarcomeres structure lead to the observed increase in twitch power. This suggests that the substrate stiffness effect on the twitch power may influence the neonatal CM transition to a more adult phenotype.22
FIG. 3.
(A) The relationship between twitch force and velocity was plotted for the twitch contraction of CMs cultured on arrays with a stiffness of 3 kPa (blue diamonds), 8 kPa (green squares), 10 kPa (red triangles), and 15 kPa (black circles). Maximum velocity versus the instantaneous force at which maximum velocity was reached; (B) Twitch power was plotted as a function of the resistive load and shows that the maximum power of 200 femtoW increased with substrate stiffness (reproduced with permission from Moorman et al.26). Color images available online at www.liebertpub.com/teb
Effect of Matrix Stiffness on Sarcomere Organization and Contractility
In addition to the matrix stiffness affecting the calcium dynamics, the sarcomere organization and contractility of CMs are greatly influenced by the matrix stiffness. A recent study demonstrated that NRVMs seeded on collagen-coated PA gel substrates with an elastic modulus (measured by atomic force microscopy [AFM]) equal to 10 kPa showed well-defined sarcomeres.6 However, the sarcomeres of those seeded on matrices with an elastic modulus greater than 10 kPa or smaller than 10 kPa were less defined and unaligned and contained stress fibers (Fig. 4).6 These results suggest that NRVMs fail to mature when plated on substrates that are stiffer than the native myocardium through a mechanism that results in lower contractile force and involves Rho-associated protein kinase (ROCK pathway), as stimulation of the ROCK pathway leads to the formation of α-actinin stress fibers.30 It was observed that hydroxy fasudil inhibits the reduction in cell traction forces on substrates stiffer than 10 kPa by blocking the ROCK pathway.6 To overcome the formation of stress fibers on stiff matrices, they were treated with fasudil, which is a Rho-kinase inhibitor and vasodilator, enabling the development of well-defined sarcomeres.6 This result coincides with the hypothesis that α-actinin stress fiber formation interferes with the development of sarcomeres and the maturation of NRVMs. On the contrary, it has been shown that the role of individual stress fibers on softer surfaces contributes more toward intracellular tension and overall cell shape (Table 2).31 A different study demonstrated that a continuous tensional balance between substrate stiffness and internal remodeling of the contractile filaments exists, determining at any given time the cytoskeletal shape (prestress).32
FIG. 4.
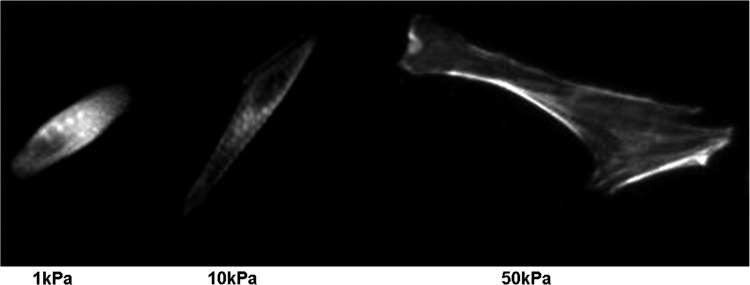
Neonatal rat ventricular myocytes on collagen-coated polyacrylamide gels and labeled for a α-actinin have poorly defined striations on soft 1 kPa substrates, well-defined and aligned striations on 10 kPa substrates, and unaligned striations with long, large stress fibers on stiff 50 kPa gels (reproduced with permission from Jacot et al.6).
Table 2.
Summary of Reported Effects of Substrate Stiffness on Cardiomyocytes Maturation and Growth
| Soft matrix (<1 kPa) | Intermediate matrix (5–10 kPa) | Stiff matrix (<30 kPa) | References |
|---|---|---|---|
| Lower spread of cells | Less spread of cells | High spread of cells | 5 |
| Poorly defined sarcomere | Well-organized sarcomere | Less-aligned sarcomere | 6,16 |
| Cells adopt an axially aligned shape | Cells adopt an axially aligned shape | Cells spread into irregular shapes | 6 |
| Low aspect ratio | Highest aspect rations | Low aspect ratio | |
| High disorganized myofibrillar architecture | Organized myofibrillar architecture | Striated myofibrils | 5,25 |
| Beating | Beating | Not beating | 5 |
In another study, inert polycaprolactone (PCL) planar layers of a different crosslinking density of varying Young's moduli ranging from 1 to 133 MPa (measured by tensile testing) were prepared. Interestingly, CMs seeded on softer substrates in the range of 0.91±0.08 and 1.53±0.16 MPa were mostly mature and had assembled sarcomeres.33 On the contrary, the CMs grown on the stiff PCL (49.67±2.56 and 133.23±8.67 MPa) expressed immature cardiac cell genes (Nkx-2.5) and proteins (GATA-4), in addition to a number of genes involved in inflammatory processes. This confirmed the hypertrophy that the differentiation of CM phenotypes is influenced by the substrate stiffness showing that softer substrates induce a more mature phenotype.33
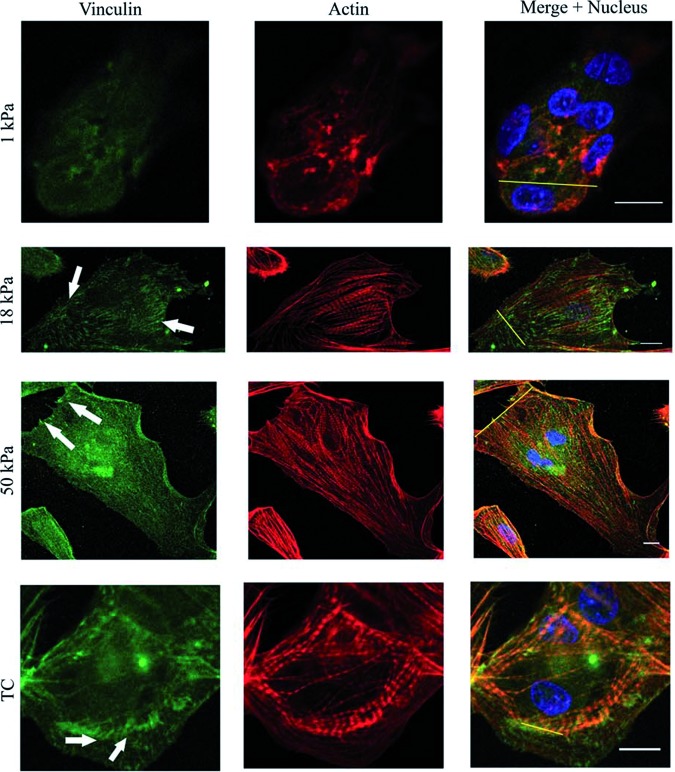
Furthermore, the effect of substrate stiffness on CM beating has also been investigated,16 by seeding CMs, derived from embryonic (day 8) chicks, on PA gel substrates of different stiffness for 1–5 days. The PA gel substrates that were considered had a stiffness of 1, 18, and 50 kPa. Initially, the CMs beat fastest on the 18 kPa substrate, confirming previous studies that myocytes beat fastest on substrates that mimic the native tissue.16 CMs beat synchronously when there is direct contact between each other, exerting more force compared with individual cells34; hence, after the fifth day of culture, the beating frequencies across the different substrates became more uniform, as the CMs had proliferated and came into contact with each other, with the fastest beating on the 50 kPa gel. This explains why some studies show that CMs function better on relatively stiffer substrates compared with other studies. The same study showed immunofluorescent imaging (Fig. 5) of the CMs focal adhesion (FA) formation and growth of the different cultures, demonstrating an increase over time in the FA area and number of myocytes on stiff matrices, and a decrease over time in the FA area and number of myocytes on soft matrices. The decrease in the FA number and area size indicates a less-organized sarcomeric cytoskeleton. Thus, soft matrices over time showed less organized sarcomeres as compared with the stiff matrices that had well-organized and aligned myofibrils.16
FIG. 5.
Relative fluorescent intensity of α-actinin and vinculin for cells on the substrates. Sections of cardiac cells expressing vinculin and α-actinin proteins on the different substrates are shown. Fluorescence intensity profiles depict the area of the line drawn in the merged images. The arrows show well-defined mature focal adhesions. Scale bar: 10 μm (reproduced with permission from Bajaj et al.16). Color images available online at www.liebertpub.com/teb
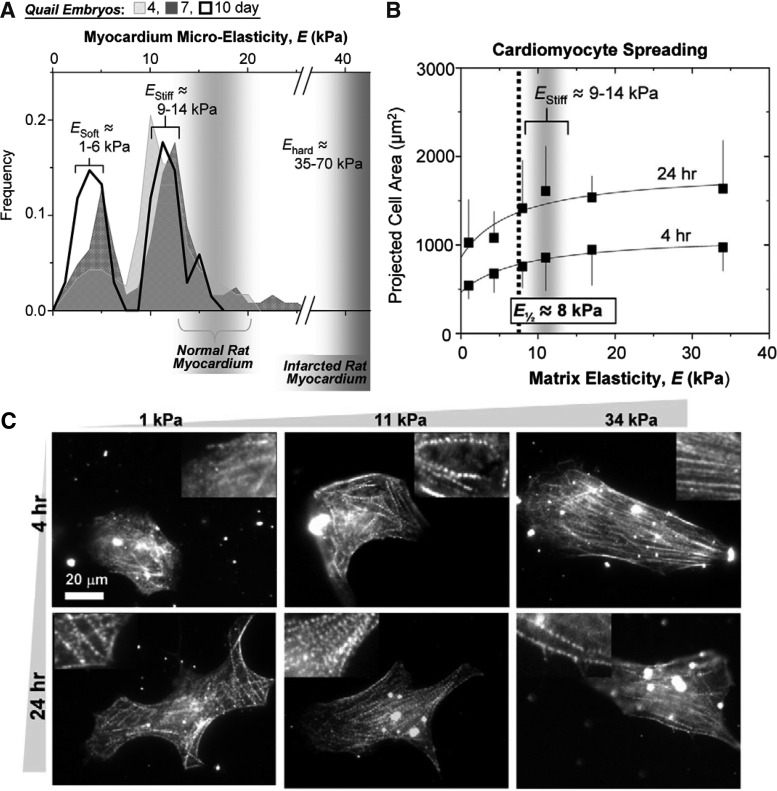
Although myofibril formation in CMs has been extensively studied in vitro, the difficulty of obtaining a rhythmically contractile phenotype on hard substrates is clearly illustrated.5 Rhythmic beating of cultured CMs depends on the efficient spreading of the cells on the substrate and on myofibril reassembly.35,36 The effect of substrate stiffness on the morphological and the functional behavior of isolated chick embryonic CMs seeded on collagen-coated PA gels has been investigated,5 indicating that cells on stiff substrates appear to be well spread (Fig. 6A and 6B); however, striation was observed in very few cells. The optimum substrate stiffness allowed the cells to reassemble their striations and transmit the maximum contractile work to the matrix. It was shown that the α-actinin for the first-day culture on the 10 kPa substrate showed the maximum sarcomeres striations of cells. Another study confirmed the effect of substrate stiffness on the beating kinetics of the CM, by introducing nano-topography to polyurethane substrates of a different elasticity in order to induce a more organized and aligned cytoskeleton. The nanotopography mainly affected the orientation and elongation of the CMs and was enhanced with an increasing groove depth. It was again demonstrated that CMs maintained better contractions on softer substrates with deep grooves.37
FIG. 6.
(A) Myocardial elasticity during embryogenesis. Histograms of elastic moduli (∼150 locations per sample) determined from quail embryos show two peaks, one indicating passive elasticity of contractile myocardium (E-Stiff) and a softer, second peak (E-Soft) that surrounds the myocardium and increases in frequency with development. Results for normal and infarcted rat myocardium are indicated for comparison5,9; (B) CMs and fibroblast spreading. CMs and pericardial fibroblasts were plated from 10-day-old embryonic myocardium without purification, and spread areas were measured after 4 or 24 h5; (C) In vitro striation of CMs. Purified CMs from 10-day-old embryonic myocardium were plated onto substrates of varying elasticity to observe striated cytoskeletal organization with skeletal α-actinin. Many cells on both soft gels and intermediate E* gels reassembled myofibrils, whereas cells on hard matrices exhibited less myofibril reassembly. Inset images show magnified views of the larger images (reproduced with permission from Engler et al.5).
CMs beat at ∼1 Hz and as they contract, they exert cyclic strains (intrinsic forces) on the substrate (Fig. 1). The amount of work done by the cells on the substrate can be estimated as the square mean matrix strain (local strain), ɛL, multiplied by the matrix elastic modulus, Em. Engler et al. approximated the work done by the cells as ½Em ɛL.5 This estimate predicts that cells seeded on substrates with stiffness below 10 kPa do little work, while cells seeded on substrates with stiffness above 10 kPa cannot strain the substrate. The strain exerted by the cells was relatively constant in substrates with a modulus of ∼10 kPa. When the substrate modulus increased above 10 kPa, the strain exerted by the cells decreased, reflecting that there must exist a rigidity beyond which the cells cannot contract. Hence, it can be suggested that an optimum matrix elastic modulus E* allows the matrix strains and cell strains to be similar in magnitude (Fig. 5), while a high elastic modulus (stiff matrices) results in straining/overstretching of the cell (Fig. 6). Optimal matrix elasticity shows a close correspondence with tissue elasticity and supports the CM function for longer times. Scar tissue arises after a myocardial infarction in adults is rigid, which is known to impede the contractile function of the heart.
It should be noted that the substrate stiffness can also affect the cytoskeleton, which can sense and react to intrinsic and extrinsic cellular mechanical stimuli. Hence, different substrate stiffnesses lead to structural and organizational changes, which control the structural and mechanical output of CMs. CMs respond to mechanical stimuli through a variety of molecular mechanisms: (1) Integrins in sarcomere and FA transmit loads from the ECM to the cytoskeleton, (2) cadherins connect myofibrils between cells at adherent junctions, and (3) sarcomere spanning proteins such as titin respond to intracellular stresses.21
CMs have many mechanosensors associated with the actomyosin cytoskeleton. At costameres, integrins and FA complexes link the ECM to the Z-disc and detect extracellular stress. Cadherin/catenin complexes link the ends of myofibrils from neighboring cells to detect intercellular loads at adherens junctions. Within the sarcomere, titin spans between the Z-disc and M-band acts as an intracellular strain sensor. Since these mechanosensors have different ligands and orientations relative to the axis of alignment, the cytoskeleton can discriminate the direction and source of mechanical inputs.21 A recent study quantified the striation of CMs on microposts through measurements of sarcomere spacing and Z-disk width. This study demonstrated that sarcomere spacing is a sign of myofibril maturity and an indicator for force output.22 Force output of the CM on different substrates fell within accepted values for mature myofibrils and showed an increase with an increase in substrate stiffness. Particularly, it was reported that the z-disc width (Fig. 7), which indicates coupling of sarcomeres within a myofibril, increases with an increase in stiffness.22
FIG. 7.
Resting sarcomere length and Z-band width increase with stiffness. (A) Cells on the softest arrays (3 kPa) and (B) on the stiffest arrays (15 kPa) were fixed and stained for α-actinin (green) and nuclei (blue). Insets: Same cell with a bright-field image showing the microposts. (C) Sarcomere length and (D) Z-band width increased with substrate stiffness. Error bars represent the 95% confidence intervals. Scale bars=5 μm (reproduced with permission from Moorman et al.26). Color images available online at www.liebertpub.com/teb
Effect of Static and Dynamic Stretching on CMs Maturation
Another important factor that was shown to increase CMs maturation and proliferation is stretching of the substrate. A brief summary on the studies that have illustrated this effect is given next. One study was concerned with the effect of cyclic strain on embryonic (day 7) white leghorn chicken CMs cultured on collagen coated rubber.38 Results showed that stretching the substrate enhanced proliferation and maturation of the CMs functional properties. In another study, embryonic (day 7) and fetal (day 14) white leghorn chicken ventricular cells were embedded in type I collagen gel, and the gel was stretched uniaxially by 8% strain for the embryonic and by 4% strain for the fetal at 0.5 Hz. The stretched cultures showed an increase in the active stress compared with the unstretched cultures. Particularly, for the fetal cell stretched cultures, an increase was observed in the passive stress, while for the embryonic cell cultures an increase was observed in proliferation.39
Others studied the effect that stretching of the substrate had on the maturation of differentiated CMs from embryonic stem cells. In one study, CMs that differentiated from embryonic stem cells were seeded on poly(lactic-co-caprolactone) scaffolds. When these scaffolds were cyclically stretched at 1.0 Hz for 2 weeks at 10% strain, an increase in cardiac α-myosin heavy chain (α-MHC), α-actinin, GATA-4, and Nkx2.5 mRNA was observed, compared with unstretched control scaffolds. Furthermore, the stretched cultures were beating in a synchronized manner and were integrated electrically into the myocardium of infarcted rat hearts, whereas on the unstretched cultures synchronized beating did not occur.40
Another study found that contractile markers in Murine embryonic stem cell-derived CMs (selected by transfection of [α-MHC]-promoter-driven gene conferring resistance to genetecin [G418] and embedded in a collagen-fibronectin scaffolds) were highly sensitive to the frequency, during 10% mechanical stretching. While the cardiac α-actinin increased with a stretch frequency of 1, 2, or 3 Hz, the skeletal α-actinin, α-MHC, and β-MHC decreased after 3 days of stretching at 1 Hz, but increased after 3 days of stretching at 3 Hz. The transcription factor GATA-4 decreased with stretching at 1 Hz, but was not significantly different after higher stretch frequencies.41
Effect of Substrate Stiffness and Mechanical Stretch on Mesenchymal Stem Cell Differentiation into CMs
Embryonic stem cells in serum containing media can differentiate spontaneously into CMs, and can further differentiate into major components of heart muscle tissue or the conduction system. In general, cardiogenesis in embryonic stem cell cultures is indicated by spontaneous beating, the shape of action potentials and calcium transients, the presence of specific ion currents, and by the expression of specific cardiac cell markers. The differentiation into cardiac tissue is denoted by the termination of certain pluripotency markers (such as Oct-3/4, fibroblast growth factor-5, and Nodal), the formation of early cardiac markers (such as the transcription factors Nkx2.5 and GATA-4, and SERCA2a), and the formation of some late-stage cardiac markers (such α-MHC, the ryanodine receptor, cardiac troponin-T [cTnT], and calsequestrin). An overview of differentiation times and markers has been reported as focusing on mouse embryonic stem cells.42
One study has shown the effect of the substrate on mesenchymal stem cell differentiation into myogenic cells. Results showed optimal marker protein expression on substrates with an elastic modulus of 10 kPa, while no marker protein expression was detected on substrates with a modulus greater than 20 kPa and below 2 kPa.43 In a recent study, PA hydrogels were used to demonstrate the sensitivity of human pluripotent stem cells (hPSCs) to substrate stiffness during early mesendoderm specification. Substrates of stiffness mimicking those of native tissue were found to promote more CM differentiation as compared with very soft or stiff substrates. However, substrate stiffness did not impact the efficiency differentiation to cTnT CMs when cells were split from tissue culture plates to hydrogels at the Nks2.5+/Isl1+ cardiac progenitor stage. This confirmed the hypothesis that human embryonic stem cells hESCs sense and respond with biased differentiation decisions in a developmental stage-specific manner to their mechanical microenvironment.44
Furthermore, studies have shown an increase in the percentage of beating cells and cells expressing sarcomeric α-actinin derived from embryonic stem cells when statically stretched for 2 h, till radial strains of 5%, 10%, 15%, and 20%. When stretched by 10% strain, the cells showed an increase in the expression of cardiac markers MEF2c and GATA-4. Angiogenesis increased with increasing the strain till 10%, and then decreased again with a further increase in the strain till 20%.45
On the contrary to the increase in beating, differentiation has been shown to be inhibited by stretching the substrates. Indeed, differentiation of human embryonic stem cells was shown to decrease when a 10% strain was applied at 10 cycles/min, keeping them in a pluripotent state.46 However, shear stresses tend to induce early cardiac and smooth muscle cell markers, all of which are downstream of a remodeling of chromatin structure; for example, vascular endothelial growth factor receptor 2, smooth muscle protein 22-α, myocyte-specific enhancer factor 2C (MEF2c), smooth muscle actinin, sarcomeric α-actinin, and platelet endothelial cell adhesion molecule-1 (PECAM-1).47
Conclusions
This review covered current investigations on the effect of mechanics (substrate stiffness and cyclic stretching) on CM structure and function development. The analysis of the literature revealed that recent research has generated considerable knowledge on the effect of substrate mechanics on CM maturation and growth. CMs beat best on substrates whose mechanical properties mimic those of native cardiac tissue, as the CMs sense the microenvironment mechanics and adapt to them by alterations in their structural and functional properties. The summary presented in this review helps in understanding the physical requirements for the preferred CM microenvironment and provides important guidance for the design of biomaterials, for example, heart patches, that can be used in cardiac tissue engineering. One of the major limitations in all of the presented in vitro studies is that they modeled the processes in 2D when in reality they occur in 3D. Mechanotransduction is very complex in the heart, due to the various dynamic mechanical loads applied to CMs, as they experience complex cyclic mechanical strains. Therefore, it is also crucial to understand the effect of the different mechanical stimulations on the CMs functional maturation.
Acknowledgment
The authors would like to acknowledge the financial support received from the European Research Council Starting Grant MINATRAN 211166 to initiate this study.
Disclosure Statement
No competing financial interests exist.
References
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., et al. . Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation 125,e2, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel J.K., and Kobashigawa J.A.Cardiology patient page. Heart transplantation. Circulation 124,E132, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Jawad H., Lyon A., Harding S.E., Ali N., and Boccaccini A.Myocardial tissue engineering. Br Med Bull 87,31, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Soonpaa M.H., and Field L.J.Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res 83,15, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Engler A., Carag-Krieger C., Johnson C., Raab M., Tang H., Speicher D., et al. . Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci 121,3794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacot J., McCulloch A., and Omens J.Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95,3479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieber S., Aubry N., Pain J., Diaz G., Kim S., and Vatner S.Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am J Physiol Heart Circ Physiol 287,H645, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Brady A.Mechanical properties of isolated cardiac myocytes. Physiol Rev 71,413, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Berry M.F., Engler A.J., Woo Y.J., Pirolli T.J., Bish L.T., Jayasankar V., Morine K.J., Gardner T.J., and Discher D.E.S.H.Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol 290,H2196, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Azeloglu E., and Costa K.Cross-bridge cycling gives rise to spatiotemporal heterogeneity of dynamic subcellular mechanics in cardiac myocytes probed with atomic force microscopy. Am J Physiol Hear. Circ Physiol 298,H853, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Borg T., and Caulfield J.The collagen matrix of the heart. Fed Proc 40,2037, 1981 [PubMed] [Google Scholar]
- 12.Murry C., Field L., and Menasché P.Cell-based cardiac repair: reflections at the 10 year point. Circulation 112,3174, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Patel A., Thakar R., Chown M., Ayala P., Desai T., and Kumar S.Biophysical mechanisms of single-cell interactions with microtopographical cues. Biomed Microdevices 12,287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De R., Zemel A., and Safran S.A.Do cells sense stress or strain? Measurement of cellular orientation can provide a clue. Biophys J 94,L29, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopalan S., Flaim C., Bhatia S., Hoshijima M., Knoell R., Chien K., et al. . Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol Bioeng 81,578, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Bajaj P., Tang X., Saif T., and Bashir R.Stiffness of the substrate influences the phenotype of embryonic chicken cardiac myocytes. J Biomed Mater Res A 95,1261, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Fukuda K., and Yuasa S.Stem cells as a source of regenerative cardiomyocytes. Circ Res 98,1002, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Menasche P.The potential of embryonic stem cells to treat heart disease. Curr Opin Mol Ther 7,293, 2005 [PubMed] [Google Scholar]
- 19.Srivastava D., and Ivey K.N.Potential of stem-cell-based therapies for heart disease. Nature 441,1097, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Dowell J., Rubart M., Pasumarthi K., Soonpaa M., and Field L.Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res 58,336, 2003 [DOI] [PubMed] [Google Scholar]
- 21.McCain M., and Parker K.Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch 462,89, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez A., Han S., Regnier M., and Sniadecki N.Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys J 101,2455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodish H., Berk A., Matsudaira P., Kaiser C.A., Krieger M., Scott M.P., et al. . Molecular Cell Biology, 4th edition. New York: WH Freeman and Company, 2000 [Google Scholar]
- 24.Asahi M., Otsu K., Nakayama H., Hikoso S., Takeda T., Gramolini A., et al. . Cardiac-specific overexpression of sarcolipin inhibits sarco(endo)plasmic reticulum Ca21 ATPase (SERCA2a) activity and impairs cardiac function in mice. Proc Natl Acad Sci U S A 101,9199, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wibo M., Bravo G., and Godfraind T.Postnatal maturation of excitation-contraction coupling in rat ventricle in relation to the subcellular localization and surface density of 1,4-dihydropyridine and ryanodine receptors. Circ Res 68,662, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Moorman A., Vermeulen J., Koban M., Schwartz K., Lamers W., and Boheler K.Patterns of expression of sarcoplasmic reticulum Ca-ATPase and phospholamban MRNAs during rat heart development. Circ Res 76,616, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Fisher D., Tate C., and Phillips S.The role of dicarboxylic anion transport in the slower Ca21 uptake in fetal cardiac sarcoplasmic reticulum. Pediatr Res 32,664, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Gomez J., Potreau D., and Raymond G.Intracellular calcium transients from newborn rat cardiomyocytes in primary culture. Cell Calcium 15,265, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Husse B., and Wussling M.Developmental changes of calcium transients and contractility during the cultivation of rat neonatal cardiomyocytes. Mol Cell Biochem 163–164,13, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Noma K., Oyama N., and Liao J.Physiological role of rocks in the cardiovascular system. Am J Physiol Cell Physiol 290,C661, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S., Maxwell I., Heisterkamp A., Polte T., Lele T., Salanga M., et al. . Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J 90,3762, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang N., Tolić-Nørrelykke I., Chen J., Mijailovich S., Butler J., Fredberg J., et al. . Cell prestress I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol 282,C606, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Forte G., Pagliari S., Ebara M., Uto K., Tam J.K., Van Romanazzo S., et al. . Substrate stiffness modulates gene expression and phenotype in neonatal cardiomyocytes in vitro. Tissue Eng Part A 18,1837, 2012. Available at www.ncbi.nlm.nih.gov/pubmed/22519549 [DOI] [PubMed]
- 34.Liu J., Sun N., Bruce M., Wu J., and Butte M.Atomic force mechanobiology of puripotent stem cell derived cardiomyocytes. PLoS One 7,e37559, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehler E., Rothen B., Hämmerle S., Komiyama M., and Perriard J.Myofibrillogenesis in the developing chicken heart: assembly of Z-disk, M-line and the thick filaments. J Cell Sci 112,1529, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Du A., Sanger J., Linask K., and Sanger J.Myofibrillogenesis in the first cardiomyocytes formed from isolated quail precardiac mesoderm. Dev Biol 257,382, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Wang P.-Y., Yu J., Lin J.-H., and Tsai W.-B.Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater 7,3285, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Miller C., Donlon K., Toia L., Wong C., and Chess P.Cyclic strain induces proliferation of cultured embryonic heart cells. In Vitro Cell Dev Biol Anim 36,633, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Tobita K., Liu L., Janczewski A., Tinney J., Nonemaker J., Augustine S., et al. . Engineered early embryonic cardiac tissue retains proliferative and contractile properties of developing embryonic myocardium. Am J Physiol Heart Circ Physiol 291,H1829, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Gwak S., Bhang S., Kim I., Kim S., Cho S., Jeon O., et al. . The effect of cyclic strain on embryonic stem cell-derived cardiomyocytes. Biomaterials 29,844, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Shimko V., and Claycomb W.Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue Eng Part A 14,49, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boheler K., Czyz J., Tweedie D., Yang H., Anisimov S., and Wobus A.Boh differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res 91,189, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Engler A., Sen S., Sweeney H., and Discher D.Matrix elasticity directs stem cell lineage specification. Cell 126,677, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Hazeltine L.B., Badur M.G., Lian X., Das A., Han W., and Palecek S.P. Temporal impact of substrate mechanics on differentiation of human embryonic stem cells to cardiomyocytes. Acta Biomater 10, 604, 2014. Available at www.ncbi.nlm.nih.gov/pubmed/24200714 [DOI] [PMC free article] [PubMed]
- 45.Schmelter M., Ateghang B., Helmig S., Wartenberg M., and Sauer H.Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J 20,1182, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Saha S., Ji L., de Pablo J., and Palecek S.Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol 206,126, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Illi B., Scopece A., Nanni S., Farsetti A., Morgante L., Biglioli P., et al. . Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ Res 96,501, 2005 [DOI] [PubMed] [Google Scholar]