Abstract
Purpose of review
The objective of this review is to 1) appraise recently published literature that describes the relationship between HIV, biologic and environmental risk factors, and CVD risk with particular emphasis on the aging HIV population and 2) to demonstrate that these biologic and environmental factors may interact to increase the risk of CVD in the HIV population.
Recent findings
The mechanisms linking HIV and CVD are multi-factorial and encompass biological and “environmental” modalities including multi-morbid conditions that co-occur with HIV, immunologic alterations associated with HIV, polypharmacy (which affects adherence and increases likelihood of adverse drug-drug interactions) and healthcare disparities in CVD risk reduction by HIV status.
Summary
Data regarding optimal treatment strategies that balance immunological restoration and CVD risk reduction are needed.
Keywords: Cardiovascular disease, HIV, multimorbidity, polypharmacy, clinical guidelines, healthcare disparities
Introduction
With the success of antiretroviral therapy (ART), HIV infection has transitioned from a rapid death sentence to a more complex chronic disease with increasing numbers of people worldwide living longer (Figure 1) without AIDS.(1) The price of this success is that people aging with HIV are at risk for similar diseases of aging as the general population.(2) HIV infection is associated with an increased risk of multiple cardiovascular diseases (CVDs) including acute myocardial infarction, coronary heart disease (CHD), ischemic stroke, and heart failure. While ART, Framingham risk factors and non-Framingham risk factors (e.g., renal disease, anemia, and hepatitis C co infection) are all associated with CVD risk among HIV+ people, these risk factors do not explain the excess risk of CVD in this population compared to uninfected people. To explain this excess risk, investigators have turned their attention to deepening our understanding of biologic (e.g. immuno-virological, multimorbidity) and environmental (e.g. polypharmacy, healthcare disparities) risk factors (Figure 2). The objective of this review is to 1) appraise recently published literature that describes the relationship between HIV, biologic and environmental risk factors, and CVD risk with particular emphasis on the aging HIV population and 2) to demonstrate that these biologic and environmental factors may interact to increase the risk of CVD in the HIV population.
Figure 1.
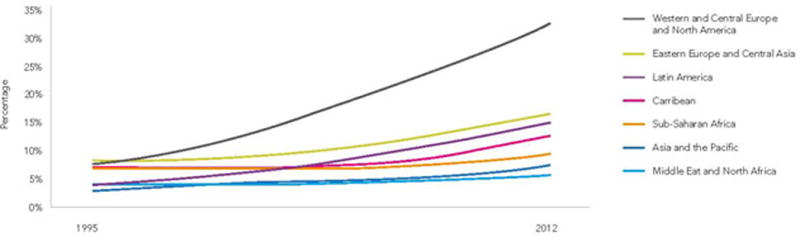
People aged 50 years or older, as a percentage of all adults 15 years or older living with HIV, by region, 1995–2012 (Image from UNAIDS https://www.un.org/apps/news//story.asp?NewsID=46393&Cr=hiv&Cr1=aids#collapseTwo).
Figure 2.
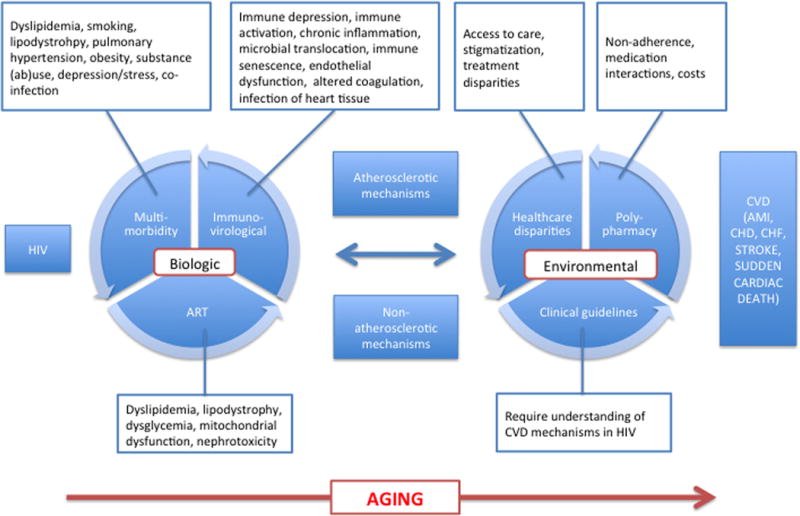
Mechanisms of CVD in HIV
HIV is associated with CVD
CVD accounts for increasing proportions of HIV mortality as AIDS mortality declines and HIV populations age.(3–7) Multiple studies show that HIV is an independent risk factor for CVD.(8*, 9) Prior reviews have discussed findings from large prospective, longitudinal studies.(9, 10) Briefly, these earlier studies show increased MI and CHD incidence among HIV infected (HIV+) compared to uninfected comparators using large administrative and clinical databases(11, 12) or population-based comparators.(13) Similar studies have also demonstrated an increased risk of heart failure,(14) stroke(15) and sudden cardiac death(16) among HIV+ people. Results from the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study support a role for antiretroviral therapy in CVD risk(17, 18) although the Strategies for the Management of Antiretroviral Therapy (SMART) study(19) clearly shows the benefits of ART outweigh this potential risk. For this review we will focus on more recent data.
Is HIV an independent predictor of CVD and atherosclerosis?
Two recent large cohort studies with demographically and behaviorally similar HIV+ and uninfected groups found strong, consistent associations of HIV with acute myocardial infarction (AMI). Silverberg et al matched 230,069 uninfected Kaiser Permanente members to 22,081 HIV+ patients identified from HIV registries.(20**) They reported a 44% increased risk of AMI among HIV+ people compared to controls independently of age, race/ethnicity, calendar era, socioeconomic status, smoking, overweight, substance abuse, diabetes, hypertension and lipid lowering therapy. Similar results were reported from the Veterans Aging Cohort Study Virtual Cohort (VACS VC), a cohort of HIV+ Veterans matched on age, race-ethnicity, sex, and clinical site to two uninfected Veterans also in clinical care.(8, 21) Adjusting for Framingham risk factors, comorbid diseases, and substance use, HIV+ Veterans were 48% more likely to experience an AMI compared to their uninfected counterparts. This association persisted after adjusting for the competing risk of death and among Veterans maintaining viral suppression (HIV-1 RNA<500 cpm) over time.
Further evidence comes from subclinical CVD studies. Some but not all of these studies – often using coronary artery calcium (CAC) or carotid intima-media thickness (cIMT) as surrogate measures of atherosclerosis – report an association between HIV and the occurrence and/or progression of subclinical CVD.
A 2009 meta-analysis from 13 studies reported similar CAC but increased cIMT (weighted mean difference was +0.04 mm 95% CI 0.02–0.06 mm, p<0.001) in HIV+ compared to uninfected participants.(22) There was significant heterogeneity among the studies and the authors concluded that HIV was not likely to be a strong independent risk factor for atherosclerosis. In more recent work, results are mixed with associations reported between HIV status and carotid lesions,(23) non-calcified plaque, (24) cIMT and cIMT progression(25) in some but not all (26–29) studies. Interestingly, Hsue et al showed increased cIMT and cIMT progression among HIV elite controllers (untreated with HIV-1 RNA <50cpm), suggesting that HIV factors separate from ART and HIV-1 RNA contribute to cIMT and its progression.(25) HIV+ children (97% vertically infected, mean age 15 years) had higher cIMT compared to a group of uninfected children of similar age, sex, ethnicity and BMI distribution.(30) Among non-smokers, Desvarieux et al reported higher cIMT among treated HIV+ compared to HIV- but similar levels among untreated HIV+ compared to the same HIV- referent group.(31) These latter two studies suggest a role of HIV infection in atherosclerosis over and above that of comorbid diseases (e.g. smoking) or pro-atherosclerotic conditions not yet present in children.
In summary, HIV infection is associated with an increased risk of CVD including AMI, CHD, heart failure, stroke and sudden cardiac death. The effect of HIV on subclinical CVD may be more dependent on, age, comorbid disease, gender, duration of infection, and modified by study design (biomarker of atherosclerosis, CAC/cIMT measurement site, subject characteristics).
Biologic Mechanisms of CVD in HIV
The studies we discuss in this section are recent studies that investigate potential mechanisms summarized in Figure 2.
Immuno-virology
Immune alteration is a shared feature of aging, CVD and HIV. Immune function declines with age and HIV infection,(32, 33) immune alterations are associated with atherosclerosis,(34, 35) and age and HIV infection are associated with CVD. Among healthy uninfected people, immune cell subsets (e.g. intermediate monocytes, CD4+ T-helper 1 cells), inflammation, T-cell activation and evidence of immunosenescence (e.g. low naïve to memory T-cell ratio) are associated with atherosclerosis.(36–38) HIV infection may alter the balance of immune cell subsets in a pro-inflammatory and pro-atherosclerotic manner. The association between HIV, immune function (including immune activation and immune cell depletion) and CVD risk was recently reviewed by Hsue et al(39**) and several important questions remain including:
Do specific immune cell subsets play a role in the pathogenesis of CVD in HIV?
Does activation of the innate and adaptive immune system mediate the effects of HIV, ART, and comorbid disease on incident CVD?
What is the role of T-cell senescence in aging related diseases like CVD? (see future directions)
More recent work addresses some of these knowledge gaps. Among HIV+ people, higher frequencies of CD16+ monocytes (intermediate and nonclassic; pro-atherosclerotic(40)) were independently associated with CAC progression. Activated CD4+ and CD8+ T-cell phenotypes were not associated with CAC progression.(41*) Funderburg et al showed that uninfected participants with acute coronary syndrome (ACS) and HIV+ patients with elevated HIV-1 RNA levels (but without ACS) had similar proportions of CD16+ monocytes. Both groups had higher proportions of CD16+ monocytes compared to uninfected participants with stable coronary artery disease (CAD).(42**) T-cell activation markers (HLA-DR+ and CD38+ CD8 cells) were lower in uninfected ACS patients compared to HIV patients with elevated HIV-1 RNA levels suggesting different sources of activation despite the similar resultant monocyte phenotype. These studies suggest a role for immune cell subsets in CVD risk in the setting of HIV infection.
Similarly, others have reported increased arterial inflammation (measured by sCD163 (43)) in HIV+ versus controls with prior atherothrombotic events or atherosclerosis. (44**) Among HIV+ elite controllers (ART naïve, HIV-1 RNA<48cpm), Pereyra et al reported significantly increased coronary plaque (i.e. stenosis >50%), sCD163, high sensitivity interleukin 6 (biomarker of inflammation), soluble CD14 (biomarker of monocyte activation), CXCL10 (promotes monocyte migration) and CD38+HLA-DR+ CD4 cells (marker of lymphocyte activation) compared to uninfected controls.(45**) Differential associations by HIV status between markers of monocyte migration/activation and cIMT have been reported (with minimal covariate adjustment) though interactions were not tested. (29*, 46*) These studies suggest a relationship between HIV and atherosclerosis, independent of CVD risk factors and ART. Whether inflammation and immune activation are causes or effects of this association remains unclear. A limitation of these cross-sectional analyses is the inflammatory nature of ACS/CAD events such that having the event alters immune cell subset distribution making causal inference challenging.
Epidemiological associations between measures of adaptive immunity (e.g. low CD4+ T-cell count) with CVD events are inconsistent.(8, 47**, 48, 49) Among HIV+ people, activated T cells (CD38+HLA-DR+) and senescent T cells (CD28-CD57+) are significantly associated with subclinical carotid atherosclerosis and carotid artery stiffness.(50–53)
In summary, recent data support at least a cross-sectional association of monocyte subset/activation and T-cell activation and CVD in HIV. Whether immune alterations mediate the relationship between HIV and CVD is still unclear.
Antiretroviral therapy (ART)
The SMART study makes it clear that the benefits of ART outweigh the cost of some regimens increasing the risk of CVD events. Additionally, ART treatment initiation studies show improvements in CVD risk factors(54–57) that may be regimen dependent.(58, 59) More cardio-protective or at least cardio-neutral ART regimens are available and clinicians should note regimens to avoid in patients at high risk for CVD.(59) Clinical management should also anticipate excess weight gain in certain subgroups initiating ART.(60) Newer or non-protease inhibitor based ART regimens have improved metabolic side effect profiles(61, 62) though some debate about PI-regimens remains.(63) Lastly, ART clinical trials rarely include older patients – who often have significant co-morbidity and reduced immune response (64) – who nonetheless currently receive these drugs. Results from ongoing studies are keenly awaited (Table 1).
Table 1.
Antiretroviral therapy trials focused on older people
| ClinicalTrials.gov identifier | Title | Estimated completion date | Sponsor |
|---|---|---|---|
| NCT01737047 | A Prospective, Observational Study to Examine the Effects of Ageing on the ‘Pharmacokinetic and Clinical Observations in People Over Fifty’ (POPPY) | 2016 | Gilead/Janssen/ViiV/Bristol Myers Squibb/Merck |
| NCT01213316 | A Study to Assess the Efficacy of Raltegravir, Administered in Combination With Other Antiretroviral Drugs as Treatment for Adults and Older Adults Infected With the Human Immunodeficiency Virus 1 (HIV-1)(MK-0518-145) (Wirksamkeit Von Isentress® Unter Praxisbedingungen) (WIP) | 2015 | Merck |
| NCT01180075 | Tenofovir, Emtricitabine, Efavirenz and Atazanavir Pharmacokinetics in the Aging HIV-Infected Population | 2014 | University of North Carolina, Chapel Hill, National Institute of Allergy and Infectious Diseases (NIAID) (collaborator) |
Treated and suppressed HIV+ people still have excess risk of CVD compared with uninfected controls.(8) This excess risk could be driven in part by high viremia exposure prior to ART initiation, ART, and/or residual low-level viral replication in ART suppressed patients. Current guidelines recommend ART initiation at HIV diagnosis regardless of CD4 count, which deals with the first source of excess risk.(59) Residual viremia is potentially important because in time updated analyses, HIV+ Veterans who maintained a viral load <500 copies per ml over time had a higher risk for acute myocardial infarction compared to uninfected Veterans after adjusting for multiple confounders.(8) HIV seroconversion and ART initiation studies suggest that some worsening in CVD risk factors that occurs after HIV infection (e.g. decreased HDL) do not improve to pre-HIV levels with ART initiation(65–67), potentially contributing to excess CVD risk. Likewise, cross-sectional studies suggest increased prevalence of coronary plaque, sCD163 and sCD14 among treated, controlled HIV+ people versus uninfected controls.(45)
A recent study described new cycles of HIV replication occurring with only modest ART non-adherence. Importantly, this residual replication is not detected as virological rebound on commercial HIV RNA assays.(68) As we improve our ability to measure residual viremia, (69) it would be useful to quantify the extent to which residual replication contributes to inflammation and how this relates to CVD in aging patients.
Comorbidity
Framingham CVD risk factors accumulate with age regardless of HIV status. These risk factors remain associated with CVD events in HIV+ populations. Whether the interaction of HIV status and CVD risk factors contributes to excess CVD risk is unclear partly because there are few studies that have large numbers of HIV+ and uninfected people with incident CVD events and detailed information on comorbid diseases. In a Veteran population, there was no significant interaction of elevated blood pressure with HIV status on the risk of acute myocardial infarction.(70*) Althoff et al reported no difference by HIV status in adjusted mean age at incident myocardial infarction (55.3 years for both groups) although HIV+ people had a greater risk of myocardial infarction.(71) In contrast, Fitch et al reported an interaction between age and HIV, and age, HIV and smoking on cIMT prevalence whereby cIMT increased more with age among HIV+ versus uninfected people.(24) Herrin et al described an interaction between HIV status and weight gain post-ART initiation on diabetes incidence. Although HIV+ people had lower risk of diabetes, the risk of diabetes associated with weight gain was 60% greater among infected versus uninfected people.(72)
Understanding whether comorbid CVD risk factors occur earlier and/or with increased severity in HIV+ compared to uninfected people will improve the effectiveness of CVD risk reduction in this group. HIV+ Veterans were more likely than uninfected controls to have renal or liver disease, substance abuse disorders and multiple simultaneous comorbidities (multimorbidity) and less likely to have hypertension, diabetes, vascular disease or psychiatric disorders. (73) Older HIV+ Veterans were more likely than older uninfected Veterans to have, liver disease, substance abuse, and multimorbidity.(73)
Additional comorbidities that are less prevalent in the general population may also contribute excess CVD risk in HIV+ cohorts. Cardiovascular implications of comorbid infections have recently been investigated.(74) Earlier work suggested that HCV mono-infection is associated with increased risk of CVD(75, 76) though the data on HCV co-infection with HIV are somewhat conflicting.(77, 78) Data from a nested case-control study of the Multicenter AIDS Cohort Study found an association between increased coronary artery calcium herpes simplex virus 2 (HSV-2) co-infection with HIV, and number of herpesviruses among HIV+ men co-infected with compared to HIV mono-infected men.(79) Parrinello et al found no association between CMV antibody titres and cIMT or carotid artery lesions among HIV+ women, though they did find an independent association between CMV antibody titres and lower carotid artery distensibility/elasticity regardless of treatment/viremia status.(80*) CMV antibodies were not associated with any vascular parameters studied among the much smaller cohort of HIV uninfected controls though no statistical interaction was found between HIV status and CMV on any of the vascular outcomes studied. Earlier work found increased CMV specific T-cell responses to be associated with cIMT.(81)
In summary, there is limited evidence that comorbid conditions other than older age modify the association between HIV and CVD events or atherosclerosis. HIV specific CVD risk quantification tools have been investigated though external validation in diverse HIV populations is still needed.(82–87) Subsequent iterations should assess whether additional HIV specific comorbid CVD risk factors and interactions between HIV status and comorbid disease improve risk assessment.
Environmental Mechanisms of CVD in HIV
Understanding and addressing non-biological mechanisms of CVD in HIV may improve the success and sustainability of interventions focused on biological mechanisms, particularly in older, more complex patients (Figure 2).
Polypharmacy, multimorbidity, and medication adherence and interaction
HIV is now a chronic condition accompanied by multiple comorbidities, each adding additional treatment regimens that often become more complex with age. The linked issues of polypharmacy and multimorbidity in HIV have recently been reviewed.(88*, 89*, 90) Common themes emerging specific to CVD and aging in HIV are:
Increased prevalence of polypharmacy in older versus younger HIV patients
Increased multimorbidity in older HIV+ patients versus older uninfected patients(73)
Increased risk of drug-drug interactions and adverse drug events in older versus younger HIV adults with a high percentage of interactions involving CVD drugs(91**, 92, 93)
- Poorer ART adherence with conditions that are either more prevalent in older HIV+ versus older uninfected people or interact with older age or HIV status to reduce adherence e.g.:
Suggestions for clinical management have been proposed,(88, 100) ART/non-ART drug interactions have been described in detail,(101) and innovative solutions have been successfully piloted.(102**, 103) However, there is a need for development of comprehensive, effective evidence-based guidelines for managing older HIV+ patients with multiple comorbidities and multiple ART and non-ART drug regimens.
Clinical guidelines
HIV clinical guidelines are evolving to recognize the increasing impact of CVD in the aging HIV population. As mentioned earlier, CVD risk quantification tools are being investigated though external validation in diverse HIV populations is needed prior to widespread implementation.(82–87) Recommended CVD risk reduction strategies are similar to those for uninfected people because there is strong evidence linking these risk factors to CVD events. However, with a fuller understanding of the mechanisms of CVD in HIV, there exists a possibility for more effective and efficient CVD risk reduction. For example, if a unit increase in a CVD risk factor is associated with a greater increase in CVD risk in HIV+ versus uninfected persons, it may be appropriate to recommend stricter control of that comorbid condition among HIV+ people. However, lessons learned from stringent glucose and blood pressure control studies in older diabetic patients (104, 105) suggest that any alterations in CVD risk reduction guidelines for HIV+ people should be accompanied by evidence from randomized clinical trials involving HIV+ people. Alternatively, given the earlier discussion of the detrimental effects of polypharmacy, it may be more effective to control the major contributors to CVD risk instead of attempting to control all known risk factors. The inclusion of older people in ART and other HIV management trials is essential to the development of appropriate evidence-based guidelines (Table 1).
Healthcare disparities
Disparities in care by HIV status compound the differential CVD risk due to biological factors. In two recent studies, only 20% of HIV+ adults who met guidelines for receipt of aspirin therapy for primary prevention of CVD actually received this therapy. Moreover, in one academic medical center and, compared to uninfected adults, HIV+ adults were significantly less likely to receive aspirin for CVD prevention.(106**, 107) Other data on medical management and outcomes following acute myocardial infarction show that HIV+ people received significantly fewer cardiovascular procedures and/or therapeutics (e.g., thrombolytic and anticoagulant agents, coronary arteriography, cardiac catheterization, CABG).(108**) In addition, the risk of in-hospital mortality following AMI was 38% higher among HIV+ adults. In spite of this, HIV+ people are likely to incur greater direct medical costs for non-infectious comorbidities (including CVD) compared to uninfected people.(109) Future studies reporting which processes of care for the prevention and management of AMI contribute to the increased risk of AMI and death after AMI among HIV+ people would likely be useful.(110–112)
Future directions and conclusion
Whether HIV+ individuals literally age faster and thus experience CVD events earlier is still unclear. Research is needed to understand whether they experience comorbidity (e.g. dyslipidemia, altered glucose metabolism, reduction in bone density, renal insufficiency, neurocognitive impairment) earlier; respond less well to comorbid disease burden than expected (e.g. decreased organ reserve); experience immune cell senescence (e.g. thymic involution, dramatic CMV specific T-cell response) at a (accelerated) rate more consistent with older age; or have “vascular ages” that are incongruent with chronological age.(113) Alternatively these may reflect HIV pathogenesis compounding the effects of aging in a more independent manner. The answers to these important question remain unclear though the latter explanation may be more likely and is probably organ specific.(114)
What is clear is that the mechanisms linking HIV and CVD are multi-factorial and encompass biological and “environmental” modalities. Controlling viremia with appropriate ART regimens, preventing and treating comorbid conditions, recognizing and avoiding healthcare disparities and the risks of polypharmacy associated with multimorbidity and aging will all likely be necessary to reduce the risk of CVD in this high risk population.
Key points.
HIV infection is associated with cardiovascular disease across age groups
The mechanisms linking HIV and CVD are multi-factorial and encompass biological and “environmental” modalities including immunologic alterations, multimorbidity, polypharmacy and healthcare disparities
Data regarding optimal treatment strategies that balance immunological restoration and CVD risk reduction are needed
Acknowledgments
We would like to thank Drs. Maria Rodriguez-Barradas, David Rimland, Sheldon Brown, Matthew Goetz, David Leaf, Kris Ann Oursler, Cynthia Gibert, Roger Bedimo and Adeel Butt for their valuable comments to improve this review.
Disclosures: Matthew Freiberg has received grant funding from the National Heart, Lung, and Blood Institute and the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health (Grant numbers: HL095136, AA021989).
Footnotes
Conflict of interest
There are no conflicts of interest.
Annotated bibliography
- Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA internal medicine. 2013 Mar;4:1–9. doi: 10.1001/jamainternmed.2013.3728. Epub 2013/03/06. Eng. *This work shows an association between HIV and myocardial infarction, a CVD outcome that is relatively distinct (minimize event ascertainment bias). Importantly, the study uses adjudicated events, clinical data, a large well characterized cohort, a demographically and behaviorally similar uninfected group, time-dependent covariate analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, et al. Immunodeficiency and Risk of Myocardial Infarction Among HIV-Positive Individuals With Access to Care. J Acquir Immune Defic Syndr. 2014 Feb 1;65(2):160–6. doi: 10.1097/QAI.0000000000000009. **This work shows an association between HIV and myocardial infarction, a CVD outcome that is relatively distinct (minimize event ascertainment bias). Importantly, the study benefits from a very large well characterized cohort, a demographically matched uninfected group, time-dependent covariate analysis. [DOI] [PubMed] [Google Scholar]
- Monroe AK, Dobs AS, Xu X, Palella FJ, Kingsley LA, Post WS, et al. Low free testosterone in HIV-infected men is not associated with subclinical cardiovascular disease. HIV Med. 2012 Jul;13(6):358–66. doi: 10.1111/j.1468-1293.2011.00988.x. *Relatively large study (N= 856) from MACS cohort looking at coronary artery calcium and HIV infection status. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC, et al. Carotid Intima-Media Thickness Progression in HIV-Infected Adults Occurs Preferentially at the Carotid Bifurcation and Is Predicted by Inflammation. Journal of the American Heart Association. 2012 Apr;1(2) doi: 10.1161/JAHA.111.000422. *Study design involved stratifiying HIV infected group by treatment status and detectable HIV viremia. Two regions of carotid artery imaged and analyzed separately and longitudinal analysis performed. Study links HIV associated inflammaiton with atherosclerosis within the same cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose H, Low H, Dewar E, Bukrinsky M, Hoy J, Dart A, et al. The effect of HIV infection on atherosclerosis and lipoprotein metabolism: a one year prospective study. Atherosclerosis. 2013 Jul;229(1):206–11. doi: 10.1016/j.atherosclerosis.2013.04.010. *Study investigates multiple steps in a proposed mechanism involving HIV, ART, lipid dysregulation and atherosclerosis within a single cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman CO, Longenecker CT, Carman TL, McComsey GA. C-Reactive Protein Predicts 96-Week Carotid Intima Media Thickness Progression in HIV-Infected Adults Naive to Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2013 Nov 11; doi: 10.1097/QAI.0000000000000063. *Study investigates multiple steps in a proposed mechanism involving HIV, inflammation and atherosclerosis within a single cohort, without potential confounding effects of antiretroviral therapy. [DOI] [PubMed] [Google Scholar]
- Hileman CO, Carman TL, Longenecker CT, Labbato DE, Storer NJ, White CA, et al. Rate and predictors of carotid artery intima media thickness progression in antiretroviral-naive HIV-infected and uninfected adults: a 48-week matched prospective cohort study. Antivir Ther. 2013;18(7):921–9. doi: 10.3851/IMP2651. *Study investigates multiple steps in a proposed mechanism involving HIV, inflammation, altered coagulation and atherosclerosis within a single cohort, without potential confounding effects of antiretroviral therapy. [DOI] [PubMed] [Google Scholar]
- Sainz T, Alvarez-Fuente M, Navarro ML, Diaz L, Rojo P, Blazquez D, et al. Subclinical atherosclerosis and markers of immune activation in HIV-infected children and adolescents: The CaroVIH Study. J Acquir Immune Defic Syndr. 2013 Aug 26; doi: 10.1097/QAI.0b013e3182a9466a. **Studies like this, among children/adolescents, minimize some of the confounding by comorbid diseases since they are either absent in children or have a duration that is closer in magnitude (or shorter than) to the duration of HIV infection. [DOI] [PubMed] [Google Scholar]
- Desvarieux M, Boccara F, Meynard JL, Bastard JP, Mallat Z, Charbit B, et al. Infection duration and inflammatory imbalance are associated with atherosclerotic risk in HIV-infected never-smokers independent of antiretroviral therapy. AIDS. 2013 Oct 23;27(16):2603–14. doi: 10.1097/QAD.0b013e3283634819. **This study minimizes confounding by an important comorbidity, smoking, to better estimate the association between HIV and atherosclerosis. It also investigates multiple steps in a proposed mechanism involving HIV associated inflammation and atherosclerosis. [DOI] [PubMed] [Google Scholar]
- Hsue PY, Deeks SG, Hunt PW. Immunologic Basis of Cardiovascular Disease in HIV-Infected Adults. J Infect Dis. 2012 Jun;205(Suppl 3):S375–82. doi: 10.1093/infdis/jis200. **Thorough review covering inflammation, altered coagulation, lipid dysregulation, immunosenescence and their potential roles in mediating CVD in the setting of HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JV, Hullsiek KH, Singh A, Wilson E, Henry K, Lichtenstein K, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS. 2013 Dec 24; doi: 10.1097/QAD.0000000000000145. *Provides support for monocyte activation as an important link in HIV-related CVD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012 Nov 29;120(23):4599–608. doi: 10.1182/blood-2012-05-433946. **Provides support for monocyte activation as an important link in HIV-related CVD. Importantly, this study includes two uninfected reference groups, and shows similar monocyte subset distributions between otherwise healthy HIV infected people and uninfected people with known atherosclerosis/atherothrmbotic events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012 Jul 25;308(4):379–86. doi: 10.1001/jama.2012.6698. **Suggests that HIV infected people with no prevalent disease resemble uninfected people with atherosclerotic disease with respect to arterial inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012 Nov 28;26(18):2409–12. doi: 10.1097/QAD.0b013e32835a9950. **This study includes elite controllers, though few, with similar CVD risk factors as HIV progressor and uninfected comparators. This enables inferences on the association between HIV and CVD independent of ART and somewhat indepnedently of comorbid conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhorpe CL, Maisa A, Spelman T, Hoy JF, Dewar EM, Karapanagiotidis S, et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol. 2013 Dec 3; doi: 10.1038/icb.2013.84. * Provides support for monocyte activation as an important link in HIV-related CVD. [DOI] [PubMed] [Google Scholar]
- Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012 Nov 15;206(10):1558–67. doi: 10.1093/infdis/jis545. *Suggests differential association between bacterial translocation (thought to contribute to chronic immune activation) and atherocslerosis by HIV status though interaction not specifically tested. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin CA, Ryom L, De Wit S, Mocroft A, Phillips AN, Worm SW, et al. Associations between immune depression and cardiovascular events in HIV infection. AIDS. 2013 Nov 13;27(17):2735–48. doi: 10.1097/01.aids.0000432457.91228.f3. **This study, from a large, well-characterized cohort, contradicts data from other cohorts that suggest immune depression is associated with cardiovascular events. [DOI] [PubMed] [Google Scholar]
- Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013 Jul;14(6):385–90. doi: 10.1111/hiv.12013. *This study contradicts other studies linking monocyte activation and atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini E, Luzi K, Suardi E, Barassi A, Cerrone M, Martinez JS, et al. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One. 2012;7(9):e46073. doi: 10.1371/journal.pone.0046073. *The use of multiple immunologic parameters within the same cohort in this study may help to minimize some of the confounding inherent in comparing associations between these parameters and atherosclerosis across different studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, et al. HIV Status, Burden of Comorbid Disease and Biomarkers of Inflammation, Altered Coagulation and Monocyte Activation. Clin Infect Dis. 2012 Apr 24; doi: 10.1093/cid/cis406. Epub 2012/04/27. Eng. *This study suggests immune depression and ongoing viral replication as the source of increased inflammation, monocyte activation and altered coagulation in HIV infected versus uninfected persons. It also highlights the important contribution of comorbid diseases to inflammation, monocyte activation and altered coagulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello CM, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis. 2012 Jun 15;205(12):1788–96. doi: 10.1093/infdis/jis276. *One of few large studies of HIV, infectious comorbidity and atherosclerosis among a cohort of women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs & aging. 2013 Aug;30(8):613–28. doi: 10.1007/s40266-013-0093-9. *An excellent review of the importance and dangers of polypharmacy in the context of an aging HIV population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega JB, Hsu AJ, Uthman OA, Spinewine A, Pham PA. Antiretroviral therapy adherence and drug-drug interactions in the aging HIV population. AIDS. 2012 Jul 31;26(Suppl 1):S39–53. doi: 10.1097/QAD.0b013e32835584ea. *Contains extensive listings of interactions of HIV with multiple classes of drugs that aging HIV patients are likely to be prescribed. [DOI] [PubMed] [Google Scholar]
- Holtzman C, Armon C, Tedaldi E, Chmiel JS, Buchacz K, Wood K, et al. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med. 2013 Oct;28(10):1302–10. doi: 10.1007/s11606-013-2449-6. **Shows older age is an independent predictor of being prescribed a contraindicated ART/non-ART combination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L, Patterson B, Scourfield A, Hughes A, de Silva S, Gazzard B, et al. A dedicated clinic for HIV-positive individuals over 50 years of age: a multidisciplinary experience. Int J STD AIDS. 2012 Aug;23(8):546–52. doi: 10.1258/ijsa.2012.011412. **Successful pilot program shows proof of concept and used targeted screening approaches relevant to the aging HIV population to detect pathologies that may have gone undetected. [DOI] [PubMed] [Google Scholar]
- Burkholder GA, Tamhane AR, Salinas JL, Mugavero MJ, Raper JL, Westfall AO, et al. Underutilization of Aspirin for Primary Prevention of Cardiovascular Disease Among HIV-Infected Patients. Clin Infect Dis. 2012 Sep 24; doi: 10.1093/cid/cis752. Epub 2012/09/04. Eng.**Highlights important disparity in primary CVD prevention by HIV status. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce D, Ani C, Espinosa-Silva Y, Clark R, Fatima K, Rahman M, et al. Comparison of In-Hospital Mortality from Acute Myocardial Infarction in HIV Sero-Positive Versus Sero-Negative Individuals. Am J Cardiol. 2012 Oct 15;110(8):1078–84. doi: 10.1016/j.amjcard.2012.05.045. **Highlights important disparity in secondary and tertiary CVD prevention by HIV status. [DOI] [PubMed] [Google Scholar]
References
- 1.Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr. 2007 Feb 1;44(2):179–87. doi: 10.1097/01.qai.0000247229.68246.c5. Epub 2006/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 2.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011 Dec;53(11):1130–9. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 3.Grinsztejn B, Luz PM, Pacheco AG, Santos DV, Velasque L, Moreira RI, et al. Changing mortality profile among HIV-infected patients in Rio de Janeiro, Brazil: shifting from AIDS to non-AIDS related conditions in the HAART era. PLoS One. 2013;8(4):e59768. doi: 10.1371/journal.pone.0059768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013 Apr;14(4):195–207. doi: 10.1111/j.1468-1293.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- 5.Ehren K, Hertenstein C, Kummerle T, Vehreschild JJ, Fischer J, Gillor D, et al. Causes of death in HIV-infected patients from the Cologne-Bonn cohort. Infection. 2013 Oct 1; doi: 10.1007/s15010-013-0535-7. [DOI] [PubMed] [Google Scholar]
- 6.Aldaz P, Moreno-Iribas C, Egues N, Irisarri F, Floristan Y, Sola-Boneta J, et al. Mortality by causes in HIV-infected adults: comparison with the general population. BMC public health. 2011;11:300. doi: 10.1186/1471-2458-11-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antiretroviral Therapy Cohort C. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010 May 15;50(10):1387–96. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA internal medicine. 2013 Mar;4:1–9. doi: 10.1001/jamainternmed.2013.3728. Epub 2013/03/06. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012 Sep;13(8):453–68. doi: 10.1111/j.1468-1293.2012.00996.x. Epub 2012/03/15. eng. [DOI] [PubMed] [Google Scholar]
- 10.Palella FJ, Jr, Phair JP. Cardiovascular disease in HIV infection. Curr Opin HIV AIDS. 2011 Jul;6(4):266–71. doi: 10.1097/COH.0b013e328347876c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003 Aug 1;33(4):506–12. doi: 10.1097/00126334-200308010-00012. Epub 2003/07/19. eng. [DOI] [PubMed] [Google Scholar]
- 12.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007 Jul;92(7):2506–12. doi: 10.1210/jc.2006-2190. Epub 2007/04/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007 Jun 15;44(12):1625–31. doi: 10.1086/518285. Epub 2007/05/23. eng. [DOI] [PubMed] [Google Scholar]
- 14.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011 Apr 25;171(8):737–43. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012 Aug 1;60(4):351–8. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012 May 22;59(21):1891–6. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friis-Moller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003 Nov 20;349(21):1993–2003. doi: 10.1056/NEJMoa030218. Epub 2003/11/25. eng. [DOI] [PubMed] [Google Scholar]
- 18.Group DADS, Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008 Apr 26;371(9622):1417–26. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–96. doi: 10.1056/NEJMoa062360. Epub 2006/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 20.Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, et al. Immunodeficiency and Risk of Myocardial Infarction Among HIV-Positive Individuals With Access to Care. J Acquir Immune Defic Syndr. 2014 Feb 1;65(2):160–6. doi: 10.1097/QAI.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 21.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006 Aug;44(8 Suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. Epub 2006/07/20. eng. [DOI] [PubMed] [Google Scholar]
- 22.Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: a systematic review and meta-analysis of observational studies. Heart. 2009 Nov;95(22):1826–35. doi: 10.1136/hrt.2009.177774. [DOI] [PubMed] [Google Scholar]
- 23.Monroe AK, Dobs AS, Xu X, Palella FJ, Kingsley LA, Post WS, et al. Low free testosterone in HIV-infected men is not associated with subclinical cardiovascular disease. HIV Med. 2012 Jul;13(6):358–66. doi: 10.1111/j.1468-1293.2011.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitch KV, Looby SE, Rope A, Eneh P, Hemphill L, Lee H, et al. Effects of aging and smoking on carotid intima-media thickness in HIV-infection. AIDS. 2013 Jan 2;27(1):49–57. doi: 10.1097/QAD.0b013e328358b29c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC, et al. Carotid Intima-Media Thickness Progression in HIV-Infected Adults Occurs Preferentially at the Carotid Bifurcation and Is Predicted by Inflammation. Journal of the American Heart Association. 2012 Apr;1(2) doi: 10.1161/JAHA.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose H, Low H, Dewar E, Bukrinsky M, Hoy J, Dart A, et al. The effect of HIV infection on atherosclerosis and lipoprotein metabolism: a one year prospective study. Atherosclerosis. 2013 Jul;229(1):206–11. doi: 10.1016/j.atherosclerosis.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hileman CO, Longenecker CT, Carman TL, McComsey GA. C-Reactive Protein Predicts 96-Week Carotid Intima Media Thickness Progression in HIV-Infected Adults Naive to Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2013 Nov 11; doi: 10.1097/QAI.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 28.Hileman CO, Carman TL, Longenecker CT, Labbato DE, Storer NJ, White CA, et al. Rate and predictors of carotid artery intima media thickness progression in antiretroviral-naive HIV-infected and uninfected adults: a 48-week matched prospective cohort study. Antivir Ther. 2013;18(7):921–9. doi: 10.3851/IMP2651. [DOI] [PubMed] [Google Scholar]
- 29.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012 Nov 15;206(10):1558–67. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sainz T, Alvarez-Fuente M, Navarro ML, Diaz L, Rojo P, Blazquez D, et al. Subclinical atherosclerosis and markers of immune activation in HIV-infected children and adolescents: The CaroVIH Study. J Acquir Immune Defic Syndr. 2013 Aug 26; doi: 10.1097/QAI.0b013e3182a9466a. [DOI] [PubMed] [Google Scholar]
- 31.Desvarieux M, Boccara F, Meynard JL, Bastard JP, Mallat Z, Charbit B, et al. Infection duration and inflammatory imbalance are associated with atherosclerotic risk in HIV-infected never-smokers independent of antiretroviral therapy. AIDS. 2013 Oct 23;27(16):2603–14. doi: 10.1097/QAD.0b013e3283634819. [DOI] [PubMed] [Google Scholar]
- 32.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005 Jun 1;174(11):7446–52. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 33.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging cell. 2004 Aug;3(4):161–7. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 34.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011 Mar;12(3):204–12. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 35.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002 Mar 5;105(9):1135–43. doi: 10.1161/hc0902.104353. Epub 2002/03/06. eng. [DOI] [PubMed] [Google Scholar]
- 36.Tracy RP, Doyle MF, Olson NC, Huber SA, Jenny NS, Sallam R, et al. T-helper type 1 bias in healthy people is associated with cytomegalovirus serology and atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association. 2013 Jun;2(3):e000117. doi: 10.1161/JAHA.113.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012 Oct 16;60(16):1512–20. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Ammirati E, Cianflone D, Vecchio V, Banfi M, Vermi AC, De Metrio M, et al. Effector Memory T cells Are Associated With Atherosclerosis in Humans and Animal Models. Journal of the American Heart Association. 2012 Feb;1(1):27–41. doi: 10.1161/JAHA.111.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsue PY, Deeks SG, Hunt PW. Immunologic Basis of Cardiovascular Disease in HIV-Infected Adults. J Infect Dis. 2012 Jun;205(Suppl 3):S375–82. doi: 10.1093/infdis/jis200. Epub 2012/05/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardali E, Waltenberger J. Monocyte function and trafficking in cardiovascular disease. Thromb Haemost. 2012 Nov;108(5):804–11. doi: 10.1160/TH12-04-0276. [DOI] [PubMed] [Google Scholar]
- 41.Baker JV, Hullsiek KH, Singh A, Wilson E, Henry K, Lichtenstein K, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS. 2013 Dec 24; doi: 10.1097/QAD.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012 Nov 29;120(23):4599–608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011 Oct 15;204(8):1227–36. doi: 10.1093/infdis/jir520. Epub 2011/09/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012 Jul 25;308(4):379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012 Nov 28;26(18):2409–12. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westhorpe CL, Maisa A, Spelman T, Hoy JF, Dewar EM, Karapanagiotidis S, et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol. 2013 Dec 3; doi: 10.1038/icb.2013.84. [DOI] [PubMed] [Google Scholar]
- 47.Sabin CA, Ryom L, De Wit S, Mocroft A, Phillips AN, Worm SW, et al. Associations between immune depression and cardiovascular events in HIV infection. AIDS. 2013 Nov 13;27(17):2735–48. doi: 10.1097/01.aids.0000432457.91228.f3. [DOI] [PubMed] [Google Scholar]
- 48.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010 Dec 15;55(5):615–9. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010 Aug 15;51(4):435–47. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 50.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013 Jul;14(6):385–90. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merlini E, Luzi K, Suardi E, Barassi A, Cerrone M, Martinez JS, et al. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One. 2012;7(9):e46073. doi: 10.1371/journal.pone.0046073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T Cell Activation and Senescence Predict Subclinical Carotid Artery Disease in HIV-Infected Women. J Infect Dis. 2011 Feb;203(4):452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011 Jul;217(1):207–13. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arildsen H, Sorensen KE, Ingerslev JM, Ostergaard LJ, Laursen AL. Endothelial dysfunction, increased inflammation, and activated coagulation in HIV-infected patients improve after initiation of highly active antiretroviral therapy. HIV Med. 2013 Jan;14(1):1–9. doi: 10.1111/j.1468-1293.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- 55.Krishnan S, Schouten JT, Atkinson B, Brown T, Wohl D, McComsey GA, et al. Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naive HIV-infected individuals. J Acquir Immune Defic Syndr. 2012 Nov 1;61(3):381–9. doi: 10.1097/QAI.0b013e3182690e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker JV, Neuhaus J, Duprez D, Freiberg M, Bernardino JI, Badley AD, et al. HIV replication, inflammation, and the effect of starting antiretroviral therapy on plasma asymmetric dimethylarginine, a novel marker of endothelial dysfunction. J Acquir Immune Defic Syndr. 2012 Jun 1;60(2):128–34. doi: 10.1097/QAI.0b013e318252f99f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young B, Squires KE, Ross LL, Santiago L, Sloan LM, Zhao HH, et al. Inflammatory biomarker changes and their correlation with Framingham cardiovascular risk and lipid changes in antiretroviral-naive HIV-infected patients treated for 144 weeks with abacavir/lamivudine/atazanavir with or without ritonavir in ARIES. AIDS Res Hum Retroviruses. 2013 Feb;29(2):350–8. doi: 10.1089/aid.2012.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hileman CO, Wohl DA, Tisch DJ, Debanne SM, McComsey GA. Short communication: initiation of an abacavir-containing regimen in HIV-infected adults is associated with a smaller decrease in inflammation and endothelial activation markers compared to non-abacavir-containing regimens. AIDS Res Hum Retroviruses. 2012 Dec;28(12):1561–4. doi: 10.1089/aid.2012.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012 Jul 25;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 60.Lakey W, Yang LY, Yancy W, Chow SC, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses. 2013 Mar;29(3):435–40. doi: 10.1089/aid.2012.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mata-Marin JA, Mendez-Cruz R, Arroyo-Anduiza CI, Mata-Marin LA, Gaytan-Martinez J, Asbun-Bojalil J. Effect of antiretroviral therapy on inflammatory markers of endothelial dysfunction in HIV treatment-naive infected patients. J Med Virol. 2013 Aug;85(8):1321–6. doi: 10.1002/jmv.23624. [DOI] [PubMed] [Google Scholar]
- 62.Overton ET, Arathoon E, Baraldi E, Tomaka F. Effect of darunavir on lipid profile in HIV-infected patients. HIV Clin Trials. 2012 Sep-Oct;13(5):256–70. doi: 10.1310/hct1305-256. [DOI] [PubMed] [Google Scholar]
- 63.Gupta SK, Shen C, Moe SM, Kamendulis LM, Goldman M, Dube MP. Worsening endothelial function with efavirenz compared to protease inhibitors: a 12-month prospective study. PLoS One. 2012;7(9):e45716. doi: 10.1371/journal.pone.0045716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agarwal S, Busse PJ. Innate and adaptive immunosenescence. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2010 Mar;104(3):183–90. doi: 10.1016/j.anai.2009.11.009. quiz 90–2, 210. [DOI] [PubMed] [Google Scholar]
- 65.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003 Jun 11;289(22):2978–82. doi: 10.1001/jama.289.22.2978. Epub 2003/06/12. eng. [DOI] [PubMed] [Google Scholar]
- 66.Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, et al. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr. 2012 May 15; doi: 10.1097/QAI.0b013e31825b03be. Epub 2012/05/18. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armah K, Lim J, Re VL, Baker J, Tracy R, Butt A, et al. Effect of HIV seroconversion and ART initiation on multiple biomarkers of organ function. Conference on Retroviruses and Opportunistic Infections; March 3–6; Atlanta, GA. 2013. [Google Scholar]
- 68.Pasternak AO, de Bruin M, Jurriaans S, Bakker M, Berkhout B, Prins JM, et al. Modest nonadherence to antiretroviral therapy promotes residual HIV-1 replication in the absence of virological rebound in plasma. J Infect Dis. 2012 Nov;206(9):1443–52. doi: 10.1093/infdis/jis502. [DOI] [PubMed] [Google Scholar]
- 69.Strain MC, Richman DD. New assays for monitoring residual HIV burden in effectively treated individuals. Curr Opin HIV AIDS. 2013 Mar;8(2):106–10. doi: 10.1097/COH.0b013e32835d811b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, et al. HIV Status, Burden of Comorbid Disease and Biomarkers of Inflammation, Altered Coagulation and Monocyte Activation. Clin Infect Dis. 2012 Apr 24; doi: 10.1093/cid/cis406. Epub 2012/04/27. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Althoff K, Wyatt C, Gibert C, oursler K, Rimland D, Rodriguez-Barradas M, et al. Cardiovascular Disease and Other Non-AIDS Events: Epidemiology and Pathogenesis. Conference on Retroviruses and Oppportunistic Infections; March 3–6; Atlanta, GA. 2013. [Google Scholar]
- 72.Tate J, Melissa Herrin, Matt Freiberg, Joyce Chang, Kristina Crothers, Cynthia Gibert, et al. Risk Of Incident Diabetes Associated With Weight Gain After Combination Antiretroviral Therapy (ART) Initiation. Conference on Retroviruses and Opportunistic Infections; March 3–6; Atlanta, GA. 2013. [Google Scholar]
- 73.Goulet JL, Fultz SL, Rimland D, Butt A, Gibert C, Rodriguez-Barradas M, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007 Dec 15;45(12):1593–601. doi: 10.1086/523577. Epub 2008/01/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011 Nov;106(5):858–67. doi: 10.1160/TH11-06-0392. [DOI] [PubMed] [Google Scholar]
- 75.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009 Jul 15;49(2):225–32. doi: 10.1086/599371. Epub 2009/06/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roed T, Lebech AM, Kjaer A, Weis N. Hepatitis C virus infection and risk of coronary artery disease: a systematic review of the literature. Clin Physiol Funct Imaging. 2012 Nov;32(6):421–30. doi: 10.1111/j.1475-097X.2012.01152.x. [DOI] [PubMed] [Google Scholar]
- 77.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010 Aug;11(7):462–8. doi: 10.1111/j.1468-1293.2009.00815.x. Epub 2010/02/19. eng. [DOI] [PubMed] [Google Scholar]
- 78.Data Collection on Adverse Events of Anti HIVDSG. Weber R, Sabin C, Reiss P, de Wit S, Worm SW, et al. HBV or HCV coinfections and risk of myocardial infarction in HIV-infected individuals: the D:A:D Cohort Study. Antivir Ther. 2010;15(8):1077–86. doi: 10.3851/IMP1681. [DOI] [PubMed] [Google Scholar]
- 79.Hechter RC, Budoff M, Hodis HN, Rinaldo CR, Jenkins FJ, Jacobson LP, et al. Herpes simplex virus type 2 (HSV-2) as a coronary atherosclerosis risk factor in HIV-infected men: multicenter AIDS cohort study. Atherosclerosis. 2012 Aug;223(2):433–6. doi: 10.1016/j.atherosclerosis.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parrinello CM, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. Cytomegalovirus immunoglobulin G antibody is associated with subclinical carotid artery disease among HIV-infected women. J Infect Dis. 2012 Jun 15;205(12):1788–96. doi: 10.1093/infdis/jis276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006 Nov 28;20(18):2275–83. doi: 10.1097/QAD.0b013e3280108704. Epub 2006/11/23. eng. [DOI] [PubMed] [Google Scholar]
- 82.D’Agostino RB., Sr Cardiovascular Risk Estimation in 2012: Lessons Learned and Applicability to the HIV Population. J Infect Dis. 2012 Jun;205(Suppl 3):S362–7. doi: 10.1093/infdis/jis196. Epub 2012/05/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Law MG, Friis-Moller N, El-Sadr WM, Weber R, Reiss P, D’Arminio Monforte A, et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med. 2006 May;7(4):218–30. doi: 10.1111/j.1468-1293.2006.00362.x. Epub 2006/04/25. eng. [DOI] [PubMed] [Google Scholar]
- 84.Moreira Guimaraes MM, Bartolomeu Greco D, Ingles Garces AH, de Oliveira AR, Jr, Bastos Foscolo R, de Campos Machado LJ. Coronary heart disease risk assessment in HIV-infected patients: a comparison of Framingham, PROCAM and SCORE risk assessment functions. Int J Clin Pract. 2010 May;64(6):739–45. doi: 10.1111/j.1742-1241.2009.02248.x. Epub 2010/06/04. eng. [DOI] [PubMed] [Google Scholar]
- 85.Edwards-Jackson N, Kerr S, Tieu H, Ananworanich J, Hammer S, Ruxrungtham K, et al. Cardiovascular risk assessment in persons with HIV infection in the developing world: comparing three risk equations in a cohort of HIV-infected Thais. HIV Med. 2011 Sep;12(8):510–5. doi: 10.1111/j.1468-1293.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 86.Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the Data collection on Adverse Effects of Anti-HIV Drugs Study. Eur J Cardiovasc Prev Rehabil. 2010 Oct;17(5):491–501. doi: 10.1097/HJR.0b013e328336a150. Epub 2010/06/15. Eng. [DOI] [PubMed] [Google Scholar]
- 87.Barros ZM, de Alencar Ximenes RA, Miranda-Filho DB, de Albuquerque Mde F, Melo HR, Carvalho EH, et al. Comparison between the Framingham and prospective cardiovascular of Munster scores for risk assessment of coronary heart disease in human immunodeficiency virus-positive patients in Pernambuco, Brazil. Metab Syndr Relat Disord. 2010 Dec;8(6):489–97. doi: 10.1089/met.2009.0100. [DOI] [PubMed] [Google Scholar]
- 88.Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs & aging. 2013 Aug;30(8):613–28. doi: 10.1007/s40266-013-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nachega JB, Hsu AJ, Uthman OA, Spinewine A, Pham PA. Antiretroviral therapy adherence and drug-drug interactions in the aging HIV population. AIDS. 2012 Jul 31;26(Suppl 1):S39–53. doi: 10.1097/QAD.0b013e32835584ea. [DOI] [PubMed] [Google Scholar]
- 90.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012 Jul 1;60(Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holtzman C, Armon C, Tedaldi E, Chmiel JS, Buchacz K, Wood K, et al. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med. 2013 Oct;28(10):1302–10. doi: 10.1007/s11606-013-2449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marzolini C, Back D, Weber R, Furrer H, Cavassini M, Calmy A, et al. Ageing with HIV: medication use and risk for potential drug-drug interactions. J Antimicrob Chemother. 2011 Sep;66(9):2107–11. doi: 10.1093/jac/dkr248. [DOI] [PubMed] [Google Scholar]
- 93.Gedela K, Vibhuti M, Pozniak A, Ward B, Boffito M. Pharmacological management of cardiovascular conditions and diabetes in older adults with HIV infection. HIV Med. 2013 Dec 18; doi: 10.1111/hiv.12116. [DOI] [PubMed] [Google Scholar]
- 94.Maiese E, Malmenäs M, Atkinson M. International Congress on Drug Therapy in HIV Infection. Glasgow, Scotland: 2012. Impact of comorbidities on HIV medication persistence: a retrospective database study using US claims data. [Google Scholar]
- 95.Barry DT, Goulet JL, Kerns RK, Becker WC, Gordon AJ, Justice AC, et al. Nonmedical use of prescription opioids and pain in veterans with and without HIV. Pain. 2011 May;152(5):1133–8. doi: 10.1016/j.pain.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Green TC, Kershaw T, Lin H, Heimer R, Goulet JL, Kraemer KL, et al. Patterns of drug use and abuse among aging adults with and without HIV: a latent class analysis of a US Veteran cohort. Drug Alcohol Depend. 2010 Aug 1;110(3):208–20. doi: 10.1016/j.drugalcdep.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ettenhofer ML, Hinkin CH, Castellon SA, Durvasula R, Ullman J, Lam M, et al. Aging, neurocognition, and medication adherence in HIV infection. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2009 Apr;17(4):281–90. doi: 10.1097/JGP.0b013e31819431bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crystal S, Akincigil A, Sambamoorthi U, Wenger N, Fleishman JA, Zingmond DS, et al. The diverse older HIV-positive population: a national profile of economic circumstances, social support, and quality of life. J Acquir Immune Defic Syndr. 2003 Jun 1;33(Suppl 2):S76–83. [PubMed] [Google Scholar]
- 99.Anema A, Weiser SD, Fernandes KA, Ding E, Brandson EK, Palmer A, et al. High prevalence of food insecurity among HIV-infected individuals receiving HAART in a resource-rich setting. AIDS Care. 2011 Feb;23(2):221–30. doi: 10.1080/09540121.2010.498908. [DOI] [PubMed] [Google Scholar]
- 100.Work Group for HIV, Aging Consensus P. Summary report from the Human Immunodeficiency Virus and Aging Consensus Project: treatment strategies for clinicians managing older individuals with the human immunodeficiency virus. Journal of the American Geriatrics Society. 2012 May;60(5):974–9. doi: 10.1111/j.1532-5415.2012.03948.x. [DOI] [PubMed] [Google Scholar]
- 101.Gleason LJ, Luque AE, Shah K. Polypharmacy in the HIV-infected older adult population. Clinical interventions in aging. 2013;8:749–63. doi: 10.2147/CIA.S37738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Waters L, Patterson B, Scourfield A, Hughes A, de Silva S, Gazzard B, et al. A dedicated clinic for HIV-positive individuals over 50 years of age: a multidisciplinary experience. Int J STD AIDS. 2012 Aug;23(8):546–52. doi: 10.1258/ijsa.2012.011412. [DOI] [PubMed] [Google Scholar]
- 103.Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996 Apr;100(4):428–37. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 104.Action to Control Cardiovascular Risk in Diabetes Study G. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Group AS, Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010 Apr 29;362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burkholder GA, Tamhane AR, Salinas JL, Mugavero MJ, Raper JL, Westfall AO, et al. Underutilization of Aspirin for Primary Prevention of Cardiovascular Disease Among HIV-Infected Patients. Clin Infect Dis. 2012 Sep 24; doi: 10.1093/cid/cis752. Epub 2012/09/04. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suchindran S, Regan S, Meigs JB, Grinspoon S, Triant VA. Comparison of Apsirin Use and Incident Myocardial Infarction Rates in HIV+ and HIV-Patients in a Large US Health Care System. Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, Georgia. 2013. [Google Scholar]
- 108.Pearce D, Ani C, Espinosa-Silva Y, Clark R, Fatima K, Rahman M, et al. Comparison of In-Hospital Mortality from Acute Myocardial Infarction in HIV Sero-Positive Versus Sero-Negative Individuals. Am J Cardiol. 2012 Oct 15;110(8):1078–84. doi: 10.1016/j.amjcard.2012.05.045. Epub 2012/07/06. eng. [DOI] [PubMed] [Google Scholar]
- 109.Guaraldi G, Zona S, Menozzi M, Carli F, Bagni P, Berti A, et al. Cost of noninfectious comorbidities in patients with HIV. ClinicoEconomics and outcomes research : CEOR. 2013;5:481–8. doi: 10.2147/CEOR.S40607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Masica AL, Richter KM, Convery P, Haydar Z. Linking joint commission inpatient core measures and national patient safety goals with evidence. Proc (Bayl Univ Med Cent) 2009 Apr;22(2):103–11. doi: 10.1080/08998280.2009.11928486. Epub 2009/04/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Redberg RF, Benjamin EJ, Bittner V, Braun LT, Goff DC, Jr, Havas S, et al. ACCF/AHA 2009 performance measures for primary prevention of cardiovascular disease in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures for Primary Prevention of Cardiovascular Disease) developed in collaboration with the American Academy of Family Physicians; American Association of Cardiovascular and Pulmonary Rehabilitation; and Preventive Cardiovascular Nurses Association: endorsed by the American College of Preventive Medicine, American College of Sports Medicine, and Society for Women’s Health Research. J Am Coll Cardiol. 2009 Sep 29;54(14):1364–405. doi: 10.1016/j.jacc.2009.08.005. Epub 2009/09/26. eng. [DOI] [PubMed] [Google Scholar]
- 112.Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, et al. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures for ST-Elevation and Non-ST-Elevation Myocardial Infarction) Developed in Collaboration With the American Academy of Family Physicians and American College of Emergency Physicians Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. J Am Coll Cardiol. 2008 Dec 9;52(24):2046–99. doi: 10.1016/j.jacc.2008.10.012. Epub 2008/12/06. eng. [DOI] [PubMed] [Google Scholar]
- 113.Guaraldi G, Zona S, Alexopoulos N, Orlando G, Carli F, Ligabue G, et al. Coronary aging in HIV-infected patients. Clin Infect Dis. 2009 Dec 1;49(11):1756–62. doi: 10.1086/648080. Epub 2009/11/06. eng. [DOI] [PubMed] [Google Scholar]
- 114.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a Model of Accelerated or Accentuated Aging? J Gerontol A Biol Sci Med Sci. 2013 Oct 24; doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]


