Abstract
BACKGROUND
Refrigeration of platelets (PLTs) offers an attractive alternative to the currently practiced storage at room temperature since it may mitigate problems associated with bacterial contamination and extend storage lifetime. Refrigeration causes a number of biophysical and biochemical changes in PLTs and decreases PLT circulation time in vivo. However, the effect of refrigeration on PLT hemostatic functions under physiologic and pathophysiologic shear conditions has not been adequately characterized.
STUDY DESIGN AND METHODS
Washed PLTs prepared from either fresh PLT-rich plasma (PRP) or PRP stored at 4°C for 2 days was mixed with exogenous von Willebrand factor (VWF) and fibrinogen and sheared in a cone-and-plate viscometer. PLT aggregation, activation, and VWF binding after shear and glycoprotein (GP) Ibα receptor expression and ristocetin-induced PLT agglutination were measured.
RESULTS
PLTs stored at 4°C for 2 days aggregated significantly more than fresh PLTs particularly at high shear rates (10,000/sec), and this increase was independent of PLT concentration or suspension viscosity. Further, refrigerated PLTs showed a greater increase in GP Ibα–dependent PLT activation under shear and also bound more VWF than fresh PLTs. However, the GP Ibα expression levels as measured by three different antibodies were significantly lower in refrigerated PLTs than in fresh PLTs, and refrigeration resulted in a modest decrease in ristocetin-induced PLT agglutination.
CONCLUSION
The combined results demonstrate that refrigeration increases PLT aggregation under high shear, but not static, conditions and also increases shear-induced VWF binding and PLT activation. Clinically, enhanced shear-induced PLT aggregation due to low temperature storage may be a beneficial strategy to prevent severe bleeding in trauma.
Platelets (PLTs) are transfused to prevent bleeding due to thrombocytopenia associated with hematologic malignancies or to manage severe blood loss during surgery or trauma. PLTs are stored at room temperature in gas-permeable bags with constant agitation for up to 5 days.1 Although millions of PLT transfusions are performed every year, supply does not match the demand. PLTs stored under current practices undergo a gradual decline in function and viability, which presumably is a result of progressive activation and an accumulation of deleterious metabolic byproducts.2,3 Other major problems associated with current storage techniques that limit the relatively short shelf life include viral and bacterial contamination despite improvements in bacterial detection and pathogen inactivation technologies.4,5
In principle, storage of PLTs under refrigeration (4°C), which is standard practice for red blood cells (RBCs), can overcome the problems associated with room temperature storage since refrigeration drastically impedes bacterial growth and reduces PLT metabolism, thus alleviating these aspects of the storage lesion.6 In addition, refrigeration would also simplify the storage and transportation of blood products in emergency use settings, such as military hospitals and civilian emergency departments, as only one storage technology would be needed for RBCs, PLTs, and thawed plasma. However, Murphy and Gardner in 19696,7 showed that the recovery and survival half-life of PLTs after 18 hours of storage at room temperature were similar to fresh PLTs at 55% and 4.0 days, respectively, while the corresponding values for storage at 4°C were 40% and 1.3 days. Several other studies have confirmed poor survival and half-life of refrigerated PLTs, leading to the current practice of storage at room temperature.8–10
PLTs stored for either short-term (1–4 hr) or long-term (2–14 days) at 4°C undergo a number of morphologic, biochemical, and functional changes collectively called the “cold storage lesion.”11 Exposure of PLTs to low temperature for 1 to 4 hours results in the loss of discoid shape due to the loss of circumferential microtubular rings around the periphery of disc-shaped PLTs12 and uncapping of actin filaments.13 Long-term refrigeration results in a number of progressive changes in PLTs: change in glycoprotein receptor (GP Ib and GP IIb/IIIa) levels,14 up regulation of PLT activation markers such as P-selectin and annexin V,15 changes in fluidity of the plasma membrane,16 altered responses to aggregating17 and disaggregating18 agents, increase in intracellular calcium concentration,19 and decreased adhesion to sub-endothelium in vivo.20 Upon transfusion, PLTs stored at 4°C for short and long term are cleared rapidly by macrophages and hepatocytes, respectively.21,22 The clearance processes are attributed to clustering and different degrees of desialylation of PLT receptor (GP) Ibα.21,23 While these studies have greatly improved our understanding of the effect of low temperature on PLT morphology and biochemistry, the effect on hemostatic function is still an unanswered question.
In this article, we have examined the effect of long-term refrigeration on in vitro PLT hemostatic function under flow. PLTs are captured from flowing blood on to injured surfaces to form a hemostatic plug through a process initiated by the binding between PLT GP Ib-IX-V complex on the PLT surface and exposed von Willebrand factor (VWF) bound to the subendothelial matrix. After this initial PLT adhesion, aggregates form through the binding of GP IIb/IIIa to fibrinogen. We hypothesized that PLTs stored at low temperature for long periods (48 hr) will function differently from fresh PLTs under shear conditions. To test this hypothesis, we evaluated PLT aggregation and activation in vitro. Our results indicate that low-temperature-stored PLTs aggregate more than fresh PLTs under shear conditions but not under static conditions. Similar phenomena may modulate hemostatic function in vivo, in response to colder temperature exposure of PLTs in peripheral circulation, as others have suggested.22
MATERIALS AND METHODS
Ethics statement
Venous blood was freshly drawn from healthy, nonsmoking adult volunteers, free from PLT-altering medications, after signing an informed consent and obtaining written ethics approval in accordance with the institutional review board (IRB) protocol (IRB #11-190, Office of Research Integrity and Compliance, UTSA).
Reagents
We purchased Factor VIII-free human VWF from Haematologic Technologies, Inc. (Essex Junction, VT), lyophilized human plasma fibrinogen from EMD Chemicals (San Diego, CA), mouse monoclonal anti-GP Ibα antibodies: unconjugated and fluorescein isothiocyanate (FITC)-conjugated AK2 (binds residues 36-59), unconjugated SZ2 (binds Residues 201-268), and VM16d (binds Residues 269-282) were obtained from Abcam (Cambridge, MA). AK2 binds to the first leucine-rich repeat 36 to 59, VM16d binds to the C-terminal flank 201 to 268, and SZ2 binds to the anionic sulfated region 269 to 282.24 Mouse mono-clonal anti-P-selectin antibody AK6, phycoerythrin (PE)-conjugated sheep polyclonal anti-VWF antibody, goat polyclonal anti-mouse FITC and PE secondary antibodies, and immunoglobulin G isotype control antibodies were all from Abcam, prostaglandin I2 sodium salt from Cayman Chemicals (Ann Arbor, MI), and 30% solution of bovine serum albumin (BSA) from Fisher Scientific (Pittsburgh, PA).
PLT isolation and storage
Whole venous blood was collected by venipuncture using specimen collection tubes (Vacutainer system, BD, Franklin Lakes, NJ), 21-gauge needle with 12-in. tubing attached into 8.5-mL sterile, glass evacuated tubes containing 1.5 mL of anticoagulant acid citrate dextrose A (tri-sodium citrate, 22.0 g/L; citric acid, 8.0 g/L; and dextrose 24.5 g/L). PLT-rich plasma (PRP) was obtained from whole blood by centrifuging at 250 × g for 20 minutes at room temperature. The PRP layer was gently aspirated under sterile conditions and placed in 15 mL sterile, conical, plastic centrifuge tubes for storage as 2-mL aliquots, taking care not to include any RBCs. PRP was used immediately or was stored without agitation in the refrigerator (4°C) for 48 hours. We used only 2-mL aliquots so that there is sufficient head space for gas exchange.
Preparation of washed PLTs
Stored or fresh PRP was centrifuged at 1000 × g for 10 minutes at room temperature in the presence of 2.7 nmol/L (final concentration) PGI2 to pellet PLTs. PLT-poor plasma was decanted and the PLT pellet gently resuspended in 2 mL of modified HEPES/Tyrode’s buffer without Ca2+ and Mg2+ (136 mmol/L NaCl, 2.7 mmol/L KCl, 12 mmol/L NaHCO3, 0.042 mmol/L NaH2PO4, 5.6 mmol/L glucose, 5 mmol/L HEPES, 0.1% BSA, pH 7.4) in the presence of 2.7 nmol/L PGI2. The PLT suspension was centrifuged at 1000 × g for 10 minutes at room temperature. After the supernatant was decanted, the washing step was repeated once more, and PLTs were resuspended in HEPES/Tyrode’s buffer with or without 2 mmol/L Ca2+/1 mmol/L Mg2+ (CaCl2/MgCl2) for assays. PLT count was obtained with a cell and particle counter (Beckman Z2 Coulter Counter, Brea, CA) and adjusted to desired concentration with HEPES/Tyrode’s buffer.
Measurement of receptor expression levels
Ten microliters of fresh or stored PRP was incubated with 25 μg/mL FITC-conjugated AK2 or unconjugated SZ2 or VM16d antibodies for 20 minutes. In case of SZ2 and VM16d, the primary antibody-bound PLTs were incubated with a PE-conjugated secondary antibody. The antibody-bound PLTs were then fixed in 400 μL of 1% formaldehyde containing 2% BSA in Dulbecco’s phosphate-buffered saline (DPBS) without Ca2+/Mg2+ for 30 minutes and analyzed by flow cytometry for percent positive cells and the mean fluorescence intensity (MFI).
Shear-induced PLT aggregation
Eighty microliters of washed PLTs resuspended to a final concentration of 1 × 107 to 4 × 107/mL in HEPES/Tyrode’s buffer with Ca2+ and Mg2+ mixed with 2 mg/mL fibrinogen and 5 μg/mL VWF was sheared at either 500, 2500, or 10,000/second for 120 seconds at 37°C in a computer-controlled 0.5° cone-and-plate rheometer (MCR 301, Anton-Paar, Ashland, VA). Ten microliters of sheared PLT suspension was immediately fixed in 100 μL of 1% formaldehyde in DPBS without Ca2+ and Mg2+ for 30 minutes, after which 300 μL of DPBS without Ca2+ and Mg2+ was added. For static controls, the suspension was left unsheared under the same conditions on the cone-and-plate rheometer. PLT aggregation was measured using a flow cytometer as an increase in the forward scatter-side scatter population which is bigger and outside the single, unactivated PLT gate (BD Biosciences, San Jose, CA).25
Shear-induced PLT activation
PLTs were sheared as described above and 10 μL was immediately removed and added to 100 μL of annexin binding buffer (10 mmol/L HEPES, 140 mmol/L NaCl, 2.5 mmol/L CaCl2) containing 1 μL FITC-conjugated annexin V. After 10 minutes of incubation the reaction was diluted with 300 μL of annexin-binding buffer and analyzed within 15 minutes on the flow cytometer. P-selectin expression levels were measured by adding 10 μL of sheared PLT suspension to 100 μL HEPES/Tyrode’s buffer containing 2% BSA and 2.5 μL PE-conjugated anti-P-selectin antibody. After 30 minutes’ incubation, 300 μL of HEPES/Tyrode’s buffer was added and the sample was analyzed within 15 minutes on the flow cytometer. In certain experiments, PLT suspension was treated with 25 μg/mL AK2 for 20 minutes to block GP Ibα before running the static-shear experiments.26,27 From a previous titration experiment with 5 to 25 μg/mL AK2 antibody, we had determined that at 25 μg/mL antibody is sufficient to bind all GP Ibα receptors on the PLT surface.
Measurement of VWF binding to PLTs
Washed PLTs were mixed with either 5 μg/mL VWF or buffer (negative control) sheared as described. Ten microliters of sheared PLT suspension was immediately fixed in 100 μL of 1% formaldehyde in DPBS without Ca2+ and Mg2+. VWF-bound PLTs were stained with PE-conjugated anti-VWF mouse antibody and washed twice before analysis. PE-positive PLTs were quantified by flow cytometry. As negative control, antibody binding to PLTs not treated with VWF was used to obtain baseline binding to PLTs.
Ristocetin-induced PLT agglutination
Washed PLTs (107/mL) were mixed with either 5 μg/mL VWF, and 0.75 or 1.5 mg/mL ristocetin, and the final volume was adjusted to 250 μL.28 The suspension was agitated in an aggregometer (Chrono-log, Havertown, PA) at 37°C for 20 minutes. The increase in light transmission was used as a measure of PLT agglutination.
Statistical analysis
Data are mean ± SEM of three to eight donors, and for each donor the means were calculated from experiments performed either in duplicate or triplicate. The differences between means of untreated and treated samples were considered significant if p values were less than 0.05 as estimated using a t test.
RESULTS
Shear-induced aggregation of fresh and stored PLTs
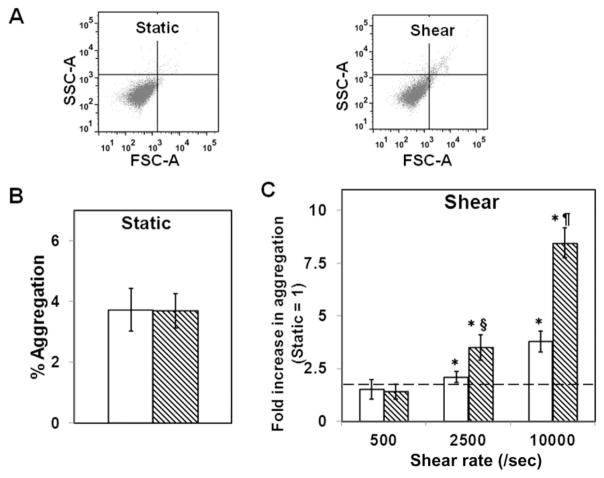
We exposed washed PLTs that were mixed with physiologic concentrations of VWF and fibrinogen to a range of constant shear stresses mimicking those of venous, arterial, and stenotic shear stresses for 120 seconds at 37°C and measured PLT aggregation.25,29 As baseline, we measured spontaneous aggregation of PLTs under similar conditions but without shear (Fig. 1A). Fresh PLTs or PLTs stored for 48 hours at 4°C showed minimal PLT aggregation (<3%) indicating that neither fresh nor stored PLTs aggregated spontaneously at our experimental conditions (Fig. 1B). When PLT suspension was sheared at a venous shear rate of 500/second, there was no significant increase in aggregation over that under static conditions (Fig. 1C). When the shear rate was increased further to an arterial shear rate of 2500/second, we observed a modest two- to threefold increase in aggregation due to shear compared with static conditions in both fresh and stored PLTs, although the stored PLTs aggregated better than fresh PLTs. Upon increasing the shear rate to a high stenotic shear rate of 10,000/second, fresh PLTs showed a 3.8-fold increase in PLT aggregation due to shear, while 4°C-stored PLTs showed 8.4-fold increase in PLT aggregation. Thus, 4°C storage enhances shear stress–induced PLT aggregation (SIPA) by more than 100% (i.e., twofold) compared to freshly isolated PLTs at high shear rates.
Fig. 1.
SIPA in fresh and 4°C-stored PLTs. Washed PLTs (107/mL) were mixed with 5 μg/mL VWF and 2 mg/mL fibrinogen and subjected to shear rates of 0, 500, 2500, or 10,000/second for 120 seconds at 37°C, and PLT aggregation was measured. (A) Flow cytometric plots for the measurement of PLT aggregates. SIPA was estimated as increase in PLT population outside the single PLT gate compared to static controls. (B) Under static conditions, fresh or stored PLTs showed similar, baseline aggregation. (C) At different shear rates, the shear-induced aggregation of fresh or stored PLTs was normalized to the baseline static levels for each donor at the corresponding condition. Both fresh (□) and stored (▧) PLTs aggregate at high shear rates (n = 8, *p < 0.05, static vs. shear), but 4°C storage enhances aggregation (n = 8, §p < 0.05, ¶p < 0.01, fresh vs. stored). FSC = forward scatter; SSC = side scatter.
Effect of cell–cell collisions and fluid shear stress on SIPA
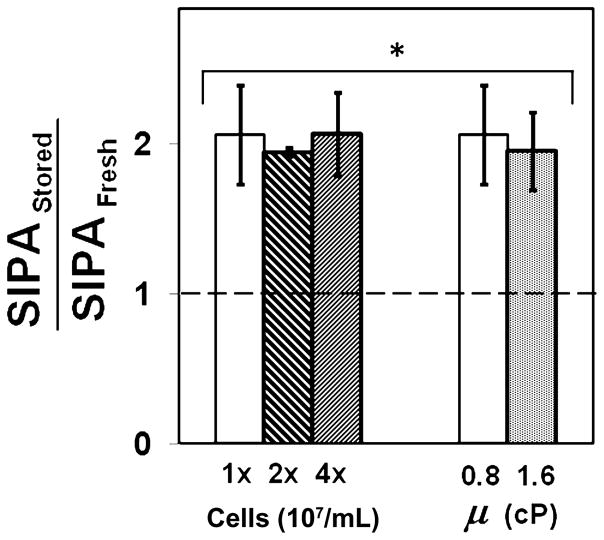
We examined if the enhanced SIPA observed due to 4°C storage in PLTs is modulated by biophysical factors that control PLT aggregation in a shear field, namely PLT–PLT collision frequency and the force experienced by aggregating PLTs.26 These factors are controlled, respectively, by shear rate (γ) and shear stress (μ) in a flow field, and they are related as τ = μγ, where μ is viscosity. Hence by independently varying shear stress and shear rate, we can vary the two biophysical factors independent of each other. An increase in PLT concentration will increase the number of PLT–PLT collisions during shear. Fresh or stored PLT concentration was increased two or four times more than the baseline value shown in Fig. 1C, and the suspension was sheared at 10,000/second for 120 seconds at 37°C. We compared enhancement in SIPA in stored PLTs over fresh PLTs when the PLT concentration in suspension was increased (Fig. 2). We found that SIPA in PLTs stored at 4°C was twofold (100%) more than fresh PLTs and this ratio maintained irrespective of PLT concentration in suspension. Next, we evaluated the effect of force on PLT aggregation by increasing the viscosity of the sheared suspension and measuring PLT aggregation at the same shear rate of 10,000/second. We doubled the viscosity of PLT suspension in HEPES buffer from the baseline 0.8 to 1.6 cP by adding 1.5% dextran and measured SIPA in stored and fresh samples after applying 10,000/second shear for 120 seconds at 37°C. At 1.6 cP suspension viscosity, PLTs stored at 4°C aggregated by twofold more than fresh PLTs, and this increase was comparable to that observed at 0.8 cP (Fig. 2). In control experiments, addition of dextran did not alter baseline aggregation or activation levels (data not shown). Thus, enhanced SIPA due to 4°C storage of PLTs is maintained at other PLT concentrations and suspension viscosities, suggesting that this phenomenon is independent of biophysical factors and instead due to the intrinsic receptor–ligand binding interactions.
Fig. 2.
SIPA in fresh and 4°C-stored PLTs at different PLT concentrations and viscosity. The PLT concentration was increased by two- or fourfold and subjected to a shear rate of 10,000/second using the protocol described in Fig. 1. In another experiment, only the suspension viscosity was increased twofold by the addition of 1.5% dextran and subjected to a shear rate of 10,000/second using the protocol described in Fig. 1. The SIPA ratio between stored and fresh PLTs was calculated for each donor and was found to be sig-nificantly increased upon storage under all conditions (n = 5, *p< 0.05, stored/fresh > 1.0).
Shear-induced binding of VWF to PLTs
It is now well established that PLT aggregation at high shear stress is primarily mediated by GP Ibα–VWF interaction.30 Shear stress increases the binding of VWF to GP Ibα on PLT surface.31,32 Hence, to address if the increase in SIPA in stored PLTs is due to an increase in VWF binding, we measured the binding of VWF to fresh or stored PLTs under static and shear conditions using a polyclonal antibody against surface-bound VWF (Fig. 3A). We observed that similar levels of VWF bind to fresh and refrigerated PLTs in static conditions, indicating no spontaneous binding of VWF to PLT surface (Fig. 3B). However, upon exposure to high shear stress, significantly more VWF bound to refrigerated PLTs than fresh PLTs. This three- to fourfold difference may contribute to enhanced SIPA seen in refrigerated PLTs.
Fig. 3.
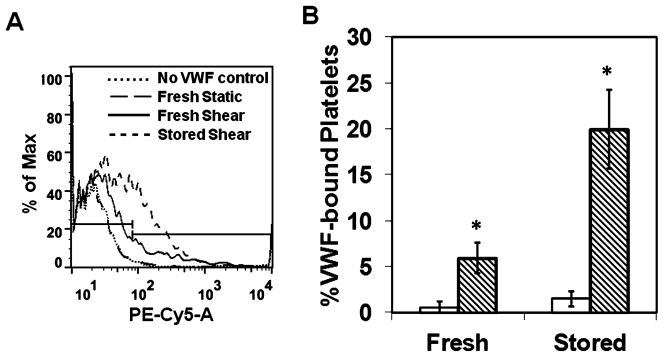
Effect of storage temperature on the binding of VWF under static (□) and shear (▧) conditions. Washed PLTs were mixed with 5 μg/mL VWF and subjected to static or shear (10,000/sec) for 120 seconds at 37°C. (A) The VWF binding was measured using a PE-conjugated anti-VWF polyclonal antibody. (B) The results are the mean of one experiment performed in triplicate, and the experiment was repeated for three donors. Shear stress increased the binding of VWF to PLTs over static incubation (n = 3, *p < 0.05, static vs. shear).
Shear-induced activation in fresh and stored PLTs
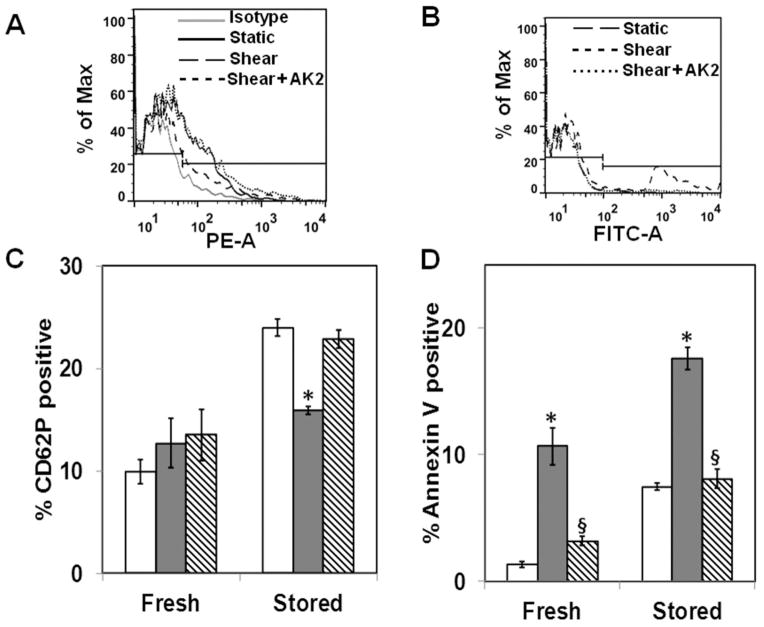
We addressed whether the increased VWF binding at high shear in PLTs stored at 4°C resulted in enhanced shear-induced PLT activation and hence contributed to enhanced PLT aggregation. First, we measured baseline PLT activation due to storage by measuring PLT P-selectin expression and phosphatidylserine (PS) exposure levels in fresh and stored samples (Figs. 4A and 4B). Under static conditions, both P-selectin levels and PS exposure are low in fresh PLTs, indicating minimum activation. Storage at 4°C activates the PLTs as evidenced by an increase in both P-selectin levels and PS exposure (Figs. 4C and 4D). Since shear stress is a potent activator of PLTs, we investigated whether shear stress increased PLT activation differentially between fresh and stored PLTs at 10,000/second. We observed that shear stress does not increase P-selectin expression in fresh PLTs, an observation consistent with previous reports (Figs. 4A and 4C).33 A significant decrease in P-selectin expression due to shear was observed in stored PLTs, suggesting shedding of these receptors. When PLTs were treated with saturating quantities of VWF-function blocking antibody AK2, the P-selectin expression levels were unchanged compared to static levels. However, shear stress induces an increase in PS exposure in both fresh and 4°C-stored PLTs, indicating that annexin V binding is a sensitive marker of shear-induced PLT activation (Figs. 4B and 4D).26 This shear-induced PLT activation is dependent on PLT GP Ibα–VWF binding since blocking GP Ibα receptor with VWF-function blocking antibody AK2 reduced annexin V binding to static levels. These results indicate that shear stress increases GP Ibα–dependent PLT activation as measured by annexin V binding significantly in 4°C-stored PLTs.
Fig. 4.
Shear-induced PLT activation in fresh and 4°C-stored PLTs. Washed PLTs were mixed with 5 μg/mL VWF and subjected to static or shear rates (10,000/second) for 120 seconds at 37°C. The samples were incubated with PE-conjugated P-selectin antibody (A and C) or annexin V (B and D) and analyzed by flow cytometry. Binding was blocked by preincubating PLTs with 25 μg/mL GP Ibα antibody AK2 for 20 minutes before shear. Shear stress decreased P-selectin binding (n = 4, *p < 0.05, static [□] vs. shear [
 ]), increased annexin V binding (n = 4, *p < 0.05, static vs. shear), and AK2 blocked annexin V binding to PLTs under shear (n = 4, §p < 0.05, shear vs. shear + AK2 [▧]).
]), increased annexin V binding (n = 4, *p < 0.05, static vs. shear), and AK2 blocked annexin V binding to PLTs under shear (n = 4, §p < 0.05, shear vs. shear + AK2 [▧]).
GP Ibα receptor expression in fresh and stored PLTs
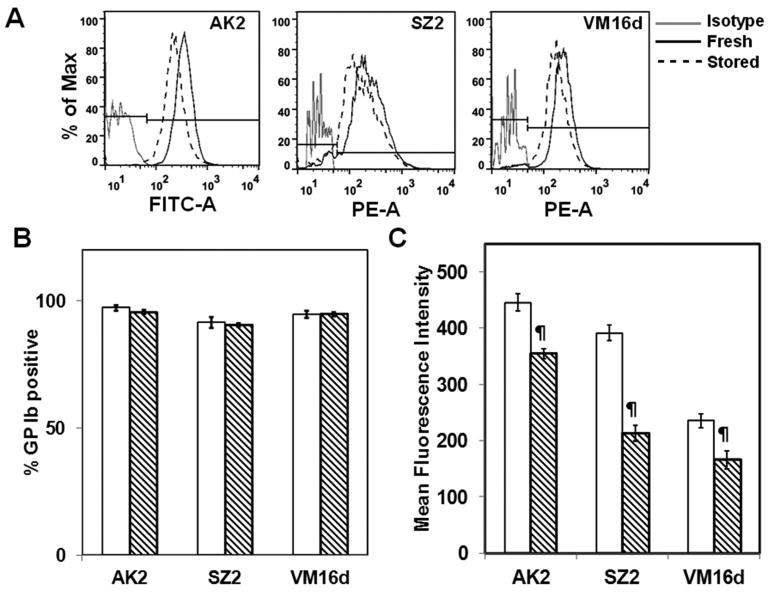
Since VWF binding under shear increased in stored PLTs, we examined the effect of cold storage on the expression levels of the VWF receptor on PLT surface receptor GP Ibα, which is critical in PLT adhesion, activation, and aggregation at high shear stress. We analyzed GP Ibα expression levels using antibodies AK2, VM16d, and SZ2, which bind to different regions along the 45-kDa N-terminal region of GP Ibα.24 Of these, AK2 has an epitope in the VWF-binding region of GP Ibα. We noticed that the antibody-binding levels decrease significantly upon 4°C storage though all PLTs express GP Ib receptor (Figs. 5A and 5B). We estimated that AK2 binding decreases by 21%, SZ2 by 44%, and VM16d by 30%—suggesting a partial loss of GP Ibα receptors from the surface (Fig. 5C). Thus, despite an increase in SIPA in stored PLTs, we observed a possible loss in GP Ibα expression levels due to storage.
Fig. 5.
GP Ibα receptor expression in fresh and 4°C-stored PLTs. Fresh (□) or stored (▧) PRP was incubated with FITC-conjugated AK2 or unconjugated SZ2 or VM16d antibodies. The unconjugated antibodies were then bound with PE-conjugated secondary antibodies and the receptor expression levels were measured by flow cytometry. (A) Flow cytometry plots of fresh and stored PLTs with respect to isotype control. (B) % GP Ib-positive PLTs. (C) MFI. The GP Ibα expression levels decreased due to storage (n = 6, ¶p< 0.01, fresh vs. stored).
Ristocetin-induced PLT agglutination
We evaluated the effect of the decrease in expression levels of GP Ibα in stored PLTs on VWF interactions using a standard static-binding assay. We estimated the effect of storage on ristocetin-induced PLT agglutination (RIPA) at two different ristocetin concentrations, namely, 0.75 and 1.5 mg/mL (Fig. 6). Ristocetin is an exogenous modulator that mediates PLT agglutination by forming dimeric bridges between VWF and PLT GP Ibα.34 We observed that at a ristocetin concentration of 1.5 mg/mL, which is typically used in clinical assays, both fresh and stored PLTs showed similar levels of agglutination, and at lower ristocetin concentration of 0.75 mg/mL the stored PLTs did not agglutinate as well as fresh PLTs. These results indicate that the decrease in GP Ibα expression levels due to storage has a modest effect on the GP Ibα–VWF interactions under static conditions and contrasts with the behavior seen under shear conditions.
Fig. 6.
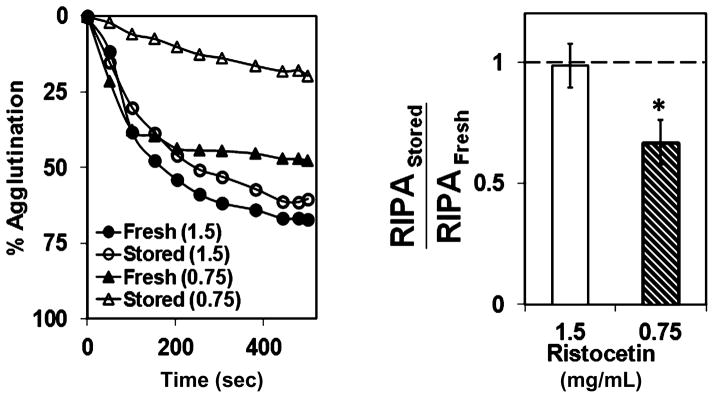
RIPA. Washed PLTs were mixed with 5 μg/mL VWF and 0.75 or 1.5 mg/mL ristocetin, and agglutination was monitored in an aggregometer. PLTs stored at 4°C agglutinated to similar levels as fresh PLTs at 1.5 mg/mL ristocetin, but were significantly lower at 0.75 mg/mL ristocetin (n = 3, *p < 0.05, fresh vs. stored).
DISCUSSION
We have demonstrated that low-temperature storage of PLTs results in enhanced shear-induced aggregation and that this enhancement may be due to an increase in shear-induced PLT activation and the binding of VWF to its receptor, GP Ibα. We also demonstrate that low-temperature storage results in a decrease in GP Ibα expression levels and in ristocetin-induced PLT agglutination.
Cold storage (4°C) of PLTs is an attractive, practical alternative to the current practice of 22°C storage for 5 days. This could potentially ameliorate the problems of the PLT storage lesion and higher risk of contamination associated with room temperature storage. PLTs are transfused to form a stable hemostatic plug that is critical to prevent bleeding. PLT activation, adhesion, and aggregation are central to hemostasis, and blood flow is a potent regulator of this process. The hemostatic function of cold-stored PLTs under physiologic flow conditions has not been adequately characterized. Further, there is a lack of in vivo data on the hemostatic function of cold-stored PLTs. In this work, we measured PLT aggregation under shear rates representative of stasis (0/sec) and flow in veins (500/sec), arteries (2500/sec) and some arterioles, and stenosed arteries and prosthetic cardiovascular devices (10,000/sec). We used washed PLTs mixed with physiologic levels of VWF and fibrinogen instead of PRP or endogenous plasma to eliminate possible confounding effects of storage on plasma proteins. Of note, since the viscosity of washed PLT suspension (0.8 cP) is four times lower than that of blood (3.2 cP) at 37°C, the shear stress experienced by PLTs in buffer at a shear rate of 10,000/second will be comparable to that experienced by PLTs physiologically in whole blood at a shear rate of 2500/second. Shear stress is a mechanical agonist that can aggregate fresh PLTs, and the aggregation depends on duration, level of exposure, and temperature. Consistent with several published studies, we observed that aggregation of fresh PLTs increased in a shear stress–dependent manner.30,33 Interestingly, the 4°C-stored PLTs showed a heightened aggregatory response to shear stress compared to fresh PLTs. Previous studies have shown that PLTs stored at 4°C long term (24–48 hr), compared to fresh PLTs, show a muted aggregation response to chemical agonists including thrombin, ADP, collagen, and epinephrine15,17,35 and reduced sensitivity to signal transduction-inhibiting agents.18 In light of these reports, results suggest that shear stress, a mechanical agonist, may act differently than chemical agonists in aggregating stored PLTs.
PLT aggregation under shear is controlled by cell–cell collision frequency and the force applied to the cells.26 These factors are directly related to cell concentration and viscosity, respectively. When the PLT concentration and viscosity were increased independently at a constant high shear rate, aggregation of 4°C-stored PLTs was greater than fresh PLTs. This suggests that neither collision frequency nor the fluid force acting on the PLTs are critical factors in modulating the response of 4°C-stored PLTs to shear stress. We surmised that PLT activation during storage may contribute to enhanced shear-induced PLT aggregation. PLTs undergo a number of activation-related biochemical changes during cold storage, albeit more slowly than at room temperature, including up regulation of P-selectin and PS exposure15 and increase in intracellular calcium levels.19 Consistent with these results, we observed an increase in P-selectin expression and PS exposure in 4°C-stored PLTs after 2 days. However, activation due to storage alone does not result in a significant increase in spontaneous PLT aggregation under static conditions but may prime the 4°C-stored PLTs for an amplified response to shear stress.
Shear stress is also an established and potent activator of PLTs. Dayananda and coworkers31 have reported that VWF-bound GP Ibα acts as a mechanoreceptor for shear stress on PLTs and that annexin V binding increases proportionally to the amount of VWF bound to PLTs. We observed that at 10,000/second shear rate, 4°C-stored PLTs binds three- to fourfold more VWF than in fresh PLTs. As more VWF binds to stored PLTs, a greater increase in GP Ibα–transduced mechanosensory signal is transmitted from outside-in to activate PLTs leading to PS exposure on the outer leaflets of the PLT surface as measured by an increase in annexinV binding. Of interest, we observed that shear stress does not alter P-selectin expression in fresh PLTs but significantly decreases in 4°C-stored PLTs. It has been reported previously that P-selectin levels are unaltered in sheared fresh PLTs, leading the authors to conclude that PLT aggregation due to shear is not accompanied by activation.29,33 P-selectin stored in α-granules is released upon stimulation in response to a number of agonists including ADP and epinephrine and is considered a quintessential marker for PLT activation. Since shear stress induces PS exposure but not P-selectin release, it is possible that shear as a mechanical agonist may operate by different pathways in activating PLTs than chemical agonists. A decrease in P-selectin levels in 4°C-stored PLTs due to shear suggests that these receptors are shed from the PLT surface. Shedding of P-selectin from PLTs occurs after several hours of increased P-selectin expression due to PLT activation and marks a highly procoagulant state.35 Taken together, we infer that shear stress may further activate stored PLTs and trigger P-selectin shedding, and blocking with AK2 antibody may down regulate this process.
To understand possible reasons for the increased binding of VWF to 4°C-stored PLTs, we measured the expression levels of surface receptor GP Ibα. GP Ibα is the primary ligand for VWF binding under high shear.30 We used three antibodies recognizing epitopes in different regions of the GP Ibα molecule and a VWF–function blocking antibody that binds to the same region on the N-terminal region of GP Ibα as VWF. The three antibodies bound 20% to 40% less to cold-stored PLTs compared to fresh PLTs suggesting a possible partial loss in GP Ibα due to cold storage. Recently, Jansen and coworkers23 reported a similar drop in binding of anti-GP Ibα antibodies to cold-stored murine PLTs and showed that this is due to the cleavage of GP Ibα from the PLT surface by metalloproteases ADAM17. These authors analyzed the integrity and/or release of GP Ibα ectodomains by immunoprecipitation from fresh and stored PLTs and corresponding plasma and showed that GP Ibα is shed from the PLT surface into the plasma during 48-hour storage at 4°C. In concordance with the decrease in GP Ibα expression levels, there was a decrease in RIPA mediated by relatively lower concentrations of ristocetin at 0.75 mg/mL due to storage. Ristocetin is believed to work as a bifunctional dimer by binding to both GP Ibα and VWF and mechanically cross-links the PLTs with VWF.34 At a ristocetin concentration of 1.5 mg/mL used in clinical assays, RIPA was comparable in fresh and stored PLTs possibly because the agglutination mechanism is more complex than a simple 1:1 stoichiometry between GP Ibα, ristocetin, and VWF. The correlation between a decrease in GP Ibα expression levels and in agglutination was not unexpected since RIPA is not a dynamic functional assay and merely quantifies physical crosslinking between the PLTs and VWF. Thus, we find that though there is a loss in surface GP Ibα levels due to refrigeration for 48 hours, the receptor binds to more VWF and is more active only under high shear stress. Further, the routinely employed static binding assay, such as ristocetin agglutination assays, may not capture the complex coupling between hydrodynamics and GP Ibα–VWF binding under physiologic conditions and may not be a good indicator of hemostasis or thrombosis in vivo.
There are several mechanisms by which refrigeration may increase GP Ibα–VWF binding under shear and subsequent PLT activation and aggregation. Cold storage for longer durations (48 hr) has two important effects on GP Ibα, namely, desialylation and clustering of GP Ibα receptors. First, cold storage releases sialidases that hydrolyze sialic acid from N-linked glycans including the two located within the leucine-rich repeat in the 45-kDa N-terminal of GP Ibα. The hydrolysis results in the exposure of β-galactose residues as measured by a small increase in the binding of fluorescently labeled lectin.21,23 The VWF-binding region is located within the leucine-rich repeat and thus desialylation may affect GP Ibα–VWF interaction. Second, and more importantly, clustering of GP Ibα during cold storage reconfigures linear arrays of surface GP Ibα in fresh PLTs to small clusters of higher local density due to changes in cytoskeleton and membrane lipid rafts.21,22,36 Such an increase will facilitate the cooperative binding of polyvalent ligand VWF to clustered GP Ibα, thus increasing the avidity of GP Ibα for VWF. As more VWF binds to GP Ibα, it increases the overall size of the globular head of GP Ibα, thus amplifying the mechanical force due to shear stress by at least one order of magnitude.31 This increase in applied force is transduced to an increase in PLT activation, which in turn results in enhanced PLT aggregation. Thus, cold-induced changes in GP Ibα play an important role not only in the rapid clearance of PLTs, but also in their enhanced functionality. Recent studies have shown that the lifetime of stored murine PLTs in vivo can be increased by enzymatically inhibiting cold-induced changes of GP Ib receptor.23 These possible hemostatic mechanisms under various cold storage conditions need to be investigated in vivo in animal models.
In conclusion, we demonstrate that low-temperature storage of PLTs results in enhanced aggregation at high shear stress but not under static conditions and also that this enhanced response may be a manifestation of increased PLT activation mediated by VWF binding to GP Ibα. There are other pathways which may also contribute to enhanced PLT aggregation under shear such as activated GP IIb/IIIa–fibrinogen interaction, particularly at lower shear stresses. The results presented in this work have interesting implications in transfusion medicine. Low-temperature storage of PLTs could be a double-edged sword: beneficial in some conditions, but harmful in others. When transfusing PLTs to support hemostasis in acute hemorrhage, the enhanced shear-mediated activity of cold-stored PLTs could help achieve faster control of bleeding and improved patient outcomes. On the other hand, prophylactic PLT transfusion strategies intended to protect thrombocytopenic patients depend for their efficacy on keeping a maximum number of functional PLTs in circulation for the longest possible interval. Cold storage, by causing accelerated clearance of transfused PLTs, would undermine this approach. Our results suggest that further exploration of cold-stored PLT function, including in vivo testing in relevant animal models and clinical settings merits consideration.
Acknowledgments
This work was partially supported by a grant from the San Antonio Life Sciences Institute.
We thank Drs Bernard P. Arulanandam and Samer Dessouky for access to instruments and Prajeeda Nair for technical assistance.
ABBREVIATIONS
- GP
glycoprotein
- PRP
platelet-rich plasma
- PS
phosphatidylserine
- RIPA
ristocetin-induced platelet agglutination
- SIPA
shear stress–induced platelet aggregation
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
References
- 1.Tynngard N. Preparation, storage and quality control of platelet concentrates. Transfus Apher Sci. 2009;41:97–104. doi: 10.1016/j.transci.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Singh H, Chaudhary R, Ray V. Evaluation of platelet storage lesions in platelet concentrates stored for seven days. Indian J Med Res. 2003;118:243–6. [PubMed] [Google Scholar]
- 3.Gulliksson H. Defining the optimal storage conditions for the long-term storage of platelets. Transfus Med Rev. 2003;17:209–15. doi: 10.1016/s0887-7963(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 4.Bjachman M, Beckers E, Dickmeiss E, Lin L, Moore G, Muylle L. Bacterial detection of platelets: current problems and possible resolutions. Transfus Med Rev. 2005;19:259–72. doi: 10.1016/j.tmrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.McCullough J, Vesole DH, Benjamin RJ, Slichter SJ, Pineda A, Snyder E, Stadtmauer EA, Lopez-Plaza I, Coutre S, Strauss RG, Goodnough LT, Fridey JL, Raife T, Cable R, Murphy S, Howard F, 4th, Davis K, Lin JS, Metzel P, Corash L, Koutsoukos A, Lin L, Buchholz DH, Conlan MG. Therapeutic efficacy and safety of platelets treated with a photo-chemical process for pathogen inactivation: the SPRINT trial. Blood. 2004;104:1534–41. doi: 10.1182/blood-2003-12-4443. [DOI] [PubMed] [Google Scholar]
- 6.Murphy S, Gardner FH. Platelet preservation—effect of storage temperature on maintenance of platelet viability—deleterious effect of refrigerated storage. N Engl J Med. 1969;280:1094–8. doi: 10.1056/NEJM196905152802004. [DOI] [PubMed] [Google Scholar]
- 7.Murphy S, Gardner FH. Platelet storage at 22°C: metabolic, morphologic, and functional studies. J Clin Invest. 1971;50:370–7. doi: 10.1172/JCI106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottschall JL, Rzad L, Aster RH. Studies of the minimum temperature at which human platelets can be stored with full maintenance of viability. Transfusion. 1986;26:460–2. doi: 10.1046/j.1537-2995.1986.26587020126.x. [DOI] [PubMed] [Google Scholar]
- 9.Slichter SJ, Harker LA. Preparation and storage of platelet concentrates II: storage variables influencing platelet viability and function. Br J Haematol. 1976;34:403–19. doi: 10.1111/j.1365-2141.1976.tb03587.x. [DOI] [PubMed] [Google Scholar]
- 10.Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. Transfus Apher Sci. 2010;42:63–70. doi: 10.1016/j.transci.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cauwenberghs S, van Pampus E, Curvers J, Akkerman JW, Heemskerk JW. Hemostatic and signaling functions of transfused platelets. Transfus Med Rev. 2007;21:287–94. doi: 10.1016/j.tmrv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 12.White JG, Krivit W. An ultrastructural basis for the shape changes induced in platelets by chilling. Blood. 1967;30:625–35. [PubMed] [Google Scholar]
- 13.Winokur R, Hartwig JH. Mechanism of shape change in chilled human platelets. Blood. 1995;85:1796–804. [PubMed] [Google Scholar]
- 14.Sandgren P, Hansson M, Guilliksson H, Shanwell A. Storage of buffy-coat-derived platelets in additive solutions at 4°C and 22°C: flow cytometry analysis of platelet glyco-protein expression. Vox Sang. 2007;93:27–36. doi: 10.1111/j.1423-0410.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 15.Babic AM, Josefsson EC, Bergmeier W, Wagner DD, Kaufman RM, Silberstein LE, Stossel T, Hartwig JH, Hoffmeister KM. In vitro function and phagocytosis of galactosylated platelet concentrates after long-term refrigeration. Transfusion. 2007;47:442–51. doi: 10.1111/j.1537-2995.2007.01134.x. [DOI] [PubMed] [Google Scholar]
- 16.Crowe JH, Tablin F, Tsvetkova N, Oliver AE, Walker N, Crowe LM. Are lipid phase transitions responsible for chilling damage in human platelets? Cryobiology. 1999;38:180–91. doi: 10.1006/cryo.1998.2137. [DOI] [PubMed] [Google Scholar]
- 17.Choi JW, Pai SH. Influence of storage temperature on the responsiveness of human platelets to agonists. Ann Clin Lab Sci. 2003;33:79–86. [PubMed] [Google Scholar]
- 18.Mondoro TH, Vostal JG. Cold temperatures reduce the sensitivity of stored platelets to disaggregating agents. Platelets. 2002;13:11–20. doi: 10.1080/09537100120111586. [DOI] [PubMed] [Google Scholar]
- 19.Oliver AE, Tablin F, Walker NJ, Crowe JH. The internal calcium concentration of human platelets increases during chilling. Biochim Biophys Acta. 1999;1416:349–60. doi: 10.1016/s0005-2736(98)00239-9. [DOI] [PubMed] [Google Scholar]
- 20.McGill M, Brindley DC. Effects of storage on platelet reactivity to arterial subendothelium during blood flow. J Lab Clin Med. 1979;94:370–80. [PubMed] [Google Scholar]
- 21.Rumjantseva V, Grewal PK, Wandall HH, Josefsson EC, Sorensen AL, Larson G, Marth JD, Hartwig JH, Hoffmeister KM. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15:1273–80. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Bergmeier W, Mayadas TN, von Andrian UH, Wagner DD, Stossel T, Hartwig JH. The clearance mechanism of chilled blood platelets. Cell. 2003;112:87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 23.Jansen AJ, Josefsson EC, Rumjantseva V, Liu QP, Falet H, Bergmeier W, Cifuni SM, Sachstein R, von Andrian UH, Wagner DD, Hartwig JH, Hoffmeister KM. Desialylation acclerates platelet clearance after refrigeration and initiates GP Ibα metalloproteinase-mediated cleavage in mice. Blood. 2012;119:1263–73. doi: 10.1182/blood-2011-05-355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong JF, Berndt MC, Schade A, McIntire LV, Andrews RK, Lopez JA. Ristocetin-dependent, but not botrocetin-dependent, binding of von Willebrand factor to the platelet glycoprotein Ib-IX-V complex correlates with shear-dependent interactions. Blood. 2001;97:162–8. doi: 10.1182/blood.v97.1.162. [DOI] [PubMed] [Google Scholar]
- 25.Fox SC, Sasae R, Janson S, May JA, Heptinstall S. Quantitation of platelet aggregation and microaggregate formation in whole blood by flow cytometry. Platelets. 2004;15:85–93. doi: 10.1080/09537100310001645979. [DOI] [PubMed] [Google Scholar]
- 26.Shankaran H, Alexandridis P, Neelamegham S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood. 2003;101:2637–45. doi: 10.1182/blood-2002-05-1550. [DOI] [PubMed] [Google Scholar]
- 27.Choi H, Aboulfatova K, Pownall HJ, Cook R, Dong JF. Shear-induced disulfide bond formation regulates adhesion activity of von Willebrand factor. J Biol Chem. 2007;282:35604–11. doi: 10.1074/jbc.M704047200. [DOI] [PubMed] [Google Scholar]
- 28.Kumar RA, Moake JL, Nolasco L, Bergeron AL, Sun C, Dong JF, McIntire LV. Enhanced platelet adhesion and aggregation by endothelial cell-derived unusually large multimers of von Willebrand factor. Biorheology. 2006;43:681–91. [PubMed] [Google Scholar]
- 29.Zhang JN, Wood J, Bergeron AL, McBride L, Ball C, Yu Q, Pusiteri AE, Holcomb JB, Dong JF. Effects of low temperature on shear-induced platelet aggregation and activation. J Trauma. 2004;57:216–23. doi: 10.1097/01.ta.0000093366.98819.fe. [DOI] [PubMed] [Google Scholar]
- 30.Goto S, Ikeda Y, Saldivar E, Ruggeri ZM. Distinct mechanisms of platelet aggregation as a consequence of different shearing flow conditions. J Clin Invest. 1998;101:479–86. doi: 10.1172/JCI973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dayananda KM, Singh I, Mondal N, Neelamegham S. von Willebrand factor self-association on platelet GpIbα under hydrodynamic shear: effect of shear-induced platelet activation. Blood. 2010;116:3990–8. doi: 10.1182/blood-2010-02-269266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konstantopoulos K, Chow T, Turner N, Hellums J, Moake JL. Shear stress-induced binding of von Willebrand factor to platelets. Biorheology. 1997;34:57–71. doi: 10.1016/S0006-355X(97)00004-8. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JN, Bergeron AL, Yu Q, Sun C, McIntire LV, Lopez JA, Dong JF. Platelet aggregation and activation under complex patterns of shear stress. Thromb Haemost. 2002;88:817–21. [PubMed] [Google Scholar]
- 34.Hoylaerts MF, Nuyts K, Peerlinck K, Deckmyn H, Vermylen J. Promotion of binding of von Willebrand factor to platelet glycoprotein Ib by dimers of ristocetin. Biochem J. 1995;306:453–63. doi: 10.1042/bj3060453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robblee LS, Shepro D, Vecchione JJ, Valeri CR. Increased thrombin sensitivity to human platelets after storage at 4°C. Transfusion. 1979;19:45–52. doi: 10.1046/j.1537-2995.1979.19179160265.x. [DOI] [PubMed] [Google Scholar]
- 36.van der Wal DE, Verhoef S, Schutgens RE, Peters M, Wu Y, Akkerman JW. Role of glycoprotein Ibα mobility in platelet function. Thromb Haemost. 2010;103:1033–43. doi: 10.1160/TH09-11-0751. [DOI] [PubMed] [Google Scholar]