Abstract
OBJECTIVES
Despite the high prevalence of covert hepatic encephalopathy (CHE) in cirrhotics without previous overt HE (OHE), its independent impact on predicting clinically relevant outcomes is unclear. The aim of this study was to define the impact of CHE on time to OHE, hospitalization, and death/transplant in prospectively followed up patients without previous OHE.
METHODS
Outpatient cirrhotics without OHE were enrolled and were administered a standard paper–pencil cognitive battery for CHE diagnosis. They were systematically followed up and time to first OHE development, hospitalization (liver-related/unrelated), and transplant/death were compared between CHE and no-CHE patients at baseline using Cox regression.
RESULTS
A total of 170 cirrhotic patients (55 years, 58% men, 14 years of education, Model for End-Stage Liver Disease (MELD 9), 53% hepatitis C virus (HCV), 20% nonalcoholic etiology) were included, of whom 56% had CHE. The entire population was followed up for 13.0±14.6 months, during which time 30% developed their first OHE episode, 42% were hospitalized, and 19% had a composite death/transplant outcome. Age, gender, etiology, the MELD score, and CHE status were included in Cox regression models for time to first OHE episode, hospitalization, death, and composite death/transplant outcomes. On Cox regression, despite controlling for MELD, those with CHE had a higher risk of developing OHE (hazard ratio: 2.1, 95% confidence interval 1.01–4.5), hospitalization (hazard ratio: 2.5, 95% confidence interval 1.4–4.5), and death/transplant (hazard ratio: 3.4, 95% confidence interval 1.2–9.7) in the follow-up period.
CONCLUSIONS
Covert HE is associated with worsened survival and increased risk of hospitalization and OHE development, despite controlling for the MELD score. Strategies to detect and treat CHE may improve these risks.
INTRODUCTION
The spectrum of neurocognitive impairment in cirrhosis (SONIC) can be divided into clinically apparent or overt hepatic encephalopathy (OHE) and the preclinical stage, known as minimal HE or covert HE (CHE) (1). CHE is characterized by subtle cognitive impairments that can only be detected through specialized testing and has been found in up to 84% of tested patients (2,3). CHE is associated with impaired health-related quality of life and can severely affect driving skills and the socioeconomic status in affected patients and caregivers (4–8). However, the prognostic significance of CHE, especially in patients who have never experienced a previous OHE episode, is not fully understood. Previous studies that show a worse prognosis for CHE patients have typically included those with previous OHE who are clearly at a higher risk for recurrence or have used the Child–Turcotte–Pugh score that includes OHE as a scoring tool or have been performed in centers where liver transplants are not offered (9–13). Therefore, this lack of clarity regarding prognostication of CHE patients without previous OHE could be one of the reasons why CHE testing is not routinely performed beyond research studies, especially in the United States. This is particularly important because treatment of CHE can improve clinical and psychosocial outcomes (4,14,15). Our aim was to prospectively measure the clinical impact of CHE, independent of age, sex, and liver severity (through the use of the Model for End-Stage Liver Disease (MELD) score), on the development of OHE, hospitalizations, and death/transplant in a prospective study of patients with cirrhosis without previous OHE.
METHODS
Study population
From November 2008 to November 2013, 170 consecutive cirrhotic patients who met the eligibility criteria, aged 18–65 years, at the outpatient clinics in the Department of Gastroenterology and Hepatology at the Virginia Commonwealth University Hospital were prospectively recruited after obtaining informed consent. All included patients had cirrhosis proven on a clinical basis involving laboratory tests, imaging findings, endoscopic findings, and liver biopsy if available. In addition, included patients were required to understand English and were not on any psychoactive medications apart from chronic antidepressants. Individuals with previous or current OHE, infection, or gastrointestinal hemorrhage within the past 6 weeks, with hepatocellular carcinoma, who were on psychoactive medications, and with recent illicit drug and alcohol use within 6 months were excluded.
Demographic data were collected for all patients. The etiology of cirrhosis was categorized into hepatitis C infection, alcoholic, and others (nonalcoholic fatty liver disease, autoimmune hepatitis, etc). The severity of liver disease at entry was assessed in all patients by MELD. Education was also recorded in years.
Diagnosis of OHE and CHE
OHE was diagnosed clinically on the basis of impaired mental status, as defined by the West Haven Criteria and impaired neuromotor function (hyperreflexia, rigidity, myoclonus, and asterixis) that required initiation of HE-related therapy with or without hospitalization and had corroboration from a caregiver (16). CHE was diagnosed if patients scored abnormally on ≥2 psychometric tests (the number connection test-A (>35 s), the number connection test-B (>99 s), the digit symbol test (<68 raw score), and block design (<28 raw score)) as recommended using cutoffs from our previous studies ( 16 ).
Outcome measures
Patients were followed up prospectively at intervals of at least 6 months for study visits and at regular intervals at Virginia Commonwealth University Medical Center. During follow-up, all episodes of OHE (which included outpatient and inpatient diagnoses), hospitalizations, transplant, and death were recorded. All patients were regularly seen at 3–6-month intervals per standard of care, and, as part of the study, patients and relatives were called every 2 months. If a patient could not be seen at our out-patient clinic during the 3–6-month follow-up intervals, patients were tracked via our electronic medical system for any episodes of OHE, hospitalizations, and death, with confirmation with telephone calls by the study staff. Time from initial screening to the first OHE event, OHE-related hospitalization and its precipitant, liver-related hospitalization and its etiology, and hospitalization for other reasons was recorded in months. Elective hospitalizations for studies and procedures were not included. Death and liver transplantation from the time of screening were also recorded in months. Total OHE events, OHE-related hospitalizations, liver-related hospitalizations, and hospitalizations for other reasons were recorded. Liver-related hospitalizations were defined as those related to cirrhosis complications (OHE, the hepatorenal syndrome, variceal bleeding, spontaneous bacterial peritonitis, and hepatic hydrothorax), whereas all others were termed liver unrelated.
Statistical analysis
Most data were presented as mean±s.d. and median interquartile range in months where deemed appropriate between CHE and no-CHE patients. Differences across categorical variables were accessed using χ2 and Fishers exact tests, whereas continuous variables were tested using t-tests. A nominal value of 0.05 was considered statistically significant. Variables that were significant were entered into the Cox regression model for hospitalization and transplants/deaths between CHE and no-CHE patients. The model consisted of age, gender, the MELD score, and CHE status that were included for time to first OHE episode, first hospitalization, death, and composite death/transplant outcomes. Two additional variables to assess the impact of etiology of cirrhosis (alcoholic vs. nonalcoholic and hepatitis C virus (HCV) vs. no-HCV) were created and tested with the above variables in separate models.
RESULTS
For this study, 256 patients potentially meeting the enrollment criteria were approached; 35 were not considered further because of previous OHE, 23 had psychoactive medications that precluded their participation, 20 patients declined participation, and 8 did not understand English. The remaining 170 were included, and at the initial screening 95 of 170 patients met the criteria for CHE (Table 1). The majority of the subjects were male (58%) with a mean age of 55±8 years and an average MELD score of 9.2±3.4. Although the CHE group had a significantly higher MELD score, both groups were compensated. The major etiologies were hepatitis C (n=90, 53%), nonalcoholic steatohepatitis (n=34, 20%), followed by alcohol (n=7, 4%) and others (n=33, 19%).
Table 1.
Patient and outcome characteristics
| CHE (N=95) | No-CHE (N=75) | P value | |
|---|---|---|---|
| Age, mean±s.d. | 56.07±6.68 | 54.04±8.77 | 0.11 |
| Gender (M/F), % | (62/33) 64 | (39/36) 52 | 0.08 |
| Etiology of cirrhosis (N (%)) | |||
| HCV | 49 (52) | 41 (55) | 0.29 |
| Alcohol | 9 (9) | 3 (4) | |
| NASH | 16 (17) | 18 (24) | |
| Other (autoimmune, and so on) | 21 (22) | 12 (16) | |
| Education (years)±s.d. | 13.56±2.19 | 13.76±2.44 | 0.58 |
| Presence of varices (%) | 37 (39%) | 28 (37%) | 0.83 |
| Serum albumin±s.d. | 3.2±2.1 | 3.5±1.6 | 0.29 |
| Serum creatinine±s.d. | 1.5±1.2 | 1.0±0.8 | 0.001 |
| Serum total bilirubin±s.d. | 2.2±2.3 | 1.5±1.1 | 0.01 |
| INR±s.d. | 1.7±1.3 | 1.1±0.9 | 0.001 |
| Median Child Score (range) | 8 (5–9) | 6 (5–8) | 0.03 |
| MELD Score±s.d. | 9.95±3.92 | 8.50±2.80 | 0.006 |
| Cognitive performance±s.d. | |||
| Number connection test-A (s) | 44.7±17.6 | 26.5±6.2 | <0.0001 |
| Number connection test-B (s) | 129.4±78.2 | 61.9±15.7 | <0.0001 |
| Digit symbol test (raw score) | 52.6±12.9 | 72.1±15.0 | <0.0001 |
| Block design test (raw score) | 21.2±12.5 | 40.2±10.3 | <0.0001 |
| Clinical outcome characteristics | |||
| Total OHE events | 73±0.77 | 35±0.47 | <0.0001 |
| Total OHE hospitalizations | 42±0.44 | 18±0.24 | 0.004 |
| Total liver-related hospitalizations | 83±0.87 | 37±0.49 | <0.0001 |
| Total liver-unrelated hospitalizations | 75±0.79 | 16±0.21 | <0.0001 |
CHE, covert hepatic encephalopathy; F, female; HCV, hepatitis C virus; INR, international normalized ratio; M, male; MELD, Model for End-Stage Liver Disease; NASH, nonalcoholic steatohepatitis; OHE, overt hepatic encephalopathy.
Patients were followed up for a mean of 13.0±14.6 months (median=8.6, interquartile range=0.1, 20.6).
During the follow-up period, 36 CHE patients (37.9%) developed at least one OHE event compared with only 13 in the no-CHE group (17.3%, P =0.001). When total OHE events during the follow-up were considered, they were significantly higher in the CHE group with 73 events (0.73 events per patient) vs. 35 in the no-CHE group (0.47 events per patient) (P≤0.001).
As not all OHE events required hospitalizations, we studied OHE hospitalizations separately. We found that a significantly higher proportion (P=0.02) of CHE patients (24 patients of the 95 total CHE patients; 25.2%) were ever hospitalized for OHE during the follow-up period compared with only 9 (12% of the 75 no-CHE patients) in the no-CHE group. Of the 24 patients with OHE-related hospitalizations in the CHE group, 18 had OHE on admission whereas 6 developed it during hospitalization for other conditions, and of the 9 patients with OHE-related hospitalizations, 8 were admitted for OHE specifically whereas 1 additional patient developed it during hospitalization for other conditions.
When the total number of OHE hospitalizations over the follow-up period was considered, this was again found to be significantly higher in the CHE group with a total of 42 hospitalizations (0.48 per patient) vs. 18 hospitalizations (0.25 per patient) in the no-CHE group (P=0.004).
The major precipitating factor for the first OHE event comprised metabolic derangements, followed by infections in both groups (24% and 16% in the CHE group and 11% and 11% in the no-CHE group, respectively).
There were more liver-related (36 vs. 16) and liver-unrelated hospitalizations (35 vs. 12) in the CHE group as compared with the no-CHE group. The most frequent etiology for liver-related hospitalization in both groups was OHE, followed by ascites management and gastroesophageal bleed (Supplementary Table S1 online). The proportion of OHE-related hospitalizations was similar across CHE and no-CHE groups, although the absolute number was higher in CHE patients. Liver-unrelated hospitalizations were mostly infections, followed by respiratory–cardiac in etiology. Moreover, we found a higher rate of death in the CHE group (17) than in the no-CHE group (4), with the most common etiology reported as liver related (renal failure, coagulopathy) followed by sepsis and multiorgan failure. The number of transplants was not significant between the two groups (8 and 4 in the CHE and no-CHE groups, respectively, P=0.435). A summary of baseline and outcome measures over time is shown in Table 1 and Figures 1–3. The cumulative incidence of OHE, hospitalization, and death/transplant for CHE and no-CHE patients is shown in Table 2a–c.
Figure 1.
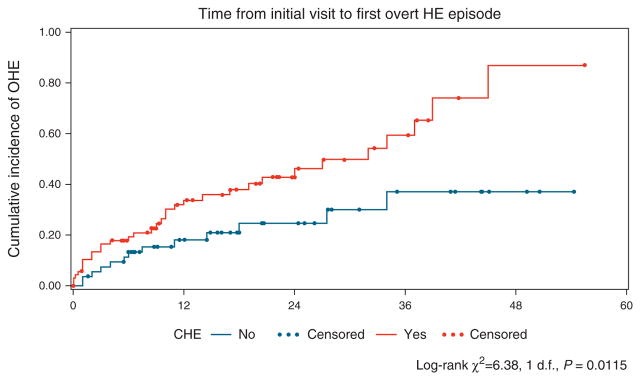
Time from initial visit to overt hepatic encephalopathy (OHE) development showed a significant difference between covert hepatic encephalopathy (CHE) and no-CHE groups (P=0.01). The Kaplan–Meier curve for estimated cumulative incidence of the particular outcome in the Y axis and months from initial entry in the X axis. The red lines depict patients with CHE, and blue lines denote patients who were CHE negative at study entry, with each dot signifying censoring due to reaching an outcome. Log-rank statistics were used to compare groups.
Figure 3.
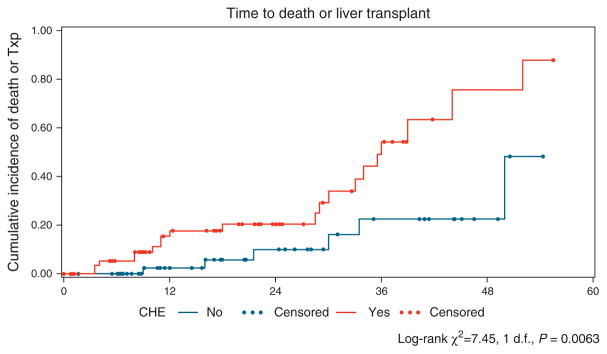
Time from initial visit to death or transplant (Txp) showed a significant difference between covert hepatic encephalopathy (CHE) and no-CHE groups (P=0.006). The Kaplan–Meier curve for estimated cumulative incidence of the particular outcome in the Y axis and months from initial entry in the X axis. The red lines depict patients with CHE, and blue lines denote patients who were CHE negative at study entry, with each dot signifying censoring due to reaching an outcome. Log-rank statistics were used to compare groups.
Table 2.
Cumulative incidence of patients who developed relevant outcomes during follow-up
| Time | CHE negative
|
CHE positive
|
||
|---|---|---|---|---|
| Cumulative incidence | 95% CI | Cumulative incidence | 95% CI | |
| (a) Time to OHE development | ||||
|
| ||||
| 1 Year | 18.1% | (9.8–2.1%) | 33.8% | (23.4–47.2%) |
|
| ||||
| 2 Years | 24.8% | (14.1–41.2%) | 46.1% | (33.4–61.0%) |
|
| ||||
| 3 Years | 37.1% | (21.2–59.5%) | 59.4% | (43.3–76.1%) |
|
| ||||
| 4 Years | 37.1% | (21.2–59.5%) | 86.9% | (60.8–98.8%) |
|
| ||||
| (b) Time to hospitalization | ||||
|
| ||||
| 1 Year | 15.2% | ( 8.2–27.2%) | 46.6% | (25.6–59.1%) |
|
| ||||
| 2 Years | 24.6% | (14.9–39.0%) | 59.9% | (47.9–72.3%) |
|
| ||||
| 3 Years | 44.3% | (28.9–63.3%) | 74.5% | (61.7–85.7%) |
|
| ||||
| 4 Years | 68.2% | (45.8–88.2%) | 88.3% | (72.8–97.1%) |
|
| ||||
| (c) Time to death or transplant | ||||
|
| ||||
| 1 Year | 2.6% | (0.4–16.8%) | 17.6% | (9.5–31.3%) |
|
| ||||
| 2 Years | 10.1% | (3.3–28.9%) | 20.4% | (11.4–34.9%) |
|
| ||||
| 3 Years | 22.5% | (9.5–48.2%) | 54.3% | (35.8–74.8%) |
|
| ||||
| 4 Years | 22.5% | (9.5–48.2%) | 75.6% | (49.7–94.5%) |
CHE, covert hepatic encephalopathy; CI, confidence interval; OHE, overt hepatic encephalopathy.
Using the aforementioned variables (CHE diagnoses, MELD score, age, gender, and etiology), Cox multivariate regression analysis was calculated. The multivariate analysis identified that CHE was significantly associated with first OHE event, first hospitalization, death, and death/transplant. The MELD score also significantly predicted first OHE event, first hospitalization, death, and death and transplant. None of the other included variables (including either HCV/no-HCV or alcohol/nonalcoholic etiology) were significant in predicting outcomes (Table 3).
Table 3.
Covert HE and development of outcomes
| Time to outcome | CHE diagnosis HR (95% CI) (P value) | MELD Score HR (95% CI) (P value) |
|---|---|---|
| First OHE episode | 2.1 (1.01–4.5) (P=0.05) | 1.15 (1.03–1.27) (P=0.01) |
| First hospitalization | 2.5 (1.4–4.5) (P=0.002) | 1.17 (1.1–1.3) (P ≤ 0.0001) |
| Death | 4.9 (1.03–23.8) (P=0.04) | 1.18 (1.03–1.35) (P=0.015) |
| Death/transplant | 3.4 (1.2–9.7) (P=0.01) | 1.18 (1.1–1.3) (P=0.0004) |
CHE, covert hepatic encephalopathy; CI, confidence interval; HE, hepatic encephalopathy; HR, hazard ratio; MELD, model for end-stage liver disease; OHE, overt hepatic encephalopathy.
Models were adjusted for gender, age, and etiology of cirrhosis.
DISCUSSION
Although most practitioners are aware of CHE, it is difficult for clinicians to justify the effort that cognitive testing requires in their busy practice to diagnose it, until there is evidence that CHE can portend clinically relevant outcomes (17). Therefore, the prediction of survival and risk of hospitalization in CHE patients without previous OHE is an important goal, as treatment can potentially reduce these risks (15,18–20). The current standard of care is therefore limited to the treatment of the OHE portion of SONIC and excludes the majority of cirrhotic patients from adequate prognostication and eligibility for HE therapy (1).
In this study, we found that CHE at baseline is associated with worsened survival and increased risk of hospitalization (OHE and liver related) and death/transplant independent of the MELD score. We also found that CHE was associated with the development of the first OHE episode, similar to previous studies (10,11,21). As expected, the MELD score was found to be a good predictor in determining the first OHE episode, hospitalization (OHE and liver related), and death/transplant. Although it is well known that one episode of OHE begets another (22,23), it is still unclear whether specific factors can predict the first OHE episode from a neurocognitive basis. This is because several previous studies of CHE and ultimate OHE development included subgroups that were being treated for OHE, often included the Child score (which has OHE in it) for prognostication, or were carried out in centers that did not offer transplant (9–12), Our study, however, extends the literature by rigorously excluding patients with previous overt HE who are usually not considered a high risk as they are perceived as being “stable” and form the majority of the clinical practice in cirrhosis clinics. Therefore, neither these patients nor their caregivers routinely receive education about what to expect or how to react if and when the patients develop their first OHE episode. The diagnosis of CHE ultimately gives the clinician an insight into the potential for future OHE development that could help in the education of the family and patients. There is also increasing evidence that even after the first OHE episode, there could be persistent cognitive deficits that may only partly reverse after a transplant (24,25), Therefore, prognostication and potential early treatment to prevent the first OHE episode could potentially prevent this persistent cognitive dysfunction from setting in (14).
Our study also includes the very important and understudied outcome of hospitalization that tremendously increases the financial burden attributable to cirrhosis ( 26 ), We found a generalized increase in hospitalizations whether liver related or unrelated in CHE patients compared with those without CHE. It is also interesting to note that not only were the OHE diagnoses more frequent in CHE patients, but this was also accompanied by a higher OHE-related hospitalization number. We found that CHE patients also had significant nonliver-related hospitalizations compared with the no-CHE group. The similar relative proportion of OHE-related hospitalizations in patients with and without CHE could be related to the overall increasing proportion of OHE as a cause of hospitalization in all cirrhotic patients (26), The increase in both types of hospitalization points toward an inherent risk of poor outcomes that are predicted by CHE that is not adequately captured by the MELD score. Although it is clear that poor cognitive performance may not directly lead to non-OHE-related hospitalizations, it may be a good “bio-marker” that reflects the underlying poor functioning. This cognitive dysfunction could be a reflection of a systemic pro-inflammatory milieu and altered microbiome that adds several layers of potential prognostication not afforded by the MELD or Child–Turcotte–Pugh score alone (27,28). Montagnese et al. (9) have added electroencephalogram to MELD to improve its accuracy of mortality prediction in unselected patients, but our study rigorously excluded previous OHE and still was able to find an additive effect of specialized testing on clinically relevant outcomes.
Our study demonstrates that even in the most stable-appearing patients, there is room for improvement in our current prognostication strategies and that it may be worthwhile to test for CHE in patients with cirrhosis to increase our ability as clinicians to counsel patients and potentially initiate therapy to prevent adverse outcomes.
Supplementary Material
Figure 2.
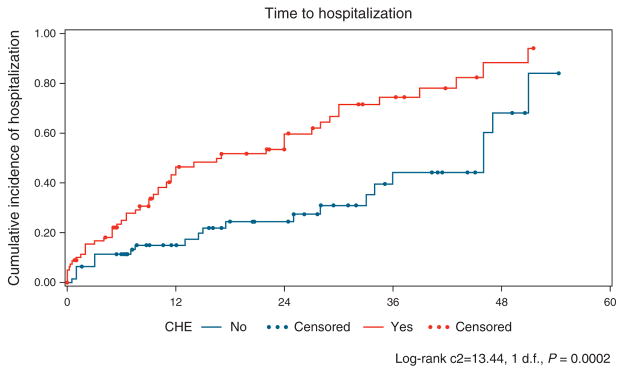
Time from initial visit to hospitalization showed a significant difference between covert hepatic encephalopathy (CHE) and no-CHE groups (P=0.0002). The Kaplan–Meier curve for estimated cumulative incidence of the particular outcome in the Y axis and months from initial entry in the X axis. The red lines depict patients with CHE, and blue lines denote patients who were CHE negative at study entry, with each dot signifying censoring due to reaching an outcome. Log-rank statistics were used to compare groups.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Covert hepatic encephalopathy (CHE) impairs daily functioning, but it is not routinely tested.
In studies that included patients with previous overt hepatic encephalopathy (HE), CHE has a higher risk of overt HE development.
It is unclear whether CHE can independently predict the first overt HE episode, hospitalizations, and survival in stable cirrhotic outpatients without any previous overt HE.
WHAT IS NEW HERE
In our patient population consisting of outpatient cirrhotics without previous overt HE, patients with CHE had a significantly higher risk of developing their first overt HE episode and associated hospitalization compared with those without CHE.
CHE was also associated with a higher risk of all-cause and liver-related hospitalizations and death or transplant independent of the Model for End-Stage Liver Disease (MELD) score.
Even in this stable, compensated, outpatient population, a diagnosis of CHE at baseline predicts clinically relevant outcomes and adds to the prognostication provided by the MELD score alone.
Acknowledgments
Financial support: This study was partly supported by the NIDDK RO1DK087013 and NIAAA RO1020203 awarded to Jasmohan S. Bajaj.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Portions of this manuscript were presented in abstract form in an oral presentation during Digestive Disease Week 2014 in Chicago.
Guarantor of the article: Jasmohan S. Bajaj, MD, MSc, FACG.
Specific author contributions: Study concept and design: J.S.B.; data analysis: L.R.T., J.S.B., and K.R.P.; manuscript preparation: K.R.P., J.S.B., J.B.W., and L.R.T.; patient recruitment and study conduct: J.S.B., R.K.S., A.J.S., R.T.S., S.C.M., M.S.S., V.A.L., M.F., M.B.W., A.B.U., and N.A.N.; manuscript review: all authors.
Potential competing interests: None.
References
- 1.Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50:2014–21. doi: 10.1002/hep.23216. [DOI] [PubMed] [Google Scholar]
- 2.Das A, Dhiman RK, Saraswat VA, et al. Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol. 2001;16:531–5. doi: 10.1046/j.1440-1746.2001.02487.x. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz M, Jacas C, Cordoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42(Suppl 1):S45–S53. doi: 10.1016/j.jhep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Kappus MR, Bajaj JS. Covert hepatic encephalopathy: not as minimal as you might think. Clin Gastroenterol Hepatol. 2012;10:1208–19. doi: 10.1016/j.cgh.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj JS, Riggio O, Allampati S, et al. Cognitive dysfunction is associated with poor socioeconomic status in patients with cirrhosis: an international multicenter study. Clin Gastroenterol Hepatol. 2013;11:1511–6. doi: 10.1016/j.cgh.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj JS, Hafeezullah M, Hoffmann RG, et al. Navigation skill impairment: another dimension of the driving difficulties in minimal hepatic encephalopathy. Hepatology. 2008;47:596–604. doi: 10.1002/hep.22032. [DOI] [PubMed] [Google Scholar]
- 7.Prasad S, Dhiman RK, Duseja A, et al. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549–59. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646–53. doi: 10.1038/ajg.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montagnese S, De Rui M, Schiff S, et al. Prognostic benefit of the addition of a quantitative index of hepatic encephalopathy to the MELD score: the MELD-EEG. Liver Int. doi: 10.1111/liv.12490. advance online publication, 12 February 2014. (e-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 10.Dhiman RK, Kurmi R, Thumburu KK, et al. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci. 2010;55:2381–90. doi: 10.1007/s10620-010-1249-7. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann IJ, Groeneweg M, Quero JC, et al. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol. 2000;95:2029–34. doi: 10.1111/j.1572-0241.2000.02265.x. [DOI] [PubMed] [Google Scholar]
- 12.Amodio P, Del Piccolo F, Marchetti P, et al. Clinical features and survival of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology. 1999;29:1662–7. doi: 10.1002/hep.510290619. [DOI] [PubMed] [Google Scholar]
- 13.Saxena N, Bhatia M, Joshi YK, et al. Auditory P300 event-related potentials and number connection test for evaluation of subclinical hepatic encephalopathy in patients with cirrhosis of the liver: a follow-up study. J Gastroenterol Hepatol. 2001;16:322–7. doi: 10.1046/j.1440-1746.2001.02388.x. [DOI] [PubMed] [Google Scholar]
- 14.Lunia MK, Sharma BC, Sharma P, et al. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12:1003–1008. e1. doi: 10.1016/j.cgh.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj JS, Heuman DM, Wade JB, et al. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology. 2011;140:478–87. e1. doi: 10.1053/j.gastro.2010.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj JS, Etemadian A, Hafeezullah M, et al. Testing for minimal hepatic encephalopathy in the United States: An AASLD survey. Hepatology. 2007;45:833–4. doi: 10.1002/hep.21515. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q, Duan ZP, Ha DK, et al. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–9. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe A, Sakai T, Sato S, et al. Clinical efficacy of lactulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology. 1997;26:1410–4. doi: 10.1053/jhep.1997.v26.pm0009397979. [DOI] [PubMed] [Google Scholar]
- 20.Sidhu SS, Goyal O, Mishra BP, et al. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial) Am J Gastroenterol. 2011;106:307–16. doi: 10.1038/ajg.2010.455. [DOI] [PubMed] [Google Scholar]
- 21.Romero-Gomez M, Boza F, Garcia-Valdecasas MS, et al. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718–23. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 22.Bajaj JS, Thacker LR, Heuman DM, et al. Cognitive performance as a predictor of hepatic encephalopathy in pretransplant patients with cirrhosis receiving psychoactive medications: a prospective study. Liver Transpl. 2012;18:1179–87. doi: 10.1002/lt.23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–81. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 24.Bajaj JS, Schubert CM, Heuman DM, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332–40. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riggio O, Ridola L, Pasquale C, et al. A simplified psychometric evaluation for the diagnosis of minimal hepatic encephalopathy. Clin Gastroenterol Hepatol. 2011;9:613–6. e1. doi: 10.1016/j.cgh.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Stepanova M, Mishra A, Venkatesan C, et al. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10:1034–41. e1. doi: 10.1016/j.cgh.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Shawcross DL, Wright G, Olde Damink SW, et al. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis. 2007;22:125–38. doi: 10.1007/s11011-006-9042-1. [DOI] [PubMed] [Google Scholar]
- 28.Bajaj JS, Ridlon JM, Hylemon PB, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


