Abstract
Several epidemiological studies have shown an association between infection or inflammation during pregnancy and increased risk of autism in the child. In addition, animal models have illustrated that maternal inflammation during gestation can cause autism-relevant behaviors in the offspring; so called maternal immune activation (MIA) models. More recently, permanent changes in T cell cytokine responses were reported in children with autism and in offspring of MIA mice; however, the cytokine responses of other immune cell populations have not been thoroughly investigated in these MIA models. Similar to changes in T cell function, we hypothesized that following MIA, offspring will have long-term changes in macrophage function. To test this theory, we utilized the poly (I:C) MIA mouse model in C57BL/6J mice and examined macrophage cytokine production in adult offspring. Pregnant dams were given either a single injection of 20 mg/kg polyinosinic–polycytidylic acid, poly (I:C), or saline delivered intraperitoneally on gestational day 12.5. When offspring of poly (I:C) treated dams reached 10 weeks of age, femurs were collected and bone marrow-derived macrophages were generated. Cytokine production was measured in bone marrow-derived macrophages incubated for 24 h in either growth media alone, LPS, IL-4/LPS, or IFN-γ/LPS. Following stimulation with LPS alone, or the combination of IFN-γ/LPS, macrophages from offspring of poly (I:C) treated dams produced higher levels of IL-12(p40) (p < 0.04) suggesting an increased M1 polarization. In addition, even without the presence of a polarizing cytokine or LPS stimulus, macrophages from offspring of poly (I:C) treated dams exhibited a higher production of CCL3 (p = 0.05). Moreover, CCL3 levels were further increased when stimulated with LPS, or polarized with either IL-4/LPS or IFN-γ/LPS (p < 0.05) suggesting a general increase in production of this chemokine. Collectively, these data suggest that MIA can produce lasting changes in macrophage function that are sustained into adulthood.
Keywords: MIA, Maternal, Immune activation, Macrophage, M1, M2, Mouse, Autism, Behavior, Inflammation
1. Introduction
There is increasing evidence suggesting that an immune insult during gestation can have a profound effect on the developing fetus (Brown. 2012). For over 30 years, epidemiological research has continued to find associations between maternal infection and increased risk of autism (Atladottir et al., 2010; Chess, 1971, 1977; Mednick et al., 1988). A recent large case-control population based study revealed an increased risk of developing autism spectrum disorder (ASD) with maternal fever, which was attenuated if pregnant mothers used a fever reducing agent (Zerbo et al., 2013). In addition, reports highlight associations between risk of having a child with autism and increased levels of inflammatory mediators in both the maternal sera and amniotic fluid. These increased inflammatory markers, including macrophage chemotactic protein (MCP)-1/CCL2, matrix metalloproteinase (MMP)-9, C-reactive protein (CRP), interleukins (IL)-4, IL-5, and interferon (IFN)-γ (Abdallah et al., 2012a,b; Brown et al., 2013; Goines et al., 2011), supporting a relationship between maternal immune activation (MIA), aberrant fetal neurodevelopment, and risk for neurodevelopmental disorders such as autism.
Murine models add further support for a role of MIA in altering fetal neurodevelopment (Patterson, 2009; Patterson et al., 2009). Many of these studies utilize either a maternal influenza infection or polyinosinic–polycytidylic acid [poly(I:C)] a synthetic toll-like receptor (TLR)-3 agonist that mimics viral infection. Maternal influenza infection or poly (I:C) induced MIA in pregnant dams produces a number of physiological changes in resultant offspring, including changes in brain morphology, decreased reelin, astrogliosis, fewer purkinje cells (PC), the presence of heterotopic PCs, and delayed migration of cerebellar granule cells (Fatemi et al., 2002a,b, 1999; Shi et al., 2009). In addition to these neurobiological effects of MIA, there are a number of behavioral alterations observed in offspring that parallel many features of autism and schizophrenia, including reduced exploratory behavior, and impaired social interaction (Malkova et al., 2012; Schwartzer et al., 2013; Shi et al., 2003).
There is a high incidence of aberrant immune responses commonly reported in individuals with autism (Onore et al., 2012), including increased monocyte numbers (Sweeten et al., 2003). Plasma cytokine profiles also suggest an increase in monocyte derived cytokine and chemokine activation (Ashwood et al., 2011a,b). Moreover, there is evidence of differential TLR signaling in autism, including increased inflammatory cytokine production following TLR4 stimulation with lipopolysaccharide (LPS) (Enstrom et al., 2010; Jyonouchi et al., 2008), further suggesting aberrant myeloid function in this disorder. Similar to immune dysfunction in human subjects, recent data in mice suggest there are long-term alterations in immune function as a result of MIA with increased numbers of CD11b+ cells in MIA offspring (Hsiao et al., 2012). Increased CD11b+ cells may implicate atypical myeloid cell activity; however, little has previously been reported regarding specific macrophage responses in MIA offspring.
Macrophage phenotypes are generally divided into major subsets characterized as M1 and M2 (Mantovani et al., 2005; Martinez et al., 2008). M1 macrophages, also known as classically activated macrophages, are associated with bacterial or viral infection (Benoit et al., 2008). Polarization to an M1 phenotype is induced by exposure to IFN-γ and results in high expression of the natural killer cell and TH1 cell activating cytokine IL-12 as well as very low levels of the anti-inflammatory cytokine IL-10. In contrast, M2 macrophages, also referred to as alternatively activated macrophages, are associated with defense against helminth infections and wound healing (Kreider et al., 2007; Rodero and Khosrotehrani, 2010). Macrophages are polarized to an M2 phenotype in the presence of IL-4 or IL-13 and generally produce high levels of IL-10, very low levels of IL-12, and generally lower levels of inflammatory cytokines compared with M1 macrophages (Zhang et al., 2008).
Growing evidence suggests that manipulating macrophage phenotype can profoundly affect normal neurodevelopment and cognitive function in animal models (Derecki et al., 2013). For example, increased M1 polarization has been implicated in several neurological diseases including multiple sclerosis and Alzheimer’s disease (Gate et al., 2010; Mikita et al., 2011), and promoting an M2 phenotype may be beneficial to cognitive function (Derecki et al., 2010a,b, 2011). Together, these data suggest that M1 macrophage polarization may have detrimental effects on normal brain development and function. Given the association between M1 polarization and neurological dysfunction and the mounting research linking MIA with altered brain and behavior function, we hypothesized that macrophages from mice born to MIA dams would be preferentially skewed towards the M1 phenotype and exhibit a pro-inflammatory cytokine profile.
To investigate the potential long term effects of MIA in offspring macrophage polarization, we utilized the poly(I:C) model of MIA in C57BL/6J mice and tested macrophage responses in vitro. Bone marrow-derived macrophages were obtained from offspring exposed to poly (I:C) [poly (I:C) group here in] or to saline control (referred to as saline group here-in) in utero. To test for specific effects on macrophage function, we measured macrophage cytokine profiles in response to TLR4 activation with and without the presence of polarizing cytokines IFN-γ (M1) and IL-4 (M2). In this study we describe a long-term effect in macrophage polarization in murine MIA offspring.
2. Methods
2.1. Mice
C57Bl/6J (C57) (Jackson Laboratory, Sacramento, CA) mice were maintained by the Campus Laboratory Animal Services, at University of California, Davis at ambient room temperature on a 12 h light/dark cycle with food and water available ad libitum. All procedures were performed with approval by the University of California, Davis Institutional Animal Care and Use Committee and in accordance with the guidelines provided by the National Institutes of Health for the ethical treatment of animals.
2.2. Maternal immune activation
Mice were mated overnight and females were checked daily for the presence of seminal plugs, noted as gestational day 0.5 (G0.5). MIA was induced as previously described (Schwartzer et al., 2013). Briefly, on gestational day 12.5, pregnant female mice were weighed and injected with a single dose (20 mg/kg body weight, intraperitoneally) of poly (I:C) (Sigma Aldrich; St. Louis, MO) or saline solution. Standard procedure for intraperitoneal injections included aspirating after needle insertion, and prior to injection, to verify needle placement in the intraperitoneal space and no entry into any of the surrounding organs. Litters receiving either saline or poly(I:C) injections appeared to be healthy, and did not display any outward signs of damage due to injection. Dams were returned to their cage and remained undisturbed until parturition. Pups remained with the mother until weaning on postnatal day (PND) 21, at which time mice were group housed 2–4 mice per cage with same sex littermates. For all behavioral and biological assays, one male and one female from each litter were used to reduce the likelihood of litter effects. A total of 40 mice were tested [poly(I:C) group, n = 22; saline group, n = 18].
2.3. Three chamber social approach
On PND70, mice were assessed for social approach behavior as previously described (Schwartzer et al., 2013). One male and one female from each litter were habituated for 10-min in the center chamber with doors closed followed by an additional 10-min habituation to the entire apparatus. Habituation sessions were video recorded and analyzed to confirm a lack of innate side preference. Following habituation, experimental mice were returned to the center chamber and a novel 129/SvImJ mouse was placed under an inverted wire cup in one side chamber and an identical empty wire cup was placed on the other side. Offspring of poly(I:C) and vehicle-treated dams were then given a 10 min test period and measured for the time spent in the chamber with the novel mouse and novel object. A sociability score was calculated as the time in social chamber minus time in novel object chamber. All testing chambers were thoroughly cleaned with 70% ethanol in between each testing session.
2.4. Marble burying
Perseverative marble-burying behavior in mice is an analogous index to the restricted repetitive patterns of behavior observed in ASD (American Psychological Association, 2013; Silverman et al., 2010; Thomas et al., 2009). Mice were habituated to a clean Plexiglas cage filled with a 4 cm thick layer of clean corncob bedding for 10 min. Following habituation, animals were returned to their home cages and 15 glass marbles were laid out in five rows of three marbles placed equidistance apart. Mice were then returned to the cages and allowed to explore under dim illumination for 10 min. At the end of the 10 min period, animals were gently removed from the testing cages and the number of marbles buried were recorded by two experimenters blind to treatment conditions. Only marbles covered by 75% or more bedding were counted as buried.
2.5. Generation of macrophage media
Confluent L929 Cells (ATCC, Manassas, VA) were cultured for 7 days in complete Dulbecco’s modified Eagles media (DMEM) F-12 (Life Technologies, Carlsbad, CA) supplemented with 10% heat inactivated FBS (Life Technologies), 100 IU/ml penicillin, and 100 IU/ml streptomycin (Sigma). The resulting L929 conditioned media was passed through a 0.2 μm filter (Millipore, Billerica, MA) to remove debris and ensure sterility. To create macrophage media, complete DMEM was supplemented 10% with filtered L929 conditioned media. L929 conditioned media was stored at −20 °C for less than 60 days before single thaw and use.
2.6. Bone marrow-derived macrophage generation
Following behavioral assessments, mice were deeply anesthetized with 4% isoflurane, and promptly euthanized by decapitation. Femurs were aseptically removed and transferred in Roswell Park Memorial Institute (RPMI) 1640 media (Life Technologies) supplemented with 10% Fetal Bovine Serum (FBS), 100 IU/ml penicillin, 100 IU/ml streptomycin, 25 μg/ml gentimycin (Sigma) prior to processing. Legs with fur and skin removed, including femur and tibia from each mouse, were washed twice in sterile cold (4 °C) Hanks buffered saline solution (HBSS) (Mediatech, Herndon, VA). Tissue was removed from the bones with sterile scissors and forceps, and bones were washed in 10 ml cold HBSS. The proximal and distal ends of both the femur and tibia were removed, and the lumens of the femurs and tibia were flushed with 10 ml of cold HBSS using a 25 gauge needle (BD Medical, Franklin Lakes, NJ). Dislodged bone marrow was agitated by aspiration and ejected with a 22 gauge needle (BD Medical). The resulting cell suspension was filtered through a 100 μm nylon mesh (BD Biosciences, Carlsbad, CA). Cells were pelleted by centrifugation at 500 g for 5 min and resuspended in macrophage media to a concentration of 1 × 105 cells/ml, plated in sterile non-cell culture treated petri dishes (BD Biosciences) at a volume of 10 ml per dish and incubated for 3 days at 37 °C, 5% CO2. After a 3 day culture, 5 ml of fresh macrophage media were added to each dish and cells were incubated for an additional 4 days at 37 °C, 5% CO2 for a total incubation time of 7 days. Petri dishes containing adherent mature bone marrow-derived macrophages were washed with 10 ml cold HBSS and incubated with 3 ml Cell Stripper™ buffer (Mediatech) per plate for 5 min at 37 °C, 5% CO2. Following incubation, cells were dislodged from the petri dishes by pipetting, diluted in an equal amount of complete DMEM and pelleted by centrifugation at 400g for 5 min. Cells were resuspended up to 1 × 106 cell/ml in complete DMEM, and 1 ml/well was plated in 12-well sterile tissue culture plate (Greiner Bio-One, Monroe, NC) and allowed to adhere overnight at 37 °C, 5% CO2.
2.7. Cytokine measurement
Following adhesion of bone marrow-derived macrophages, complete DMEM was aspirated and replaced with the following 8 conditions: media alone, 2 ng/ml recombinant mouse IL-4 (R&D, Minneapolis, MN), 150 ng/ml recombinant mouse IFN-γ (R&D), 10 ng/ml LPS (Sigma), 2 ng/ml recombinant mouse IL-4 plus 10 ng/ml LPS, or 150 ng/ml recombinant mouse IFN-γ plus 10 ng/ml LPS. The addition of LPS to the polarizing condition was necessary to produce detectable amounts of IL-4 and IL-12(p40). Cells were incubated under these conditions for 24 h, at which point supernatants were collected and stored at −80 °C until assayed. The quantification of interleukin (IL)-1β, IL-6, IL-10, IL-12(p40), CCL2, Tumor Necrosis Factor (TNF)-α, and CCL3 in supernatants was determined using mouse reactive Milliplex™ multiplexing bead immunoassays (Millipore). Samples were run in accordance with the instructions of the manufactures protocol. In brief, 25 μL of supernatant was incubated with antibody-coupled beads. In some cases, the supernatant was diluted in order to decrease the concentration of the cytokine or chemokine into the detectable range of the assay. After a series of washes, a biotinylated detection antibody was added to the beads, and the reaction mixture was detected by the addition of streptavidin conjugated to phycoerythrin. The bead sets were analyzed using a flow-based Luminex™ 100 suspension array system (Bio-Plex 200; Bio-Rad Laboratories, Hercules, CA). Unknown sample cytokine concentrations were calculated by Bio Plex Manager software using a standard curve derived from the known reference cytokine concentrations supplied by the manufacturer. A five-parameter model was used to calculate final concentrations and values were expressed in pg/ml. The sensitivity of this multiplex immunoassay allowed the detection of cytokine concentrations with the following minimal detectable limits: IL-1β (2 pg/ml), IL-6 (1.8 pg/ml), IL-10 (3.3 pg/ml), IL-12(p40) (4.9 pg/ml), CCL2 (5.3 pg/ml), CCL3 (8.7 pg/ml), CCL4 (1.8 pg/ml), CXCL10 (10.1 pg/ml) and TNF-α (1 pg/ml).
2.8. Statistical analysis
For behavioral analyses, social approach was assessed within each treatment group using separate paired-samples Student’s t-test. Differences in social score and percent of marbles buried between offspring of poly(I:C) and saline-treated dams was determined using independent samples t-tests. Cytokine/chemokine levels between MIA and saline treated groups were conducted with Student’s t-test. To identify associations between macrophage phenotype and behavioral deficits, cytokine/chemokine levels were correlated with behavioral measures using Pearson’s r correlation. All analyses were two-tailed, and values of p < 0.05 were considered statistically significant. Unadjusted p-values are presented (Rothman, 1990).
3. Results
3.1. Bone marrow-derived macrophages
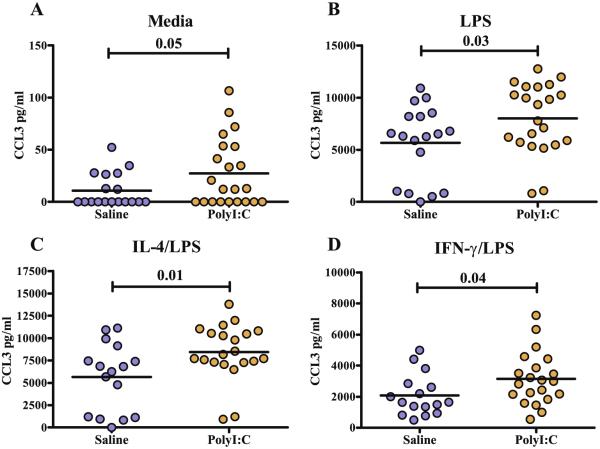
To determine the cytokine response to LPS with and without polarization, bone marrow macrophages were incubated in media alone, LPS, IL-4 (a M2 polarizing cytokine), IFN-γ (a M1 polarizing cytokine) or either IL-4 or IFN-γ with LPS (Mosser and Zhang, 2008). The majority of cytokines tested did not show significant differences between the poly (I:C) and saline groups; however, differences were repeatedly seen in the hierarchal M1 cytokine IL-12(p40) and the chemokine CCL3 (Tables 1 and 2). Following stimulation with LPS (p < 0.04), or exposure to IFN-γ/LPS (p < 0.01), macrophages from offspring of dams treated with poly(I:C) produced higher levels of IL-12(p40) compared with offspring of saline-treated dams (Fig. 1). Even without the presence of a polarizing cytokine or LPS stimulus, macrophages from offspring of dams treated with poly(I:C) exhibit an increased production of CCL3 (p < 0.05) (Fig. 2A). Regardless of whether the LPS stimulation was given or in the presence of LPS with the polarizing cytokines IL 4 or IFN-γ, macrophages from offspring of dams treated with poly(I:C) consistently produced significantly more CCL3 than their saline counterparts (Fig. 2B–D). Interestingly, saline treated mice demonstrated higher levels of CCL4 in baseline/unstimulated conditions (Table 1); however, there were no differences in CCL4 levels between groups following LPS stimulations or under either M1 or M2 polarizing conditions.
Table 1.
All cytokine levels in unstimulated and LPS stimulated macrophages.
| Cytokines (pg/ml) | Media (Mean ± SEM) |
LPS (Mean ± SEM) |
||||
|---|---|---|---|---|---|---|
| Saline (n = 18) | Poly(I:C) (n = 22) | p-Value | Saline (n = 18) | Poly(I:C) (n = 22) | p-Value | |
| IL-1β | BLD | BLD | N/A | 9.54 ± 2.99 | 14.91 ± 3.00 | 0.21 |
| IL-6 | BLD | BLD | N/A | 2981.00 ± 508.20 | 3689.00 ± 473.10 | 0.31 |
| IL-10 | BLD | BLD | N/A | 408.30 ± 56.21 | 425.6 ± 37.96 | 0.79 |
| IL-12p40 | BLD | BLD | N/A | 3.44 ± 0.63 | 7.19 ± 1.51 | 0.04a |
| TNF-α | 3.37 ± 0.50 | 2.85 ± 0.497 | 0.47 | 568.00 ± 51.93 | 724.10 ± 86.82 | 0.15 |
| CXCL10 | 160.5 ± 27.37 | 98.51 ± 18.77 | 0.06 | 12,366 ± 1913 | 10,372 ± 988.3 | 0.34 |
| CCL2 | 10.96 ± 3.42 | 10.12 ± 2.32 | 0.83 | 1164.00 ± 166.70 | 1485.00 ± 166.90 | 0.18 |
| CCL3 | 10.76 ± 3.79 | 27.41 ± 6.93 | 0.05a | 5651.00 ± 837.90 | 8007.00 ± 714.60 | 0.03a |
| CCL4 | 128.7 ± 14.55 | 93.28 ± 7.808 | 0.03a | 16,690 ± 2134 | 20,012 ± 2081 | 0.28 |
Mean cytokine measurements and standard error of mean (SEM) in pg/ml.
BLD, below level of detection; N/A, not applicable; p-values could not be calculated.
Significant p-values.
Table 2.
All cytokine levels after M2 or M1 polarization.
| Cytokines (pg/ml) | IL-4/LPS (Mean ± SEM) |
IFN-γ/LPS (Mean ± SEM) |
||||
|---|---|---|---|---|---|---|
| Saline (n = 17) | Poly(I:C) (n = 21) | p-Value | Saline (n = 17) | Poly(I:C) (n = 21) | p-Value | |
| IL-1β | 9.476 ± 2.967 | 19.57 ± 3.480 | 0.04a | 11.98 ± 2.620 | 14.91 ± 3.350 | 0.51 |
| IL-6 | 1282 ± 243.5 | 1683 ± 199.8 | 0.20 | 17,193 ± 6348 | 88,038 ± 40,946 | 0.14 |
| IL-10 | 507.4 ± 69.98 | 497.2 ± 53.65 | 0.90 | 43.26 ± 5.576 | 37.90 ± 5.110 | 0.48 |
| IL-12p40 | BLD | BLD | N/A | 304.9 ± 75.67 | 732.5 ± 134.0 | 0.01a |
| TNF-α | 498.3 ± 43.26 | 596.8 ± 56.42 | 0.19 | 1361 ± 141.6 | 1379 ± 153.9 | 0.93 |
| CXCL10 | 7480 ± 882.9 | 7504 ± 719.8 | 0.98 | 30,983 ± 8168 | 29,781 ± 6446 | 0.91 |
| CCL2 | 2502 ± 249.0 | 2898 ± 192.9 | 0.20 | 1486 ± 192.9 | 1901 ± 192.4 | 0.14 |
| CCL3 | 5649 ± 948.9 | 8453 ± 683.2 | 0.01a | 2082 ± 332.9 | 3144 ± 369.2 | 0.04a |
| CCL4 | 19,679 ± 2539 | 24,720 ± 2623 | 0.18 | 7805 ± 1103 | 10,031 ± 1269 | 0.21 |
Mean cytokine measurements and standard error of mean (SEM) in pg/ml.
BLD: Below level of detection, N/A: Not applicable, p-values could not be calculated.
Significant p-values.
Fig. 1.
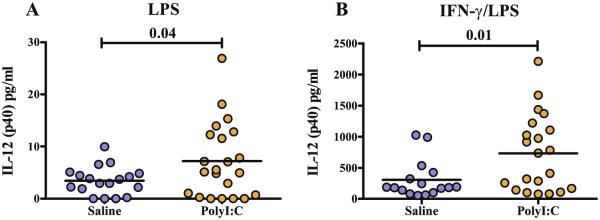
Legend: levels of IL-12(p40) cytokine production from macrophages of poly(I:C) and saline exposed offspring. Scatter graphs with mean bars of IL-12(p40) after 24 h in vitro incubation with media containing LPS (A), or IFN-γ/LPS (B). Cytokine produced by Poly(I:C) offspring macrophages produced significantly more IL-12(p40) after LPS and IFN-γ/LPS stimulation than saline control macrophages.
Fig. 2.
Legend: levels of CCL3 cytokine production from macrophages of poly(I:C) and saline exposed offspring. Scatter graphs with mean bars of CCL3 after 24 hours in vitro incubation with media alone (A), LPS (B), IL-4/LPS (C) or IFN-γ/LPS (D). Poly(I:C) macrophages produce significantly higher levels of CCL3 following exposure to LPS, IL-4/LPS and IFN-γ/LPS media conditions compared to saline macrophages.
3.2. Behavioral assessments
As previously shown by our group (Schwartzer et al., 2013) and others we found deficits in social behavior and marble burying in the offspring of poly (I:C) treated dams. Offspring from poly(I:C) dams exhibited atypical social approach behavior as defined by more time spent in the chamber with a novel object compared with the chamber with the novel mouse and buried more marbles compared with offspring of saline-treated mice (data not shown). We next examined whether there were associations between the M1 and M2 related cytokine production and behavioral outcome in the offspring. Under M2 skewing cytokine conditions after IL-4/LPS stimulation there were associations between less frequent marble burying and M2 relevant cytokines such that there was increased IL-10 production (r2 = −0.47, p = 0.01) and CCL2 production (r2 = −0.447, p = 0.02). This data suggested an association between improvements in restricted repetitive patterns of behavior as assessed by marble burying and increased production of M2 related cytokines. In contrast, the production of pro-inflammatory cytokine TNF-α was associated with increased marble burying (r2 = 0.41, p = 0.04), suggesting TNF-α is associated with more impaired behavior. For social approach behavior there was a trend to increased M1 related cytokine IL-12(p40) production, under LPS/IFN-γ culture conditions, and decreased social approach, suggesting that M1 cytokine production may be associated with more impaired social behavior but this trend did not reach statistical significance (r2 = −0.31, p = 0.1).
4. Discussion
While there is evidence that maternal immune activation may be a risk factor for neurodevelopmental disorders such as schizophrenia and autism, little is known about the long term physiological effects in offspring exposed to MIA. Separately, there is evidence of immune dysfunction in individuals with neurodevelopmental disorders, but a relationship between in utero MIA exposure and long term immune dysfunction has not been established. In this study, we describe increased production of IL-12(p40) and CCL3 from macrophages of adult offspring of a mouse model of MIA. This increase in cytokine production induced by gestational poly(I:C) exposure suggests that maternal inflammation could lead to long-term effects on myeloid cell function. Macrophages serve an important role as first responders to infection, as phagocytes, and orchestrators of adaptive responses. Dysregulation of CNS macrophages likely play an important role in a number of neurological disorders such as multiple sclerosis and Alzheimer’s disease (Hawkes and McLaurin, 2009). Of note, macrophages in the CNS are regularly replaced from the circulatory myeloid pool (Bechmann et al., 2001) suggesting that changes in macrophage activity observed in the bone-marrow (i.e., the source of circulatory macrophages) may be indicative of persistent dysregulation in macrophages residing in the CNS.
In this study, we found that macrophages derived from offspring of poly(I:C)-treated dams produced significantly more IL-12(p40) after stimulation with either LPS or under M1 skewing conditions with LPS/IFN-γ compared with offspring of dams treated with saline. The increased production of IL-12(p40) is consistent with M1 polarization in macrophages and with increased chemokine production. IL-12(p40) is a subunit of both IL-12(p70) (Presky et al., 1996) and IL-23 (Oppmann et al., 2000), which promote the activity of type 1 CD4+ T helper lymphocytes (TH1) and survival of IL-17 producing T helper lymphocytes (TH17) cells, respectively (Harrington et al., 2005; Hsieh et al., 1993). IL-12 activates STAT4 in TH1 cells and natural killer cells inducing the expression of IFN-γ (Thierfelder et al., 1996), which reinforces the M1 phenotype in macrophages, and promotes the expression of reactive oxygen intermediates and inflammatory cytokines (Benoit et al., 2008). IFN-γ can also act directly on neurons, activating the p38 MAPK pathway and is reported to induce dendrite spine retraction (Andres et al., 2008; Kim et al., 2002). The observed M1 skew is also consistent with an inflammatory phenotype previously observed in splenic CD4+ T cells in offspring of MIA treated dams, including increased production of IL-17 (Hsiao et al., 2012), and the promotion of TH17 cells (Mandal et al., 2011). Notably, macrophage polarization towards an M1 phenotype is implicated in a number of neuroinflammatory diseases (Mikita et al., 2011).
In contrast to M1 polarization, M2 polarization is considered somewhat beneficial in neuroinflammation. M2 polarization is characterized by production of the anti-inflammatory cytokine IL-10, and a reduction in IL-12 production. The secreted IL-10 inhibits the activity of M1 macrophages, and promotes TH2 cell development. Polarization towards an M2 phenotype produces positive effects on cognitive function, possibly through the promotion of an anti-inflammatory neurological environment by M2 skewed perivascular macrophages (Derecki et al., 2011). Considering both the exaggerated M1 response observed in offspring of poly (I:C)-treated dams and the neuroprotective effects associated with increased M2 polarization, and in this study the association between M2 cytokine IL-10 and CCL2 with fewer repetitive behaviors as assessed by marble burying, the neurological deficits associated with MIA may be mitigated by restoring the balance in M1/M2 phenotypes.
In addition to elevated IL-12(p40) production, macrophages of the offspring of poly (I:C) treated dams consistently produced significantly more CCL3 following stimulation with LPS, IL-4/LPS, or IFN-γ/LPS (Fig. 2). CCL3 is produced by macrophages as well as lymphocytes and is a chemoattractant for a number of different cell subsets including lymphocytes, macrophages and neutrophils (Maurer and von Stebut, 2004). High levels of this chemokine are associated with neuroinflammatory diseases including multiple sclerosis and Alzheimer’s disease (Tripathy et al., 2007; Zhang et al., 2000). Potentially relevant to the observation of increased CCL3 in this study, elevated levels of CCL3 have been reported in both prenatal and postnatal brain homogenates of offspring of MIA dams (Garay et al., 2012; Meyer et al., 2006); however, it was not determined whether or not macrophages were the major source of this chemokine. Interestingly, high serum levels of chemokines have also been reported in children with autism where they are associated with increased severity of autism-associated behaviors (Ashwood et al., 2011b).
Collectively, these data suggest that maternal immune activation with poly(I:C) promotes an inflammatory macrophage phenotype in adult offspring characterized by a shift towards an IL-12(p40) producing M1 phenotype and increased production of the chemokine CCL3. These data expand on previous reports of persistent immune dysregulation in offspring following MIA and provide a potential mechanism by which dysregulated M1 macrophage skewing may be influencing altered T cell cytokine profiles (Hsiao et al., 2012; Mandal et al., 2011). Although the mechanism by which a transient activation of the maternal immune system can exert lasting alterations in offspring immune function remains unknown, the data presented herein may implicate epigenetic modifications in innate immune cell function (Fernandez-Morera et al., 2010). Stimulation with IFN-γ or IFN-β induces STAT1 signaling and shifts macrophages towards a classical M1 phenotype. In contrast, the activation of transcription factors STAT6 and STAT3 promote the expression of scavenger receptors and IL-10, promoting an alternative M2 phenotype (Mantovani and Locati, 2013). In addition, stimulation with IL-4 also promotes M2 polarization while inhibiting M1 polarization by inducing M2 favorable chromatin restructuring via the histone demethylase JMJD3 (Sica and Mantovani, 2012). While it is unclear whether such epigenetic modifications play a role in the immune phenotype observed in the adult MIA offspring described above, it suggests epigenetic influences may be a promising area for future investigation. In this article we demonstrate a link between maternal inflammation and macrophage function in MIA affected offspring and illustrate the long-term consequences of in utero exposure to a maternal immune insult.
Acknowledgments
This work was supported by NIH T32MH073124, Jane Botsford Johnson Foundation, Peter Emch Foundation, Barbara and Michael Bass Foundation, the Brain & Behavior Research Foundation (formerly known as National Alliance for Research on Schizophrenia and Depression) and the Autism Research Institute. This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, Hougaard DM. Amniotic fluid chemokines and autism spectrum disorders: an exploratory study utilizing a danish historic birth cohort. Brain Behav. Immun. 2012a;26:170–176. doi: 10.1016/j.bbi.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Abdallah MW, Pearce BD, Larsen N, Greaves-Lord K, Norgaard-Pedersen B, Hougaard DM, Mortensen EL, Grove J. Amniotic fluid MMP-9 and neurotrophins in autism spectrum disorders: an exploratory study. Autism Res. 2012b;5:428–433. doi: 10.1002/aur.1254. [DOI] [PubMed] [Google Scholar]
- American Psychological Association Diagnostic and Statistical Manual of Mental Disorders (fifth ed.) 2013 [Google Scholar]
- Andres DA, Shi GX, Bruun D, Barnhart C, Lein PJ. Rit signaling contributes to interferon-gamma-induced dendritic retraction via p38 mitogen-activated protein kinase activation. J. Neurochem. 2008;107:1436–1447. doi: 10.1111/j.1471-4159.2008.05708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011a;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 2011b;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Kwidzinski E, Kovac AD, Simburger E, Horvath T, Gimsa U, Dirnagl U, Priller J, Nitsch R. Turnover of rat brain perivascular cells. Exp. Neurol. 2001;168:242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J. Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 2012;72:1272–1276. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Sourander A, Hinkka-Yli-Salomaki S, McKeague IW, Sundvall J, Surcel HM. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol. Psychiatry. 2013;19:259–264. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess S. Autism in children with congenital rubella. J. Autism Child Schizophr. 1971;1:33–47. doi: 10.1007/BF01537741. [DOI] [PubMed] [Google Scholar]
- Chess S. Follow-up report on autism in congenital rubella. J. Autism Child Schizophr. 1977;7:69–81. doi: 10.1007/BF01531116. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 2010a;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Privman E, Kipnis J. Rett syndrome and other autism spectrum disorders – brain diseases of immune malfunction? Mol. Psychiatry. 2010b;15:355–363. doi: 10.1038/mp.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav. Immun. 2011;25:379–385. doi: 10.1016/j.bbi.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Kipnis J. The role of microglia in brain maintenance: implications for Rett syndrome. Trends Immunol. 2013;34:144–150. doi: 10.1016/j.it.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav. Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S, Bailey K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol. Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell. Mol. Neurobiol. 2002a;22:25–33. doi: 10.1023/a:1015337611258. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, Earle JA, Thuras P. Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol. Psychiatry. 2002b;7:633–640. doi: 10.1038/sj.mp.4001046. [DOI] [PubMed] [Google Scholar]
- Fernandez-Morera JL, Calvanese V, Rodriguez-Rodero S, Menendez-Torre E, Fraga MF. Epigenetic regulation of the immune system in health and disease. Tissue Antigens. 2010;76:431–439. doi: 10.1111/j.1399-0039.2010.01587.x. [DOI] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav. Immun. 2012;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T. Macrophages in Alzheimer’s disease: the blood-borne identity. J. Neural. Transm. 2010;117:961–970. doi: 10.1007/s00702-010-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol. Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. USA. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. USA. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Cushing-Ruby A, Quraishi H. Impact of innate immunity in a subset of children with autism spectrum disorders: a case control study. J. Neuroinflamm. 2008;5:52. doi: 10.1186/1742-2094-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Beck HN, Lein PJ, Higgins D. Interferon gamma induces retrograde dendritic retraction and inhibits synapse formation. J. Neurosci. 2002;22:4530–4539. doi: 10.1523/JNEUROSCI.22-11-04530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider T, Anthony RM, Urban JF, Jr., Gause WC. Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Marzouk AC, Donnelly R, Ponzio NM. Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav. Immun. 2011;25:863–871. doi: 10.1016/j.bbi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler. Thromb. Vasc. Biol. 2013;33:1478–1483. doi: 10.1161/ATVBAHA.113.300168. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C, Petry KG. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult. Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Zhang X. Activation of murine macrophages. Curr. Protoc. Immunol. 2008 doi: 10.1002/0471142735.im1402s83. Chapter 14, Unit 14 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav. Immun. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Patterson PH, Xu W, Smith SEP, Devarman BE. Maternal immune activation, cytokines and autism. Autism. 2009:289–307. [Google Scholar]
- Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY, Gately MK, Gubler U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 2010;3:643–653. [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl. Psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav. Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeten TL, Posey DJ, McDougle CJ. High blood monocyte counts and neopterin levels in children with autistic disorder. Am. J. Psychiatry. 2003;160:1691–1693. doi: 10.1176/appi.ajp.160.9.1691. [DOI] [PubMed] [Google Scholar]
- Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D, Thirumangalakudi L, Grammas P. Expression of macrophage inflammatory protein 1-alpha is elevated in Alzheimer’s vessels and is regulated by oxidative stress. J. Alzheimers Dis. 2007;11:447–455. doi: 10.3233/jad-2007-11405. [DOI] [PubMed] [Google Scholar]
- Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J. Autism Dev. Disord. 2013;43:25–33. doi: 10.1007/s10803-012-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GX, Baker CM, Kolson DL, Rostami AM. Chemokines and chemokine receptors in the pathogenesis of multiple sclerosis. Mult. Scler. 2000;6:3–13. doi: 10.1177/135245850000600103. [DOI] [PubMed] [Google Scholar]
- Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008 doi: 10.1002/0471142735.im1401s83. Chapter 14, Unit 14 11. [DOI] [PMC free article] [PubMed] [Google Scholar]