Abstract
BACKGROUND
Whole blood (WB) has been used in combat since World War I as it is readily available and replaces every element of shed blood. Component therapy has become standard; however, recent military successes with WB resuscitation have revived the debate regarding wider WB use. Characterization of optimal WB storage is needed. We hypothesized that refrigeration preserves WB function and that a pathogen reduction technology (PRT) based on riboflavin and ultraviolet light has no deleterious effect over 21 days of storage.
STUDY DESIGN AND METHODS
WB units were stored for 21 days either at 4°C or 22°C. Half of each temperature group underwent PRT, yielding four final treatment groups (n = 8 each): CON 4 (WB at 4°C); CON 22 (WB at 22°C); PRT 4 (PRT WB at 4°C); and PRT 22 (PRT WB at 22°C). Testing was at baseline, Days 1–7, 10, 14, and 21. Assays included coagulation factors; platelet activation, aggregation, and adhesion; and thromboelastography (TEG).
RESULTS
Prothrombin time (PT) and partial thromboplastin time increased over time; refrigeration attenuated the effects on PT (p ≤ 0.009). Aggregation decreased over time (p ≤ 0.001); losses were attenuated by refrigeration (p ≤ 0.001). Refrigeration preserved TEG parameters (p ≤ 0.001) and PRT 4 samples remained within normal limits throughout the study. Refrigeration in combination with PRT inhibited fibrinolysis (p ≤ 0.001) and microparticle formation (p ≤ 0.031). Cold storage increased shear-induced platelet aggregation and ristocetin-induced platelet agglutination (p ≥ 0.032), as well as GPIb-expressing platelets (p ≤ 0.009).
CONCLUSION
The in vitro hemostatic function of WB is largely unaffected by PRT treatment and better preserved by cold storage over 21 days. Refrigerated PRT WB may be suitable for trauma resuscitation. Clinical studies are warranted.
INTRODUCTION
Transfusion medicine was born on the battlefields of World War I with the lifesaving infusion of citrated whole blood (WB) into severely injured combat casualties. WB remained the product of choice for preventing and treating hemorrhagic shock through World War II, the Korean War, and the Vietnam War. WB was used because it was readily available from in-theater donors—the so-called “walking blood bank”—and because it replaced all components of shed blood required for oxygen transport and hemostasis.1 Low hemagglutinin-titer Type O WB, stored in citrate-phosphate-dextrose anticoagulant (CPD) and refrigerated, was used as a universal resuscitation fluid by US Forces throughout the Vietnam conflict. This universal WB product, as well as type-specific WB, was collected from donor centers in the United States and Japan, shipped to Vietnam, and used over the course of a 21-day storage period.2 Current US military guidance for using WB in the treatment of life-threatening bleeding requires ABO blood type specificity to the recipient because of concerns of hemolytic reaction. Other investigators address this controversial policy in detail within this supplement.3–5
The wars in Iraq and Afghanistan, the largest military engagements since the Vietnam War, have been fought in the age of component therapy and an era of heightened awareness of the risks of transfusion-transmitted disease (TTD). The overwhelming majority of transfusions to combat casualties in the recent conflicts have been component based,6 although the apparent success of fixed-ratio transfusion to approximate WB (1:1:1 platelet [PLT] : fresh frozen plasma [FFP] : red blood cell [RBC]) and the use of fresh WB suggest that a larger role for WB could reasonably be incorporated into future trauma resuscitation strategies.7–12 A number of challenges remain to the wider use of WB. These include implementing pathogen risk mitigation, determining the role and timing of PLT-sparing leukoreduction, and optimal storage conditions.13
In the US Military, WB is used for emergency transfusions within 24 hours of collection, before results of complete, post hoc pathogen testing are available. A pathogen reduction technology (PRT), which uses riboflavin and ultraviolet (UV) light to damage the nucleic acids of white blood cells (WBCs), parasites, bacteria, and viruses, is being considered for TTD risk mitigation. The effects of this technology and subsequent storage conditions on the hemostatic properties of WB are poorly understood but must be characterized if PRT-treated WB is to be used to treat hemorrhage in severely wounded service members.
Hemostasis is a complex process involving the interaction of PLTs with RBCs and plasma proteins and is locally regulated by both biochemical factors such as PLT secretory mediators and biophysical properties such as local blood flow. We hypothesized that pathogen inactivation with riboflavin and UV light has no deleterious effect on the hemostatic function of WB stored either at 4°C or room temperature (RT; 22°C) over several days. To this end, we assessed hemostatic function at multiple levels, including changes in levels of coagulation factors in plasma, PLT activation status, PLT response to chemical agonists, PLT adhesion receptor levels, PLT function under static and flow conditions, and overall clot properties. Our comprehensive analyses demonstrate that hemostatic function in vitro is mostly unaffected by PRT treatment and is better preserved at 4°C versus RT storage.
METHODS
Institutional review board approval was obtained for blood collection from 32 healthy adult donors, and standard blood donation guidelines were followed. Thirty-two units (450 ± 50 mL) of WB were placed in commercially available bags containing 63 mL of CPD anticoagulant (Teru-flex, Terumo Medical Corporation, Somerset, NJ). PRT was performed on half of the units collected (16 of 32; see Fig. 1). Each unit was drained into the citrated polyvinyl chloride illumination bag (attached to a storage bag; set manufactured by Terumo BCT, Lakewood, CO), and ribo-flavin solution was added (35 mL of 500-μM riboflavin in 0.9% NaCl; Terumo BCT), roughly a 5%–10% dilution compared with control bags. The illumination/storage set was weighed, the hematocrit (HCT) of the contents was determined, and the set was placed in the illuminator and exposed to UV light (265–400 nm of phosphor; Mirasol System, Terumo BCT) for a dose of 80 J/mLRBC. The majority (99%) of the total energy reaching the product through the plastic is in the UV-B (280–315 nm) and UV-A (315–400 nm) spectral regions. After treatment, the contents of the illumination bag were drained into the attached storage bag, and the illumination bag was removed. All WB units were stored for 21 days under two temperature conditions, resulting in four treatment groups (Fig. 1): CON 4, untreated WB stored at 4°C (n = 8); CON 22, untreated WB stored at 22°C (n = 8); PRT 4, pathogen-reduced WB stored at 4°C (n = 8); and PRT 22, pathogen-reduced WB stored at 22°C (n = 8). WB was tested at baseline and on Days 1–7, 10, 14, and 21. Between sampling, WB units were protected from light and UV radiation within a metal-lined storage container.
Fig. 1.
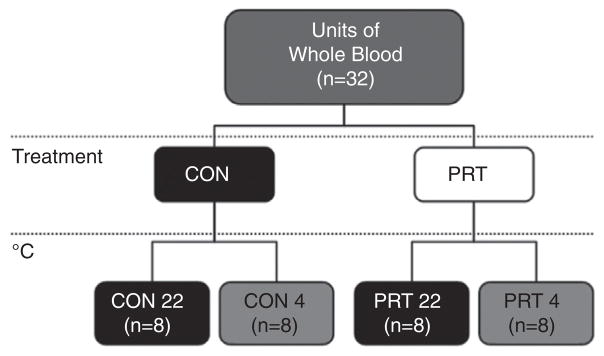
Study design. WB units (n = 32) were divided into control (CON: no pathogen reduction performed) and treatment (PRT: pathogen reduction performed) and then stored for 21 days under two temperature conditions, resulting in four treatment groups: CON 4, untreated WB stored at 4°C (n = 8); CON 22, untreated WB stored at 22°C (n = 8); PRT 4, pathogen-reduced WB stored at 4°C (n = 8); and PRT 22, pathogen-reduced WB stored at 22°C (n = 8). WB was tested at baseline, after PRT, and on Days 1–7, 14, and 21.
At each testing point, a Luer lock access port was attached with the use of a sterile technique and a 14-mL aliquot of WB collected. Tests performed included: complete blood count (Coulter Ac-T diff2 Analyzer, Beckman Coulter, Inc., Brea, CA); prothrombin time (PT) and activated partial thromboplastin time (aPTT; STart-4 Analyzer, Diagnostica Stago, Inc., Parsippany, NJ); and plasma-free hemoglobin (Hb; Plasma/Low Hb Photometer, HemoCue, Ängelholm, Sweden) for determination of sample hemolysis, as a measure of WB integrity. Throm-boelastography (TEG; Haemonetics Corporation, Brain-tree, MA) with kaolin activation measured WB hemostatic characteristics. Parameters recorded were clotting time (R), clot formation (K), alpha angle (α-angle), maximum amplitude (MA), clot lysis at 30 and 60 minutes (LY30 and LY60, respectively), and total thrombin generation (TTG). TTG was estimated by taking the first derivative of the TEG tracing as previously described.14
PLT function was assessed with multiple electrode impedance aggregometry (Multiplate 5.0 Analyzer, Dynabyte Medical, Munich, Germany), and samples were stimulated with adenosine diphosphate (ADP), collagen (COL), thrombin receptor-activating peptide-6 (TRAP), arachidonic acid agonist (ASPI), and two concentrations of ristocetin (ristocetin low [RL]: 0.2 mg/mL; and ristoce-tin high [RH]: 0.77 mg/mL, respectively). Multiple electrode aggregometry data were reported in arbitrary units defined as area under the curve. Shear effects and von Willebrand factor (vWF)-mediated PLT adhesion and aggregation were measured with a cone-and-plate analyzer (DiaMed Impact-R, Bio-Rad Laboratories, Hercules, CA). Adhesion was measured as percent surface coverage (%SC), and aggregation as aggregate size (AS; in square micrometer). A commercial analyzer (STA-R Evolution Hemostasis System, Diagnostica Stago, Inc.) measured d-dimer, antithrombin III (ATIII), fibrinogen (FIB), factor V (FV), factor VIII (FVIII), protein C, and vWF. Results for PRT-treated samples were corrected for the dilution due to the addition of riboflavin. Soluble CD40 ligand (sCD40L) released by PLTs into stored blood was measured with an enzyme-linked immunosorbent assay kit (human sCD40L Platinum [extra sensitive], eBioscience, Inc., San Diego, CA).
Microparticles (MPs) in platelet-poor plasma (PPP), prepared by centrifugation (3000 × g) for 10 minutes, were quantified and characterized as to their cellular origin, antigen expression, and size distribution by flow cytom-etry (BD FACS Canto II, BD Biosciences, San Jose, CA) with a forward scatter (FSC) photomultiplier tube (PMT) resolution of 200-nm events. Bead mixtures (201, 390, 505, 794, and 990 nm; Bangs Laboratories, Fishers, IN) were used to generate a standard curve against the median FSC PMT data from which MP sizes were determined. An MP gate was set between 990 and 201 nm. Hank’s balanced salt solution (HBSS) was filtered through a sterile 0.1-μm membrane, and sheath fluid was filtered through two inline 0.1-μm membranes.
For identification of cellular origin and antigen expression, an antibody panel was prepared with fluores-cein isothiocyanate conjugated lactadherin (phosphati-dylserine [PS] expression; Haematologic Technologies, Inc., Essex Junction, VT); PE-Cy7 conjugated anti-human CD45 (WBCs; eBioscience); PE conjugated anti-human CD142 (tissue factor [TF]; BD Biosciences), PE-Cy-5 conjugated anti-human CD62P (P-Selectin [P-Sel], PLTs, BD Biosciences), APC conjugated anti-human CD235a (glyco-phorin A, RBC; BD Biosciences), and APC-H7 conjugated anti-human CD41a (glycoprotein IIb [GPIIb], PLTs; BD Biosciences) with the use of the recommended volumes from the manufacturers.
Appropriate isotype control for each antibody was combined in a separate tube. Five microliters of PPP was added to the antibody and isotype panels, incubated for 30 minutes on ice, and 900 μL of filtered HBSS was added to each tube. The sample was analyzed by flow cytometry, and electronic noise was excluded from the MP gate by setting the appropriate instrument threshold. FSC PMT and side scatter data were acquired with the use of logarithmic settings. The concentration of MPs was measured with the use of BD TruCount absolute counting tubes (BD Biosciences) with a known number of beads. For absolute MP counts, 5 μL of PPP and 995 μL of HBSS were added to each TruCount tube, and data collected until 1000 beads were counted. Data analysis was performed with the use of BD FACS Diva software (BD Biosciences).
A second set of experiments was undertaken to further characterize the effects of PLT storage on shear-induced platelet aggregation (SIPA) following a protocol described by Montgomery and colleagues15 Platelet-rich plasma (PRP) was obtained via a standard protocol and stored without agitation at 4°C or 22°C. Samples were tested at baseline and on Days 2 and 7. Briefly, 80 μL of washed PLTs was resuspended to a final concentration of 1–5 × 107/mL in 4-(2-hydroxyethyl)-1-piperazineethane-sulfonic acid (HEPES)/Tyrode’s buffer with Ca2+, Mg2+, 2 mg/mL FIB (EMD Chemicals, Billerica, MA), and 5 μg/mL vWF (Haematologic Technologies), and exposed to shear forces of 2500 or 10,000 s−1 for 120 s at 37°C in a computer-controlled 0.5° cone-and-plate rheometer (Anton-Paar, Graz, Austria). Ten-microliter samples were immediately fixed in 1% formaldehyde, and PLT aggregation was measured by flow cytometry.15 Glycoprotein Ib alpha (GPIbα) receptor expression levels were estimated by the binding of the fluorescently conjugated anti-GPIbα monoclonal antibody AK2 (Abcam, Boston, MA) to stored PLTs. Ristocetin-induced platelet agglutination (RIPA) was performed based on our standard protocol with the use of washed PLTs with 0.75-mg/mL ristocetin and 5-μg/mL vWF.15
Statistical analysis was performed with SAS 9.2 software (SAS Institute, Cary, NC). Data were reported as mean values ± standard error of the mean, unless noted otherwise. Differences between treatment groups were evaluated with repeated measures analysis of variance (RM-ANOVA) with analysis of individual effects (time, PRT treatment, and temperature storage) to determine significance, which was set at p < 0.05. Reported p values reflect RM-ANOVA with analysis of individual effects, unless explicitly stated otherwise. When a single p value was given without reference to individual effects, the p value applies to all three, and the mathematical symbol “≤” or “≥” was used. Post hoc analysis for within-time-point comparisons was made via the Holm–Sidak method. Interactions were evaluated by one-way or two-way ANOVA, as appropriate.
RESULTS
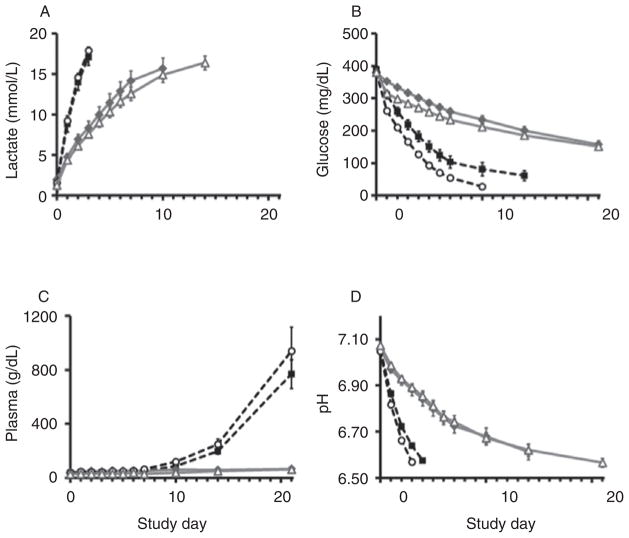
Multiple measures assessed sample quality over time. Lactate, a by-product of anaerobic respiration, increased significantly with storage, but refrigeration slowed accumulation (p ≤ 0.001; Fig. 2A). Glucose, analyzed as a measure of metabolic activity, decreased significantly; however, losses were attenuated by refrigeration (p ≤ 0.001; Fig. 2B). Hemolysis, a reflection of RBC integrity, was higher in samples stored at RT, particularly after Day 7 (p = 0.014; Fig. 2C), and remained essentially unchanged in refrigerated samples (Fig. 2C). Storage at 4°C slowed the decrease in pH over time (p ≤ 0.001; Fig. 2D). Differences due to PRT were only significant for glucose measurements (glucose: p ≤ 0.008, Fig. 2B; lactate, hemolysis, and pH: p ≥ 0.270, Fig. 2A, C–D).
Fig. 2.
Sample quality over time. Multiple measures assessed sample quality: (A) lactate, (B) glucose, (C) hemolysis, and (D) pH changed significantly over time; however, alterations were attenuated by refrigeration (A–D: p ≤0.001). Glucose differences due to PRT were small but significant (B: p ≥ 0.008). The other parameters were not significantly altered by PRT (A, C–D: p ≥ 0.270).
 = CON 22;
= CON 22;
 = CON 4;
= CON 4;
 = PRT 22;
= PRT 22;
 = PRT 4.
= PRT 4.
PLT count decreased with both refrigeration and PRT (p ≤ 0.007, Table 1). Temperature storage differences in WBCs among treatment groups were significant but not clinically relevant (p = 0.037, Table 1). Granulocytes decreased over time, a process accelerated by RT storage and PRT (p ≤ 0.047, Table 1). HCT, Hb, and RBCs did not change relative to baseline or differ between groups (p ≥ 0.817, Table 1). Soluble CD40L, a potent inducer of inflammatory responses and an indication of decreased PLT viability when elevated,16 was not significantly altered by storage temperature, PRT, or storage duration (p ≥ 0.231), and mean values remained within normal subject levels in all groups (minimum: 2.3 ± 1.0 ng/ mL; maximum: 5.7 ± 1.0 ng/mL; eBio-science product insert normal subject range: nondetectable, 7.5 ng/mL).
TABLE 1.
Complete blood count
| Study day | CON 4 | CON 22 | PRT 4 | PRT 22 | CON 4 | CON 22 | PRT 4 | PRT 22 |
|---|---|---|---|---|---|---|---|---|
| WBC (×103/μL) | Granulocytes (×103/μL) | |||||||
| 0 | 5.0 ± 0.5 | 5.4 ± 0.5 | 4.9 ± 0.3 | 6.6 ± 0.9 | 2.9 ± 0.3 | 3.2 ± 0.4 | 3.0 ± 0.2 | 4.3 ± 0.8 |
| 1 | 5.8 ± 0.4 | 5.6 ± 0.5 | 4.8 ± 0.2 | 6.2 ± 0.9 | 3.2 ± 0.3 | 3.1 ± 0.4 | 2.8 ± 0.2 | 2.5 ± 0.4 |
| 2 | 5.5 ± 0.4 | 5.4 ± 0.5 | 4.8 ± 0.3 | 6.1 ± 0.8 | 2.8 ± 0.3 | 2.6 ± 0.4 | 2.8 ± 0.2 | 0.9 ± 0.2 |
| 3 | 5.8 ± 0.5 | 5.4 ± 0.4 | 4.9 ± 0.3 | 6.1 ± 0.9 | 2.9 ± 0.5 | 2.1 ± 0.3 | 2.4 ± 0.3 | 0.4 ± 0.1 |
| 4 | 5.3 ± 0.5 | 5.3 ± 0.4 | 4.6 ± 0.2 | 5.7 ± 1.1 | 2.1 ± 0.4 | 1.6 ± 0.2 | 1.9 ± 0.3 | 0.3 ± 0.0 |
| 5 | 5.5 ± 0.5 | 5.3 ± 0.4 | 5.2 ± 0.4 | 5.9 ± 0.8 | 1.6 ± 0.3 | 1.4 ± 0.2 | 2.0 ± 0.5 | 0.6 ± 0.4 |
| 6 | 5.5 ± 0.5 | 5.5 ± 0.5 | 5.0 ± 0.3 | 5.9 ± 0.8 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.4 ± 0.4 | 0.2 ± 0.1 |
| 7 | 4.9 ± 0.6 | 5.3 ± 0.6 | 4.2 ± 0.6 | 5.2 ± 0.4 | 0.9 ± 0.2 | 0.8 ± 0.2 | 1.2 ± 0.3 | 0.2 ± 0.0 |
| 10 | 5.2 ± 0.4 | 5.5 ± 0.6 | 5.0 ± 0.3 | 5.8 ± 0.8 | 0.5 ± 0.1 | 0.9 ± 0.5 | 0.7 ± 0.2 | 0.4 ± 0.1 |
| 14 | 4.9 ± 0.4 | 5.2 ± 0.5 | 4.5 ± 0.3 | 5.7 ± 0.8 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.9 ± 0.2 |
| 21 | 4.9 ± 0.4 | 5.3 ± 0.5 | 4.4 ± 0.3 | 5.7 ± 0.8 | 0.3 ± 0.0 | 0.7 ± 0.2 | 0.3 ± 0.0 | 1.5 ± 0.2 |
| RBC (×103/μL) | Hb (g/dL) | |||||||
| 0 | 4.1 ± 0.2 | 4.1 ± 0.2 | 4.1 ± 0.1 | 4.2 ± 0.1 | 13 ± 0 | 13 ± 1 | 13 ± 0 | 13 ± 1 |
| 1 | 4.2 ± 0.2 | 4.0 ± 0.2 | 3.7 ± 0.2 | 4.0 ± 0.4 | 13 ± 1 | 12 ± 1 | 12 ± 0 | 13 ± 1 |
| 2 | 3.9 ± 0.2 | 4.1 ± 0.1 | 3.8 ± 0.1 | 3.9 ± 0.1 | 12 ± 1 | 13 ± 1 | 12 ± 0 | 12 ± 0 |
| 3 | 4.1 ± 0.1 | 3.9 ± 0.2 | 3.8 ± 0.2 | 4.0 ± 0.1 | 13 ± 0 | 12 ± 1 | 12 ± 1 | 12 ± 1 |
| 4 | 3.9 ± 0.2 | 4.1 ± 0.2 | 3.6 ± 0.1 | 4.1 ± 0.3 | 12 ± 1 | 13 ± 1 | 12 ± 0 | 13 ± 1 |
| 5 | 4.0 ± 0.2 | 4.0 ± 0.2 | 3.6 ± 0.2 | 3.9 ± 0.4 | 13 ± 1 | 12 ± 1 | 12 ± 1 | 12 ± 1 |
| 6 | 4.1 ± 0.2 | 4.1 ± 0.2 | 3.9 ± 0.1 | 3.9 ± 0.1 | 13 ± 0 | 13 ± 1 | 12 ± 0 | 12 ± 1 |
| 7 | 4.1 ± 0.2 | 4.1 ± 0.2 | 3.6 ± 0.2 | 3.9 ± 0.2 | 13 ± 0 | 13 ± 1 | 12 ± 1 | 12 ± 1 |
| 10 | 3.9 ± 0.2 | 4.1 ± 0.2 | 4.0 ± 0.2 | 3.8 ± 0.2 | 12 ± 1 | 13 ± 1 | 13 ± 1 | 12 ± 1 |
| 14 | 3.8 ± 0.3 | 4.0 ± 0.2 | 3.7 ± 0.1 | 3.8 ± 0.2 | 12 ± 1 | 12 ± 1 | 12 ± 0 | 12 ± 1 |
| 21 | 4.0 ± 0.1 | 4.2 ± 0.2 | 3.8 ± 0.1 | 3.9 ± 0.1 | 13 ± 0 | 13 ± 1 | 12 ± 0 | 12 ± 0 |
| HCT (%) | PLT (×103/μL) z | |||||||
| 0 | 37 ± 1 | 37 ± 2 | 37 ± 1 | 38 ± 1 | 156 ± 16 | 171 ± 17 | 150 ± 16 | 183 ± 25 |
| 1 | 38 ± 2 | 36 ± 2 | 33 ± 1 | 36 ± 3 | 142 ± 21 | 191 ± 11 | 140 ± 9 | 164 ± 18 |
| 2 | 35 ± 2 | 37 ± 1 | 34 ± 1 | 35 ± 1 | 160 ± 17 | 189 ± 15 | 132 ± 10 | 174 ± 13 |
| 3 | 37 ± 1 | 36 ± 2 | 34 ± 2 | 36 ± 1 | 165 ± 15 | 195 ± 16 | 122 ± 13 | 170 ± 13 |
| 4 | 35 ± 1 | 38 ± 2 | 33 ± 1 | 37 ± 3 | 146 ± 18 | 189 ± 13 | 108 ± 14 | 163 ± 16 |
| 5 | 37 ± 2 | 37 ± 2 | 33 ± 2 | 36 ± 3 | 151 ± 16 | 192 ± 15 | 118 ± 9 | 166 ± 16 |
| 6 | 37 ± 1 | 38 ± 2 | 36 ± 1 | 36 ± 1 | 146 ± 14 | 187 ± 14 | 108 ± 11 | 165 ± 10 |
| 7 | 37 ± 1 | 38 ± 2 | 33 ± 2 | 36 ± 2 | 145 ± 13 | 190 ± 21 | 107 ± 10 | 164 ± 16 |
| 10 | 35 ± 2 | 38 ± 2 | 37 ± 2 | 36 ± 2 | 138 ± 11 | 177 ± 16 | 98 ± 9 | 162 ± 16 |
| 14 | 35 ± 2 | 36 ± 2 | 34 ± 1 | 36 ± 2 | 128 ± 10 | 178 ± 17 | 88 ± 7 | 171 ± 16 |
| 21 | 37 ± 1 | 38 ± 2 | 35 ± 0 | 39 ± 1 | 110 ± 7 | 166 ± 19 | 94 ± 10 | 173 ± 17 |
PLT count decreased with both refrigeration and PRT (p ≤0.007). Temperature storage differences in WBCs among treatment groups were significant but not clinically relevant (p = 0.037; Table 1); PRT effects did not differ between groups (p = 0.275). Granulocytes decreased over time, a process accelerated by RT storage and PRT (p ≤ 0.047; Table 1). HCT, Hb, and RBCs did not change relative to baseline or differ between groups (p ≥0.817).
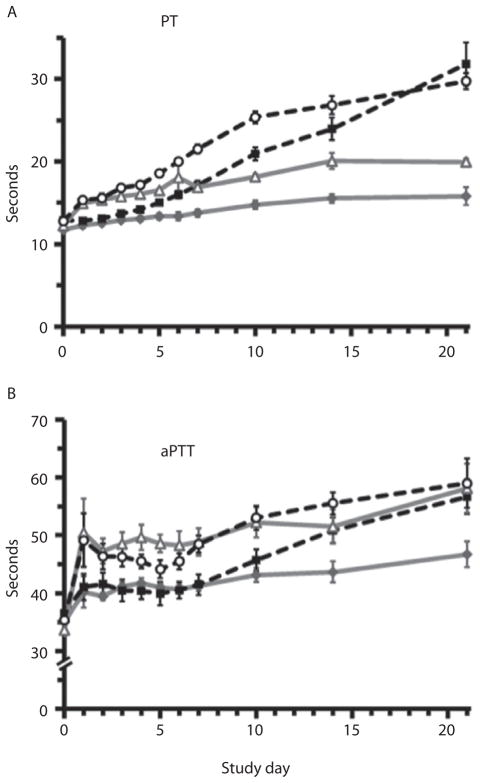
Coagulation results differed between the type of test performed and between subject groups. PT and partial thromboplastin time (PTT) increased over time, but refrigeration attenuated the effect on PT (p ≤ 0.009; Fig. 3A). Mean PT levels were within the reference ranges determined by our clinical laboratory (PT: 12.1–14.7 sec) through Day 7 in the CON 4 group compared with Day 4 for CON 22. PRT prolonged both PT and PTT (p ≤ 0.009; Fig. 3A–B), and mean values were above reference ranges by Posttreatment Day 1.
Fig. 3.
PT and aPTT. PT and aPTT increased over time; however, refrigeration attenuated the effect on PT (A: p ≤ 0.009). Mean PT levels were within reference ranges through Day 7 in the CON 4 group compared with Day 4 for CON 22. PRT prolonged both PT and PTT (A–B: p ≤ 0.009), and mean values were above reference ranges by posttreatment Day 1.
 = CON 22;
= CON 22;
 = CON 4;
= CON 4;
 = PRT 22;
= PRT 22;
 = PRT 4.
= PRT 4.
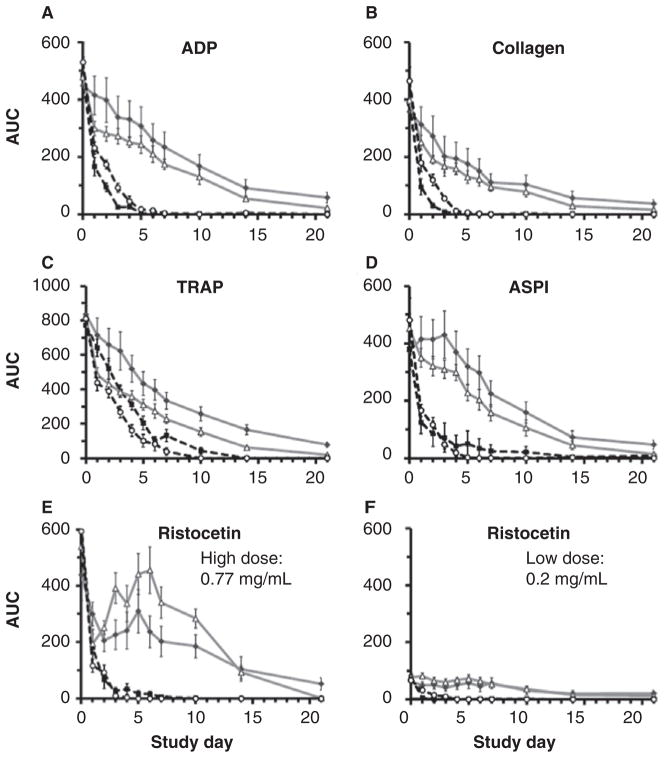
A repeated measures analysis demonstrated that agonist-stimulated impedance aggregation decreased over time in all four treatment groups (p ≤ 0.001; Fig. 4A–F); however, losses were attenuated in samples stored at 4°C compared with those stored at 22°C (p ≤ 0.001; Fig. 4A–F). PRT-treated samples demonstrated lower COL-, TRAP-, and RL-stimulated aggregation compared with controls (p ≤ 0.033; Fig. 4B, C, F), but differences were not significant in ADP-, ASPI-, and RH-stimulated samples (p ≥ 0.242; Fig. 4A, D–F).
Fig. 4.
Multiple electrode PLT aggregometry. A repeated measures analysis demonstrated that agonist-stimulated aggregation decreased over time in all four treatment groups (A–F: p ≤ 0.001); however, losses were attenuated in samples stored at 4°C compared with those stored at 22°C (A–F: p ≤ 0.001). PRT-treated samples demonstrated lower COL-, TRAP-, and RL-stimulated aggregation compared with controls (B, C, F: p ≤ 0.033), but differences were not significant in ADP-, ASPI-, and RH-stimulated samples (A, D, E: p ≥ 0.242). Aggregation immediately after PRT, with the exception of ADP and TRAP, were within one standard deviation of healthy subject ranges at our center.
 = CON 22;
= CON 22;
 = CON 4;
= CON 4;
 = PRT 22;
= PRT 22;
 = PRT 4.
= PRT 4.
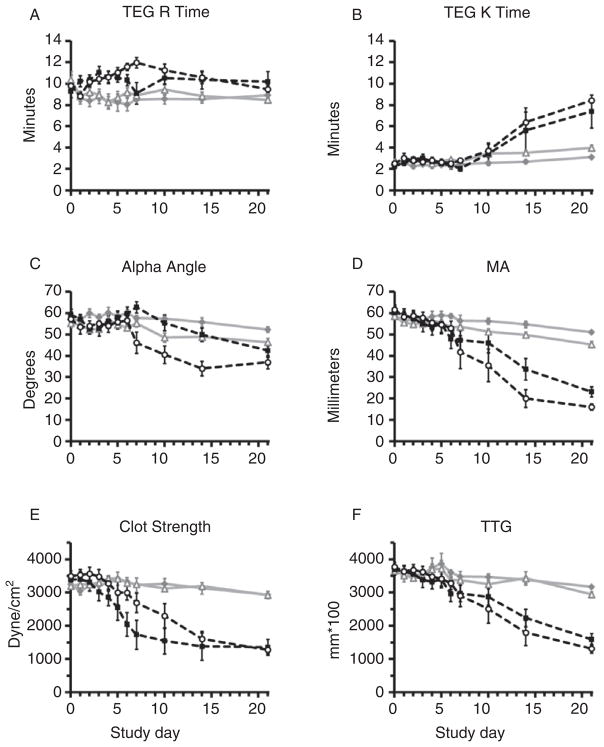
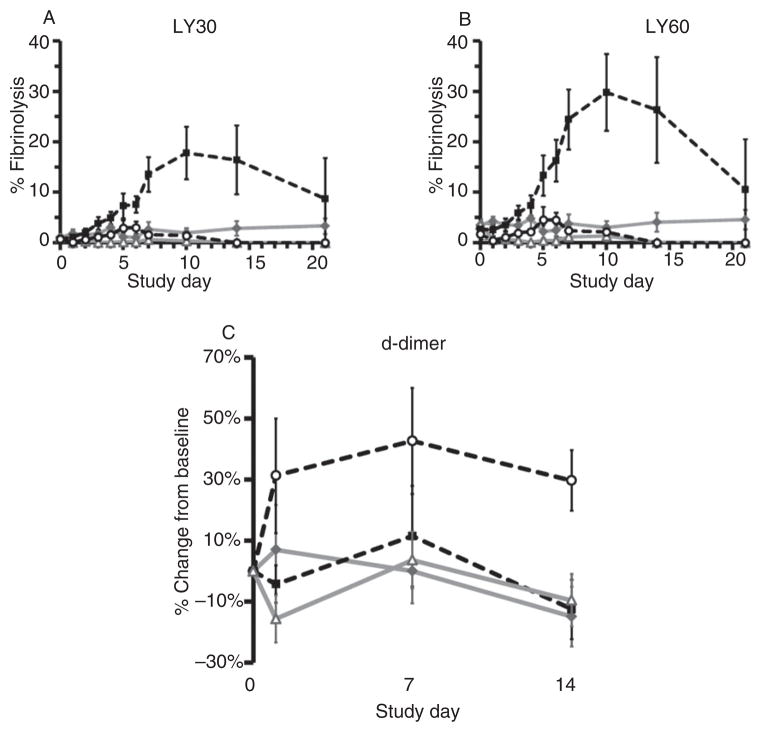
Storage at 4°C preserved TEG R, K, MA, clot strength, and TTG relative to samples stored at RT (p ≤ 0.001; Fig. 5A–F). Differences in TEG clot strength and TTG were not significant after PRT compared with controls by RM-ANOVA (p ≥ 0.237; Fig. 5E, F). R, K, α-angle, and MA were altered by PRT treatment, but results for samples in cold storage, including those that underwent PRT, remained within normal subject limits throughout most of the study.17 Fibrinolysis as measured by TEG was inhibited by both cold storage and PRT compared with control samples stored at RT (LY30: p = 0.012; LY60: p ≤ 0.001; Fig. 6A, B). There was no difference between groups in d-dimer accumulation over storage time, and most values were approximately within ± 30% of baseline (p = 0.412; Fig. 6C).
Fig. 5.
TEG. Storage at 4°C preserved TEG R time, K, α-angle, MA, clot strength, and TTG relative to samples stored at RT (A–F: p ≤ 0.001). Differences in TEG clot strength and TTG were not significant after PRT treatment compared with controls by RM-ANOVA (E–F: p ≥ 0.237). R, K, α-angle, and MA were altered by PRT treatment (A–D: p ≤ 0.045), but results for samples in cold storage, including those that underwent PRT, remained within normal subject limits throughout the study.
 = CON 22;
= CON 22;
 = CON 4;
= CON 4;
 = PRT 22;
= PRT 22;
 = PRT 4.
= PRT 4.
Fig. 6.
Fibrinolysis: (A) LY30; (B) LY60; and (C) d-dimer. Fibrinolysis as measured by TEG was inhibited by both cold storage and PRT compared with control samples stored at RT (A: LY30 p = 0.012; B: LY60 p p ≤ 0.001). d-dimer did not accumulate in unstimulated stored blood; the CON 22 samples did not differ significantly from other groups, and most values were approximately within ±30% of baseline (C: p = 0.412).
 = CON 22;
= CON 22;
 = CON 4;
= CON 4;
 = PRT 22;
= PRT 22;
 = PRT 4.
= PRT 4.
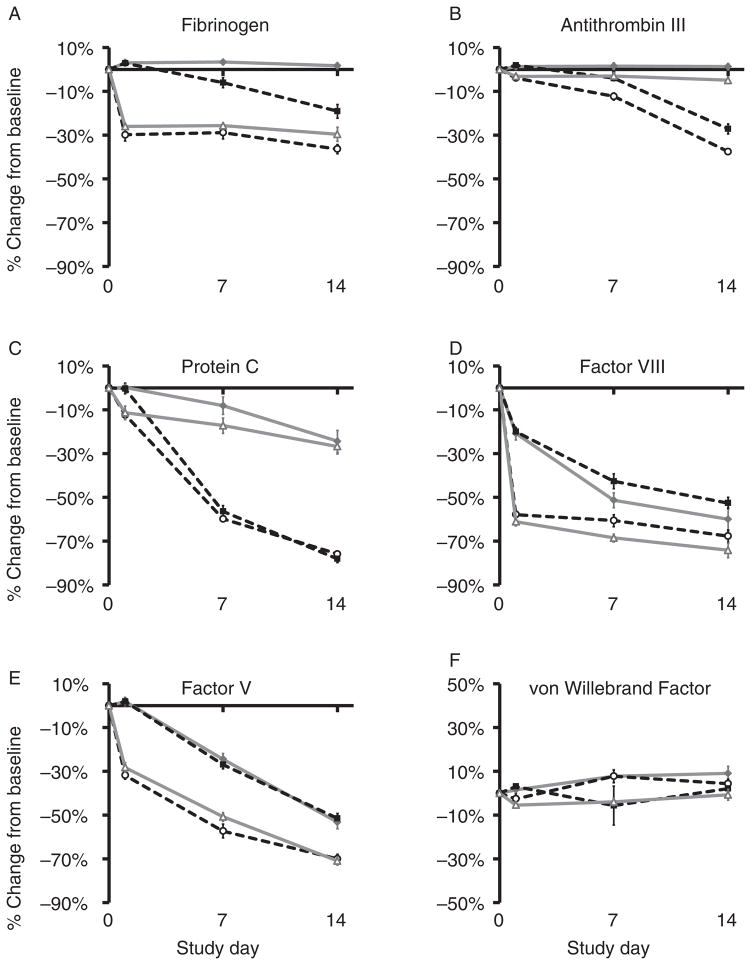
The effects of PRT and temperature storage conditions on coagulation factors were variable. ABO blood type distribution was not equal between groups (CON 4: 100% type O; CON 22: 25% type O, 37.5% type A, 25% type B, 12.5% type AB; PRT 4: 62.5% type O, 37.5% type A; and PRT 22: 50% type O, 50% type A). Mean baseline values for most plasma measurements differed between groups (p ≤ 0.05), possibly due to the differences in distribution of ABO blood groups because of donor scheduling constraints; therefore, results are shown as percent change from baseline. Refrigeration prevented decline in FIB and ATIII levels. PRT caused an initial drop in FIB (26%–30%; PRT: p ≤ 0.001; Fig. 7A); however, mean values remained within normal subject ranges (145–348 mg/dL) for all groups (lowest: 173 ± 6 mg/dL, Day 1, PRT 4).18 Temperature did not have a significant effect (TEMP: p = 0.364; Fig. 7A). PRT had no immediate effect on ATIII, and losses over time were attributable to storage at RT (p ≤ 0.001; Fig. 7B). Differences between treated and untreated groups stored at the same temperature were not signifi-cant in pairwise comparisons of any given day (p ≥ 0.471; Fig. 7B). Protein C levels decreased over time and due to storage at RT (p ≤ 0.001; Fig. 7C) but not due to PRT treatment (p = 0.529). Refrigeration diminished losses. FV and FVIII decreased due to PRT and over time (p ≤ 0.001; Fig. 7D, E). Storage temperature had no effect on FV (p = 0.243), but cold storage affected FVIII adversely (p = 0.036). Levels of vWF remained within ± 10% of baseline (Fig. 7F).
Fig. 7.
Plasma analysis. The effects of PRT treatment and temperature storage conditions on coagulation factors were variable. PRT caused an initial drop in FIB, which decreased by 26%–30% but temperature did not have a significant effect (A: PRT p ≤ 0.001; TEMP p = 0.364). PRT had no immediate effect on ATIII, and losses were attributable to storage at RT (B: PRT p ≥ 0.471 by pairwise comparison; TEMP p ≤ 0.001). Protein C levels decreased over time, but refrigeration diminished losses, and PRT treatment had no effect (C: TEMP p ≤ 0.001; PRT 0.529). FV and FVIII decreased due to PRT and over time (D–E: PRT p ≤ 0.001; DAY p ≤ 0.001). Storage temperature had no effect on FV (p = 0.243). PRT did not change vWF, and levels remained within 10% of baseline (F: TEMP p = 0.007; PRT & DAY p ≤ 0.091).
 =CON 22;
=CON 22;
 = CON 4;
= CON 4;
 = PRT 22;
= PRT 22;
 = PRT 4.
= PRT 4.
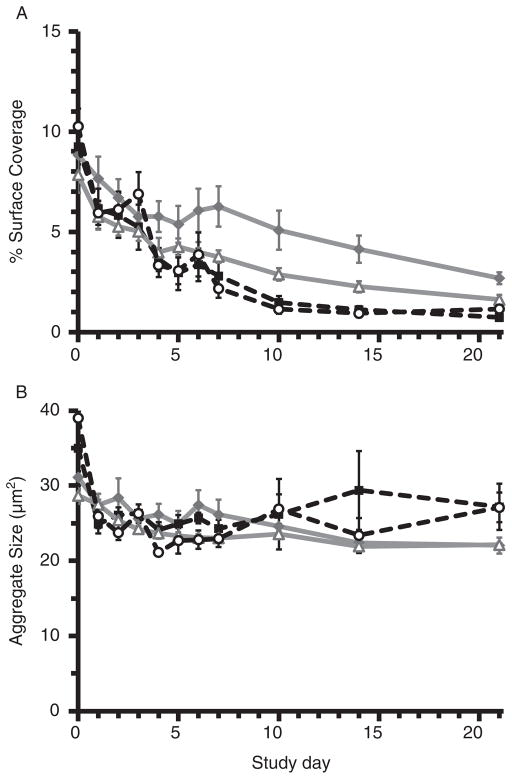
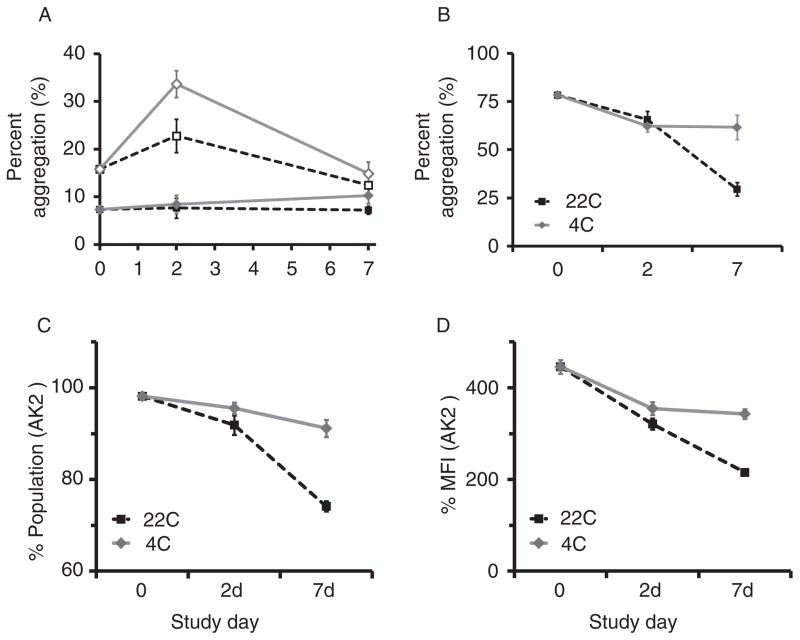
Cone-and-plate aggregometry measures GPIb–vWF PLT adhesion reported as %SC, followed by GPIIb/IIIa–FIB-mediated aggregation expressed as AS. Refrigeration significantly attenuated the decline in %SC over time (p ≥0.001; Fig 8A), whereas PRT effects were not signifi-cant (p = 0.058). Diminished AS, reflecting loss of GPIIb/ IIIa and FIB-mediated PLT aggregation, was attenuated by refrigeration (p ≤0.019; Fig 8B); PRT-related differences were not significant (p ≥0.125). Mean values were within healthy subject limits throughout the period studied. These findings were consistent with results from ristocetin-induced impedance aggregometry as described above. We observed a similar pattern in platelet concentrates (PCs) derived from PRP and stored at 4°C versus 22°C for 7 days (Fig. 9). SIPA and RIPA levels were both higher after cold storage compared with RT (SIPA Day 2: p = 0.032; RIPA Day 7: p = 0.002; Fig. 9A, B). Cold storage increased both the percentage of GPIb-expressing PLTs and the expression level, as measured by AK2, by Day 7 compared with RT storage (p ≤≤0.009; Fig. 9C, D).
Fig. 8.
Cone-and-plate aggregometry. (A) Comparison of the %SC as a measure of vWF and GPIb-mediated PLT adhesion in the four storage conditions demonstrated that refrigeration significantly attenuated the loss of function observed over time (%SC: TEMP and DAY p ≤ 0.001). Differences in the PRT samples were not significant (%SC: PRT p = 0.058). CON 4 values were within healthy subject limits through Day 14 compared with 3–4 days in WB stored at 22°C. (B) Change in AS, a measure of GPIIb/IIIa and FIB-mediated PLT aggregation, was attenuated by refrigeration (AS: TEMP and DAY p ≤ 0.019). Treatment-related differences between groups were not significant (p ≥ 0.125), and mean values were within healthy subject limits throughout the period studied.
 = CON 22;
= CON 22;
 = CON 4;
= CON 4;
 = PRT 22;
= PRT 22;
 = PRT 4.
= PRT 4.
Fig. 9.
(A) SIPA, (B) RIPA, and (C–D) measures of GPIbα expression. PC stored at 4°C versus 22°C for 7 days demonstrated higher SIPA and RIPA results after cold storage compared with RT (SIPA Day 2: p = 0.032; RIPA Day 7: p = 0.002). GPIb-expressing PLTs, as measured by AK2, were more abundant by Day 7 in refrigerated PLTs (C: p ≤ 0.001), and mean fluorescence intensity was significantly higher at Day 7 (D: p = 0.004).
 = 22C 2500 s-1;
= 22C 2500 s-1;
 = 4C 2500 s-1;
= 4C 2500 s-1;
 = 22C 10,000 s-1;
= 22C 10,000 s-1;
 = 4C 10,000 s-1.
= 4C 10,000 s-1.
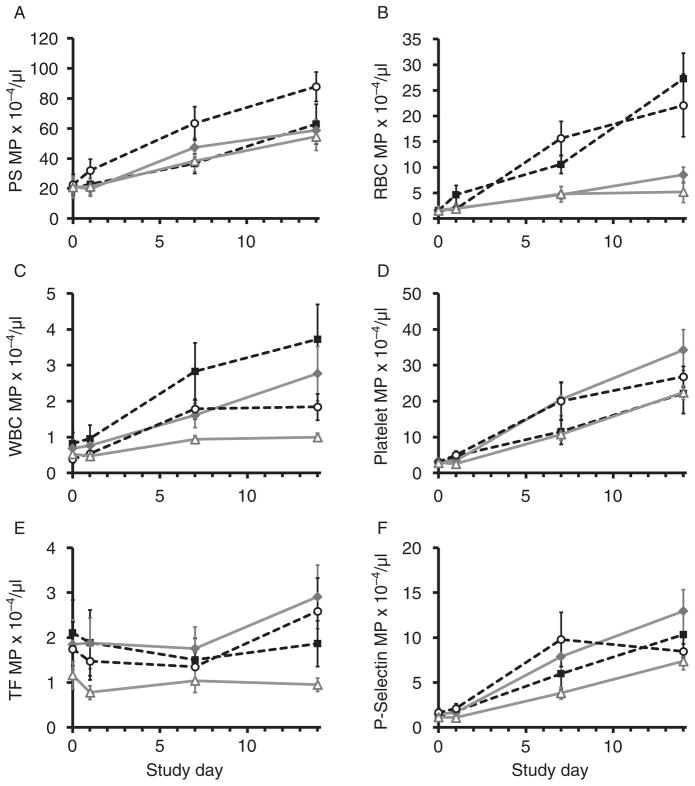
MP subpopulations increased over time in most treatment groups (p ≥ 0.05, n = 6–8 per treatment group; Fig. 10). The combination of PRT and 4°C was associated with the lowest MP levels; otherwise, temperature and PRT effects varied among MP subpopulations (Fig. 10). Size distributions fell into two ranges: 201–330 and 330–1000 nm. Abundance ratios were approximately 90% (201–330 nm) and 10% (330–1000 nm), respectively, throughout the study. The PS-expressing subpopulation was the most abundant MPs found (up to 31% of events; Fig. 10A). As a group, PS MPs were not significantly altered by either storage temperature or pathogen reduction, but up to 68% of this population was positive for other markers, the overwhelming majority of which were PLTs in origin (50% of PS MP). RBC-, WBC-, and PLT-derived MP distributions responded to refrigeration and PRT treatment in divergent ways (Fig. 10B–E). Cold storage significantly reduced RBC-derived MP accumulation (p ≤ 0.001; Fig. 10B). A similar effect was seen in WBC, PLT, and TF-expressing PLT MP but only when combined with PRT (p ≤ 0.031; Fig. 10C–E). Interestingly, increases in PLT MP were also minimized by RT storage in the absence of PRT. PLT MPs were further examined for P-Sel membrane expression; temperature and PRT effects were not significant in this population (p = 0.154; Fig. 10F).
Fig. 10.
MP analysis. MP distribution was variably affected by both temperature and PRT, whereas MP subpopulations increased over time (p ≥ 0.05). (A) PS-expressing MPs were in greatest abundance and increased 2.5- to 4-fold by Day 14 (all groups, p ≤ 0.012). Differences due to temperature storage and PRT were not significant (p ≥ 0.05). (B) RBC MP were three to four times higher in samples stored at 22°C compared with 4°C (p ≤ 0.001), whereas PRT did not cause significant effects (p = 0.148). (C) PRT and refrigeration in combination significantly reduced WBC MP accumulation (p ≤ 0.011); values in the PRT 4 group did not differ from baseline over time (p = 0.063). (D) PRT and refrigeration in combination significantly reduced PLT MP accumulation (p ≤ 0.015). Interestingly, increases in PLT MP were also minimized by RT storage in the absence of PRT. (E) PRT and refrigeration in combination significantly reduced TF MP accumulation (p ≤ 0.031). (F) Changes due to PRT and refrigeration were not significant in P-Sel MP formation (p = 0.154).
 = CON 22;
= CON 22;
 = CON 4;
= CON 4;
 = PRT 22;
= PRT 22;
 = PRT 4.
= PRT 4.
DISCUSSION
The experience of military and civilian surgeons since World War I has demonstrated that treatment of severe hemorrhage requires blood replacement. Among civilian and military trauma surgeons, component therapy to treat massive hemorrhage has recently moved toward reapproximating WB with RBCs, FFP, and PLTs after reports of improved survival.6,9,10,19–24 This approach is complicated by the logistical challenges of 1) managing RBC inventory age; 2) balancing thawed plasma and FFP inventories to provide plasma in a timely manner; and 3) ensuring suffi-cient PLT products, which are limited by a 5-day shelf life and the risks associated with RT storage. Bacterial contamination of PLTs remains a common problem, despite scientific investigations spanning decades and involving considerable finan-cial investments; more problematic still is the human cost in severe septic events and fatalities.25,26 To minimize sepsis risk, PLTs are quarantined for 24 hours while awaiting culture results, which effectively limits the shelf life to 4 days. PLTs are stored at RT to maximize PLT circulation time, as refrigeration leads to PLT clearance within 48 hours of transfusion.27 Unfortunately, this practice comes at the cost of a high risk of contamination and may have clinical consequences of increased morbidity.28
Currently, WB is used by the military as a rescue product for severe hemorrhage when component therapy is unavailable or ineffective.6,7,10–13 Storage is limited to 24 hours at RT. This is due to concern for PLT viability and because, as an emergency product, it does not undergo comprehensive in-theater infectious disease testing. Both military and civilian clinicians have reported promising results in patients who were treated with WB, possibly related to the avoidance of unbalanced resuscitation strategies characterized by early RBC and late hemostatic plasma and PLT transfusions.9,10,12,19,29 The use of WB for wider applications has an inherent teleological rationale, as it replaces what is lost during hemorrhage, but the important considerations that must be addressed before such a strategy is broadly applied include pathogen mitigation, storage temperature and time, and maintenance of hemostatic capacity.
PLTs are integral to the hemostatic potential of WB, and recent reviews of the military experience demonstrate that they have a closer association with beneficial outcomes than other blood components.6 Expansion of WB use would necessitate storage for more than 24 hours, and cold storage would be beneficial in inhibiting bacterial growth. However, refrigeration causes accelerated PLT elimination due to two mechanisms: clustering and desia-lylation of the GPIbα receptor with subsequent clearance via Mac-1 integrin binding by hepatic and splenic macrophages, and GPIbα receptor binding to Ashwell–Morell receptors on hepatocytes.30,31 PLT activation and accelerated clearance is also seen in vivo after exposure to cold temperatures;30 these mechanisms have been the rationale for eliminating the use of cold PLTs after the phenomenon was first observed more than five decades ago.30,32 Severe traumatic hemorrhage is a highly acute problem limited by total blood volume and flow rate; however, the question of whether a highly active but transient PLT population might be useful for massive bleeding was once debated but not pursued for reasons that remain unclear.33
The practical use of WB would require storage beyond 24 hours and thus presents a conundrum: while refrigeration promotes the accelerated clearance of PLTs, storage at RT poses the risk of bacterial contamination as well as decreased blood cell viability due to metabolic stress.25,26,34 Methods to nonspecifically reduce pathogens are currently under investigation and may hold the key to a safe, cost-effective, and hemostatic WB product,13,35–44 either with or without refrigeration. Here, we have comprehensively evaluated the in vitro effects of PRT treatment, refrigeration, and RT storage on coagulation and hemo-stasis over a 21-day period.
The data from these experiments indicate that loss of PLT function over time can be attenuated by refrigeration. A limitation of this study is that we did not try to balance ABO blood type between groups. As a result, all the blood in the CON 4 group was type O, which is associated with lower levels of vWF and FVIII.45,46 Because this group displayed the best aggregation and coagulation function over time, it is unlikely that differences in the experimental data associated with cold storage were attributable to unequal ABO distribution.
Pathogen reduction causes an initial drop in several measures of coagulation; however, PRT WB does not subsequently differ from control in many assays. Flow cytometry demonstrated that pathogen reduction in combination with cold storage prevents or reduces MP formation from several cell types and confirmed that antibody detection of GPIbα was preserved with refrigeration. Release of the PLT-derived inflammatory mediator sCD40L was minimal and not significantly increased from baseline in all conditions tested. PLT viability after pathogen reduction has been shown to correlate with a low glucose consumption rate,36,47 and we found that both PRT and refrigeration delayed reductions in glucose plasma levels, evidence of slowed metabolism in the cellular constituents of WB. Hemolysis, a measure of RBC lysis, was minimal in all groups through Day 7 and minimized after that by storage at 4°C with or without PRT treatment.
PT and aPTT are widely used measures of coagulation function that have been criticized as poor predictors of bleeding. These two parameters were greatly affected by time and PRT treatment, but PT prolongation was largely prevented for a week by refrigeration. As expected, the prolongations in PT and PTT were associated with decreases in the activities of a number of coagulation factors, in particular FV and FVIII. Similarly, agonist-stimulated PLT aggregation, as measured by impedance aggregometry, was decreased by time and RT storage, but unlike PT and PTT, PRT-related decreases were less marked and not significant in the case of ADP, ASPI, and RH. A better measure of PLT aggregation under flow, namely, shear-mediated PLT aggregation, was relatively preserved at 4°C, as measured by cone-and-plate aggregometry in both WB and PC, suggesting that PLT hemostatic function is more dependent on temperature than the storage solution. Taken together, these data suggest that refrigerated PRT WB could support primary hemostasis and retain utility during extended storage.
In contrast to the results of the reductionist methods of blood component analysis described above, most TEG parameters for cold-stored WB remained largely within normal limits over the 21-day WB storage period.17 These data indicate that PLT and coagulation function in cold-stored WB may be sufficient to provide hemostatic resuscitation, although this needs to be validated with in vivo studies. The TEG finding of diminished clot lysis associated with pathogen reduction and refrigeration is intriguing and suggests that PRT WB may complement the beneficial effects of the antifibrinolytic, tranexamic acid in trauma resuscitation, particularly in the most coagulopathic patients who demonstrate hyperfibrinolysis.
The clinical significance of these findings has yet to be established. These data indicate that PRT-treated, refrigerated WB can maintain hemostatic function when stored for several weeks, making it a potentially useful adjunct in austere environments when components from US blood banks cannot be obtained. If PRT WB provides adequate PLT function and coagulation factor activity to support hemostasis in the setting of severe bleeding, physicians could prepare it in anticipation of mass casualties with refrigerated, treated blood donated by soldiers in a forward blood bank. Our TEG results suggest that refrigerated PRT WB may be sufficient to restore hemostasis, even at 21 days of storage; however, this remains to be studied in in vivo models. Further refinements to PRT WB such as inhibition of glycosyltransferases could prevent or delay PLT clearance.32,48–51 In summary, these data demonstrate that refrigerated PRT WB retains substantial in vitro hemostatic activity over 21 days of storage, suggesting the viability of an expanded role for WB in hemorrhage resuscitation. Clinical studies of its safety and efficacy are warranted.
Acknowledgments
Supported by: the MRMC, Terumo BCT Biotechnologies. doi: 10.1111/trf.12048
ABBREVIATIONS
- aPTT
activated partial thromboplastin time
- ASPI
arachidonic acid agonist
- COL
collagen
- GPIbα
glycoprotein Ib alpha
- K
clot formation
- LY30
clot lysis at 30 minutes
- LY60
clot lysis at 60 minutes
- MA
maximum amplitude (clot strength)
- PC
platelet concentrates
- PMT
photomultiplier tube
- PPP
platelet-poor plasma
- PRP
platelet-rich plasma
- PRT
pathogen reduction technology
- PT
prothrombin time
- R
clotting time
- RH
high ristocetin concentration
- RIPA
ristocetin-induced platelet agglutination
- RL
low ristocetin concentration
- RT
room temperature
- SIPA
shear-induced platelet aggregation
- TEG
thromboelastography
- TRAP
thrombin receptor-activating peptide-6
- TTG
total thrombin generation
- WB
whole blood
- α-angle
alpha angle (angle of clot formation)
Footnotes
CONFLICT OF INTEREST
This study was conducted under a protocol reviewed and approved by the US Army Medical Research and Materiel Command Institutional Review Board and in accordance with the approved protocol.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
References
- 1.Kendrick BG. Blood program in World War II. 1964 [cited 2012 Sep 5]. Available from: URL: http://history.amedd.army.mil/booksdocs/wwii/blood/default.htm.
- 2.Neel S., Major General Medical support of the US Army in Vietnam 1965–1970. 1991 [cited 2012 Sep 5]. Available from: URL: http://history.amedd.army.mil/booksdocs/vietnam/medicalsupport/default.html.
- 3.Berseus O, Boman K, Nessen SC, West-erberg LA. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion. 2013;53(Suppl 1):114S–23S. doi: 10.1111/trf.12045. [DOI] [PubMed] [Google Scholar]
- 4.Gerhardt RT, Strandenes G, Cap AP, Rentas FJ, Glassberg E, Mott J, Dubick MA, Spinella PC for the THOR Network and RemTORN Study Group. Remote damage control resuscitation and the Solstrand Conference: defining the need, the language, and a way forward. Transfusion. 2013;53(Suppl 1):9S–16S. doi: 10.1111/trf.12030. [DOI] [PubMed] [Google Scholar]
- 5.Nessen SC, Eastridge BJ, Cronk D, Craig RM, Berséus O, Ellison R, Remick K, Seery J, Shah A, Spinella PC. Fresh whole blood use by forward surgical teams in Afghanistan is associated with improved survival compared to component therapy without platelets. Transfusion. 2013;53(Suppl 1):107S–13S. doi: 10.1111/trf.12044. [DOI] [PubMed] [Google Scholar]
- 6.Pidcoke HF, Aden JK, Mora A, Borgman M, Spinella PC, Dubick M, Blackbourne L, Cap AP. 10-year analysis of transfusion in Operation Iraqi Freedom and Operation Enduring Freedom: increased plasma and platelet use correlates with improved survival. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S445–52. doi: 10.1097/TA.0b013e3182754796. [DOI] [PubMed] [Google Scholar]
- 7.Perkins JG, Cap AP, Spinella PC, Shorr AF, Beekley AC, Grathwohl KW, Rentas FJ, Wade CE, Holcomb JB. Comparison of platelet transfusion as fresh whole blood versus apheresis platelets for massively transfused combat trauma patients (CME) Transfusion. 2011;51:242–52. doi: 10.1111/j.1537-2995.2010.02818.x. [DOI] [PubMed] [Google Scholar]
- 8.Perkins JG, Cap AP, Spinella PC, Blackbourne LH, Grath-wohl KW, Repine TB, Ketchum L, Waterman P, Lee RE, Beekley AC, Sebesta JA, Shorr AF, Wade CE, Holcomb JB. An evaluation of the impact of apheresis platelets used in the setting of massively transfused trauma patients. J Trauma. 2009;66(Suppl):S77–84. doi: 10.1097/TA.0b013e31819d8936. discussion S84–5. [DOI] [PubMed] [Google Scholar]
- 9.Cap AP, Spinella PC, Borgman MA, Blackbourne LH, Perkins JG. Timing and location of blood product transfusion and outcomes in massively transfused combat casualties. J Trauma Acute Care Surg. 2012;73(Suppl 1):S89–94. doi: 10.1097/TA.0b013e318260625a. [DOI] [PubMed] [Google Scholar]
- 10.Spinella PC, Dunne J, Beilman GJ, O’Connell RJ, Borgman MA, Cap AP, Rentas F. Constant challenges and evolution of US military transfusion medicine and blood operations in combat. Transfusion. 2012;52:1146–53. doi: 10.1111/j.1537-2995.2012.03594.x. [DOI] [PubMed] [Google Scholar]
- 11.Spinella PC, Strandenes G, Rein EB, Seghatchian J, Hervig T. Symposium on fresh whole blood for severe hemorrhagic shock: from in-hospital to far forward resuscitations. Transfus Apher Sci. 2012;46:113–7. doi: 10.1016/j.transci.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66(Suppl):S69–76. doi: 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spinella PC, Reddy HL, Jaffe JS, Cap AP, Goodrich RP. Fresh whole blood use for hemorrhagic shock: preserving benefit while avoiding complications. Anesth Analg. 2012;115:751–8. doi: 10.1213/ANE.0b013e318261f40e. [DOI] [PubMed] [Google Scholar]
- 14.Rivard GE, Brummel-Ziedins KE, Mann KG, Fan L, Hofer A, Cohen E. Evaluation of the profile of thrombin generation during the process of whole blood clotting as assessed by thrombelastography. J Thromb Haemost. 2005;3:2039–43. doi: 10.1111/j.1538-7836.2005.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery RK, Reddoch KM, Evani SJ, Cap AP. Rama-subramanian AK. Enhanced shear-induced platelet aggregation due to low temperature storage. Transfusion. 2013 doi: 10.1111/j.1537-2995.2012.03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenzel F, Hohlfeld T, Giers G. Soluble CD40L release as test for functional platelet loss. Clin Lab. 2012;58:337–42. [PubMed] [Google Scholar]
- 17.Jobes D, Wolfe Y, O’Neill D, Calder J, Jones L, Sesok-Pizzini D, Zheng XL. Toward a definition of “fresh” whole blood: an in vitro characterization of coagulation properties in refrigerated whole blood for transfusion. Transfusion. 2011;51:43–51. doi: 10.1111/j.1537-2995.2010.02772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oswald MW, Hunt HH, Lazarchick J. Normal range of plasma fibrinogen. Am J Med Technol. 1983;49:57–9. [PubMed] [Google Scholar]
- 19.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 20.Bhangu A, Nepogodiev D, Doughty H, Bowley DM. Meta-analysis of plasma to red blood cell ratios and mortality in massive blood transfusions for trauma. Injury. 2012:pii. doi: 10.1016/j.injury.2012.07.193. S0020–1383(12)00296–3. [DOI] [PubMed] [Google Scholar]
- 21.Johansson PI, Oliveri RS, Ostrowski SR. Hemostatic resuscitation with plasma and platelets in trauma. J Emerg Trauma Shock. 2012;5:120–5. doi: 10.4103/0974-2700.96479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutton RP. Resuscitative strategies to maintain homeosta-sis during damage control surgery. Br J Surg. 2012;99(Suppl 1):21–8. doi: 10.1002/bjs.7731. [DOI] [PubMed] [Google Scholar]
- 23.Kautza BC, Cohen MJ, Cuschieri J, Minei JP, Brackenridge SC, Maier RV, Harbrecht BG, Moore EE, Billiar TR, Peitz-man AB, Sperry JL. Changes in massive transfusion over time: an early shift in the right direction? J Trauma Acute Care Surg. 2012;72:106–11. doi: 10.1097/TA.0b013e3182410a3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holcomb JB, Zarzabal LA, Michalek JE, Kozar RA, Spinella PC, Perkins JG, Matijevic N, Dong JF, Pati S, Wade CE, Cotton BA, Brasel KJ, Vercruysse GA, MacLeod JB, Dutton RP, Hess JR, Duchesne JC, McSwain NE, Muskat PC, Johan-nigamn JA, Cryer HM, Tillou A, Cohen MJ, Pittet JF, Marin B, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(Suppl 3):S318–28. doi: 10.1097/TA.0b013e318227edbb. [DOI] [PubMed] [Google Scholar]
- 25.Harm SK, Delaney M, Charapata M, Aubuchon JP, Triulzi DJ, Yazer MH. Routine use of a rapid test to detect bacteria at the time of issue for nonleukoreduced, whole blood-derived platelets. Transfusion. 2013 doi: 10.1111/j.1537-2995.2012.03818.x. [DOI] [PubMed] [Google Scholar]
- 26.Engelfriet CP, Reesink HW, Blajchman MA, Muylle L, Kjeldsen-Kragh J, Kekomaki R, Yomtovian R, Hocker P, Stiegler G, Klein HG, Soldan K, Barbara J, Slopecki A, Rob-inson A, Seyfried H. Bacterial contamination of blood components. Vox Sang. 2000;78:59–67. doi: 10.1159/000031151. [DOI] [PubMed] [Google Scholar]
- 27.Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability—deleterious effect of refrigerated storage. N Engl J Med. 1969;280:1094–8. doi: 10.1056/NEJM196905152802004. [DOI] [PubMed] [Google Scholar]
- 28.Inaba K, Branco BC, Rhee P, Blackbourne LH, Holcomb JB, Spinella PC, Shulman I, Nelson J, Demetriades D. Impact of the duration of platelet storage in critically ill trauma patients. J Trauma. 2011;71:1766–73. doi: 10.1097/TA.0b013e31823bdbf9. discussion 1773–4. [DOI] [PubMed] [Google Scholar]
- 29.Brasel KJ, Vercruysse G, Spinella PC, Wade CE, Black-bourne LH, Borgman MA, Zarzabal LA, Du F, Perkins JG, Maegele M, Schreiber M, Hess JR, Jastrow KM, 3rd, Gonza-lez EA, Holcomb JB, Kozar R, MacLeod J, Dutton RP, Duch-esne JC, McSwain NE, Muskat P, Johannigamn J, Cryer HM, Pittet JF, Marin B, et al. The association of blood component use ratios with the survival of massively transfused trauma patients with and without severe brain injury. J Trauma. 2011;71(Suppl 3):S343–52. doi: 10.1097/TA.0b013e318227ef2d. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmeister KM, Felbinger TW, Falet H, Denis CV, Berg-meier W, Mayadas TN, von Andrian UH, Wagner DD, Stossel TP, Hartwig JH. The clearance mechanism of chilled blood platelets. Cell. 2003;112:87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmeister KM. The role of lectins and glycans in platelet clearance. J Thromb Haemost. 2011;9(Suppl 1):35–43. doi: 10.1111/j.1538-7836.2011.04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmeister KM, Josefsson EC, Isacc NA, Clausen H, Hartwig JH, Stossel TP. Glycosylation restores survival of chilled blood platelets. Science. 2003;301:1531–4. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 33.Valeri CR. Circulation and hemostatic effectiveness of platelets stored at 4°C or 22°C: studies in aspirin-treated normal volunteers. Transfusion. 1976;16:20–3. doi: 10.1046/j.1537-2995.1976.16176130832.x. [DOI] [PubMed] [Google Scholar]
- 34.Hillyer CD, Josephson CD, Blajchman MA, Vostal JG, Epstein JS, Goodman JL. Bacterial contamination of blood components: risks, strategies, and regulation: joint ASH and AABB educational session in transfusion medicine. Hematology Am Soc Hematol Educ Program. 2003:575–89. doi: 10.1182/asheducation-2003.1.575. [DOI] [PubMed] [Google Scholar]
- 35.Cancelas JA, Rugg N, Fletcher D, Pratt PG, Worsham DN, Dunn SK, Marschner S, Reddy HL, Goodrich RP. In vivo viability of stored red blood cells derived from riboflavin plus ultraviolet light-treated whole blood. Transfusion. 2011;51:1460–8. doi: 10.1111/j.1537-2995.2010.03027.x. [DOI] [PubMed] [Google Scholar]
- 36.Marschner S, Goodrich R. Pathogen reduction technology treatment of platelets, plasma and whole blood using ribo-flavin and UV light. Transfus Med Hemother. 2011;38:8–18. doi: 10.1159/000324160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodrich RP, Doane S, Reddy HL. Design and development of a method for the reduction of infectious pathogen load and inactivation of white blood cells in whole blood products. Biologicals. 2010;38:20–30. doi: 10.1016/j.biologicals.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Galan AM, Lozano M, Molina P, Navalon F, Marschner S, Goodrich R, Escolar G. Impact of pathogen reduction technology and storage in platelet additive solutions on platelet function. Transfusion. 2011;51:808–15. doi: 10.1111/j.1537-2995.2010.02914.x. [DOI] [PubMed] [Google Scholar]
- 39.Marschner S, Fast LD, Baldwin WM, III, Slichter SJ, Goo-drich RP. White blood cell inactivation after treatment with riboflavin and ultraviolet light. Transfusion. 2010;50:2489–98. doi: 10.1111/j.1537-2995.2010.02714.x. [DOI] [PubMed] [Google Scholar]
- 40.Fast LD, Nevola M, Tavares J, Reddy HL, Goodrich RP, Mar-schner S. Treatment of whole blood with riboflavin plus ultraviolet light, an alternative to gamma irradiation in the prevention of transfusion-associated graft-versus-host disease? Transfusion. 2013 doi: 10.1111/j.1537-2995.2012.03715.x. [DOI] [PubMed] [Google Scholar]
- 41.Tonnetti L, Thorp AM, Reddy HL, Keil SD, Goodrich RP, Leiby DA. Evaluating pathogen reduction of Trypanosoma cruzi with riboflavin and ultraviolet light for whole blood. Transfusion. 2012;52:409–16. doi: 10.1111/j.1537-2995.2011.03285.x. [DOI] [PubMed] [Google Scholar]
- 42.Thiele T, Sablewski A, Iuga C, Bakchoul T, Bente A, Gorg S, Volker U, Greinacher A, Steil L. Profiling alterations in platelets induced by amotosalen/UVA pathogen reduction and gamma irradiation—a LC-ESI-MS/MS-based proteomics approach. Blood Transfus. 2012;10(Suppl 2):s63–70. doi: 10.2450/2012.010S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinmann E, Gravemann U, Friesland M, Doerrbecker J, Muller TH, Pietschmann T, Seltsam A. Two pathogen reduction technologies—methylene blue plus light and shortwave ultraviolet light—effectively inactivate hepatitis C virus in blood products. Transfusion. 2013 doi: 10.1111/j.1537-2995.2012.03858.x. [DOI] [PubMed] [Google Scholar]
- 44.Seltsam A, Muller TH. UVC irradiation for pathogen reduction of platelet concentrates and plasma. Transfus Med Hemother. 2011;38:43–54. doi: 10.1159/000323845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Preston AE, Barr A. The plasma concentration of factor VIII in the normal population. II. The effects of age, sex and blood group. Br J Haematol. 1964;10:238–45. doi: 10.1111/j.1365-2141.1964.tb00698.x. [DOI] [PubMed] [Google Scholar]
- 46.O’Donnell J, Boulton FE, Manning RA, Laffan MA. Amount of H antigen expressed on circulating von Willebrand factor is modified by ABO blood group genotype and is a major determinant of plasma von Willebrand factor antigen levels. Arterioscler Thromb Vasc Biol. 2002;22:335–41. doi: 10.1161/hq0202.103997. [DOI] [PubMed] [Google Scholar]
- 47.Goodrich RP, Li J, Pieters H, Crookes R, Roodt J, Heyns Adu P. Correlation of in vitro platelet quality measurements with in vivo platelet viability in human subjects. Vox Sang. 2006;90:279–85. doi: 10.1111/j.1423-0410.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 48.Babic AM, Josefsson EC, Bergmeier W, Wagner DD, Kaufman RM, Silberstein LE, Stossel TP, Hartwig JH, Hoffmeister KM. In vitro function and phagocytosis of galactosylated platelet concentrates after long-term refrigeration. Transfusion. 2007;47:442–51. doi: 10.1111/j.1537-2995.2007.01134.x. [DOI] [PubMed] [Google Scholar]
- 49.Rumjantseva V, Grewal PK, Wandall HH, Josefsson EC, Sorensen AL, Larson G, Marth JD, Hartwig JH, Hoffmeister KM. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15:1273–80. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. Transfus Apher Sci. 2010;42:63–70. doi: 10.1016/j.transci.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorensen AL, Hoffmeister KM, Wandall HH. Glycans and glycosylation of platelets: current concepts and implications for transfusion. Curr Opin Hematol. 2008;15:606–11. doi: 10.1097/MOH.0b013e328313e3bd. [DOI] [PubMed] [Google Scholar]