Summary
A “hot spot” magnetic resonance (MR) imaging cell tracking technique has been developed that allows direct detection of dysprosium- or thulium-1,4,7,10-tetraazacyclododecane-α,α′,α″,α‴-tetramethyl-1,4,7,10-tetraacetic acid (DOTMA)–labeled protons inside cells. These highly shifted protons may allow specific detection of multiple cell types because it does not rely on acquiring the proton signal from bulk water.
The Setting
According to www.clinicaltrials.gov, there are currently more than 25 000 clinical trials registered with the National Institute of Health that involve the use of cell therapy. This form of treatment is aimed at regenerating tissue by using stem cells, attacking cancer by using immune cells, or suppressing autoimmune disease by using both. Although the results of many of these studies have shown improvement of disease outcomes, researchers have realized that cells must be tracked in vivo to further optimize cell therapy and achieve maximum benefit. MR imaging appears to be ideally suited for this purpose because it can provide detailed anatomic information, uses no radiation, and is widely available. MR cell tracking has become an actively pursued field of bio-medicine. It involves labeling the cells of interest (1), with the currently available labels being paramagnetic chelates, paramagnetic chemical exchange saturation transfer agents, superparamagnetic iron oxide particles, and fluorinated agents. None of these agents is perfect, because each provides either low sensitivity or low specificity. An agent that delivers true specificity for the cells of interest while maintaining sufficient sensitivity is highly desirable. In this issue, Schmidt et al (2) describe an approach toward achieving this goal by detecting labeled intracellular protons specifically, with sensitivity to approximately 100 µmol/L.
The Science
The principle behind highly shifted proton (HSP) MR imaging is that certain lanthanides, in particular thulium (Tm) and dysprosium (Dy), cause a chemical shift of proton resonance frequency far away (100 ppm) from the water peak (from which the MR imaging signal is normally collected) (Figure). This phenomenon has been known since the early days of nuclear MR as applied in chemistry. The authors have used a customized pulse sequence, termed “ultrashort” echo-time MR imaging, to take advantage of the dramatically shortened T1 value of the protons bound to the chelate, which is approximately 2–5 msec at 9.4 T. By applying selective windowing, they were able to collect the MR imaging signal of the 12 highly shifted chelate-bound protons specifically. This contrast mechanism should not be confused with paramagnetic chemical exchange saturation transfer imaging (3), where a saturation pulse is applied off resonance and the reduced bulk water proton signal is collected after proton chemical exchange.
Figure.
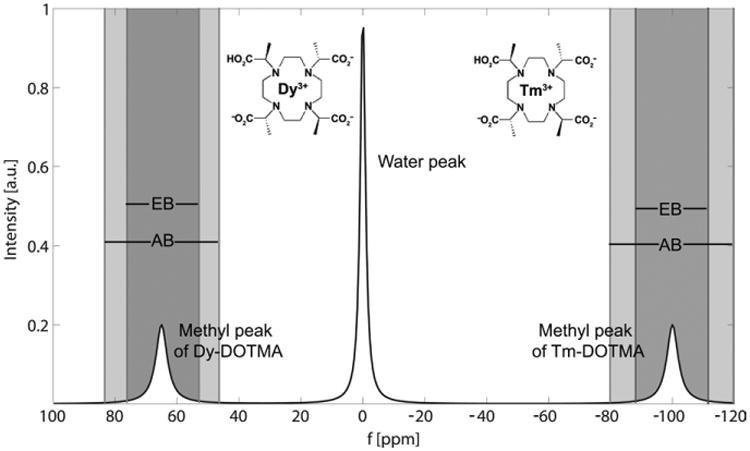
To test proof-of-concept, two different experiments were performed. In the first case, human fibrosarcoma tumor cells were labeled with Tm-DOTMA by means of electroporation. Four million cells were then injected into the flank of nude mice. HSP MR imaging was performed at 9.4 T, with the DOTMA methyl group protons separated from bulk water by using both a narrow acquisition and an excitation bandwidth placed at the center of the methyl proton peak. The tumor could be detected clearly as a hot spot on HSP MR images, and overlays with conventional T2-weighted MR images allowed their anatomic localization. The results were validated by means of elemental bioimaging of excised tumor tissue, which showed an excellent match.
In the second experiment, macrophages were labeled by means of spontaneous pinocytosis of Tm-DOTMA and were injected intravenously in mice that were implanted with polyacrylamide gel pellets. In this granuloma model, the injected macrophages were expected to home in on areas of inflammation. Cells were clearly visible as hot spots on HSP MR images for a period of at least 1 week. The liver and spleen, organs rich in macrophage-type cells, also showed a substantial presence of highly shifted protons. Again, the imaging results were well correlated with histologic and bioelemental imaging results. Furthermore, under the used labeling conditions, the lanthanide labeling did not appear to affect cell viability or cell function as assessed by means of measurement of cell death, cell adhesion, phagocytosis, or nitroxide radical production.
The Practice
Clinical use
Hot spot imaging without background signal, as shown here, may greatly improve the specificity and interpretation of MR imaging cell tracking (4), particularly in cases in which a priori overall cell distribution is unknown (eg, after systemic administration). In this context, HSP MR imaging is somewhat comparable to fluorine 19 (19F) imaging, in which the MR imaging signal comes only from fluorinated cells. In a comparison of sensitivity, HSP MR imaging and 19F MR imaging are also a good match, with an approximate lower cell detection number of 1 × 104 cells. This number is still far lower than that with the use of superparamagnetic iron oxide–labeled cells, which has now been researched in multiple trials of clinical MR imaging cell tracking (5). While the lower field strength of clinical MR imagers will decrease detection sensitivity for HSP MR imaging, it also remains to be seen if HSP MR imaging cell tracking can be safely conducted in patients. A long-term retention of lanthanides gives rise to toxicity concerns when the label is not rapidly cleared from the body, as exemplified in the case of gadolinium chelates in patients with impaired kidney function.
Future opportunities and challenges
Schmidt et al introduced an additional feature that may be used to fulfill the wish of many biologists: to pursue cell tracking of multiple cell types simultaneously. When using Dy-DOTMA, which has a large chemical shift from Tm-DOTMA on the opposite side of the water peak, the authors of this study showed that, by choosing the appropriate excitation and acquisition window, it is possible to distinguish clearly the two lanthanides from each other. Analogous approaches for dual-cell labeling include the use of two perfluorocarbons (6) or two paramagnetic chemical exchange saturation transfer agents (7), where the differential resonance frequencies are exploited. Additional dual-cell labeling experiments will be needed, but the ever-present requirements of specificity and sensitivity appear to have been met.
Footnotes
Disclosures of Conflicts of Interest: J.W.M.B. Activities related to the present article: patent US8236572 issued. Activities not related to the present article: founder and owner of SenCEST. Other relationships: disclosed no relevant conflicts of interest.
References
- 1.Ahrens ET, Bulte JW. Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol. 2013;13(10):755–763. doi: 10.1038/nri3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt R, Nippe N, Strobel K, et al. Highly shifted proton MR imaging: cell tracking by using direct detection of paramagnetic compounds. Radiology. 2014;272(3):785–795. doi: 10.1148/radiol.14132056. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Winter P, Wu K, Sherry AD. A novel europium(III)-based MRI contrast agent. J Am Chem Soc. 2001;123(7):1517–1518. doi: 10.1021/ja005820q. [DOI] [PubMed] [Google Scholar]
- 4.Bulte JW. Hot spot MRI emerges from the background. Nat Biotechnol. 2005;23(8):945–946. doi: 10.1038/nbt0805-945. [DOI] [PubMed] [Google Scholar]
- 5.Bulte JWM. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol. 2009;193(2):314–325. doi: 10.2214/AJR.09.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partlow KC, Chen J, Brant JA, et al. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 2007;21(8):1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 7.Aime S, Carrera C, Delli Castelli D, Geninatti Crich S, Terreno E. Tunable imaging of cells labeled with MRI-PARACEST agents. Angew Chem Int Ed Engl. 2005;44(12):1813–1815. doi: 10.1002/anie.200462566. [DOI] [PubMed] [Google Scholar]


