SUMMARY
Treslin helps to trigger the initiation of DNA replication by promoting integration of Cdc45 into the replicative helicase. Treslin is a key positive-regulatory target of cell cycle control mechanisms; activation of Treslin by cyclin-dependent kinase is essential for the initiation of replication. Here we demonstrate that Treslin is also a critical locus for negative regulatory mechanisms that suppress initiation. We found that the checkpoint-regulatory kinase Chk1 associates specifically with a C-terminal domain of Treslin (designated TRCT). Mutations in the TRCT domain abolish binding of Chk1 to Treslin and thereby eliminate Chk1-catalyzed phosphorylation of Treslin. Significantly, abolition of the Treslin-Chk1 interaction results in elevated initiation of chromosomal DNA replication during an unperturbed cell cycle, which reveals a function for Chk1 during a normal S-phase. This increase is due to enhanced loading of Cdc45 onto potential replication origins. These studies provide important insights into how vertebrate cells orchestrate proper initiation of replication.
INTRODUCTION
In eukaryotic cells, duplication of the genome depends upon the intricate, stepwise assembly of protein complexes onto origins of DNA replication (Sclafani and Holzen, 2007; Siddiqui et al., 2013; Tanaka and Araki, 2013). Initially, the origin recognition complex (ORC) and Cdc6 associate with potential origins and thereupon recruit Cdt1 and the mini-chromosome maintenance (MCM) complex. The MCM complex serves as the core of the replicative helicase that unwinds the DNA strands for replication. A key regulatory juncture in replication involves the concerted binding of additional proteins to the MCM complex to form the mature, activated version of the helicase. In particular, the Cdc45 and GINS proteins associate with the MCM proteins and thereby form the CMG (Cdc45-MCM-GINS) complex, which corresponds to the fully constituted helicase.
In vertebrates, integration of Cdc45 and GINS with the MCMs depends upon TopBP1 and a recently discovered TopBP1-binding protein called Treslin (also known as Ticrr)(Kumagai et al., 2010; Sansam et al., 2010). Importantly, formation of the TopBP1-Treslin complex requires phosphorylation of Treslin by the S-phase cyclin-dependent kinase (S-CDK)(Boos et al., 2011; Kumagai et al., 2011). Consequently, this phosphorylation helps to explain how the cell cycle control system dictates the timing of S-phase. An analogous situation exists in budding yeast where phosphorylation of the Treslin homologue Sld3 by S-CDK is also critical for replication (Labib, 2010; Siddiqui et al., 2013; Tanaka and Araki, 2013).
Treslin is a relatively large protein (220 kD) that is approximately three times bigger than yeast Sld3. Thus, Treslin may have acquired new properties that allow it to meet the more complex demands of higher eukaryotes. To obtain further insight into established and potentially novel functions of Treslin, we have engaged in a search for Treslin-interacting proteins in human cells. These studies resulted in the identification of Chk1, an effector kinase in checkpoint control mechanisms. Chk1 is best known for its role in blocking activation of CDKs in cells with incompletely replicated or damaged DNA (Perry and Kornbluth, 2007; Toledo et al., 2011).
Significantly, further studies have indicated that Chk1 also plays a role during a seemingly normal cell cycle. For example, a number of observations have implicated Chk1 in the control of replication during an unperturbed S-phase (Maya-Mendoza et al., 2007; McIntosh and Blow, 2012; Miao et al., 2003; Syljuasen et al., 2005). The mechanism by which Chk1 exerts these effects is obscure. Chk1 also participates in suppressing endoreplication in trophoblast stem (TS) cells (Ullah et al., 2011). Moreover, Chk1 appears to function in the system that monitors correct attachment of chromosomes to the mitotic spindle (Zachos et al., 2007). Overall, these observations suggest that Chk1 has diverse roles in cell cycle regulation, which may help to explain why it is essential for viability (Liu et al., 2000). Therefore, it will be important to understand how cells control the participation of Chk1 in these varied functions.
In this report, we have investigated the molecular mechanism and functional consequences of the Treslin-Chk1 interaction in both human cells and Xenopus egg extracts. We show that Chk1 negatively regulates the Treslin-mediated loading of Cdc45 onto chromatin and thereby serves to antagonize the initiation of replication. These studies provide an important new perspective on how vertebrate cells control the initiation of DNA replication through opposing negative and positive regulatory mechanisms. Furthermore, these experiments reveal the mechanistic basis for a critical function of Chk1 apart from its role in checkpoint responses to damaged DNA.
RESULTS
Chk1 Is a Treslin-interacting Protein
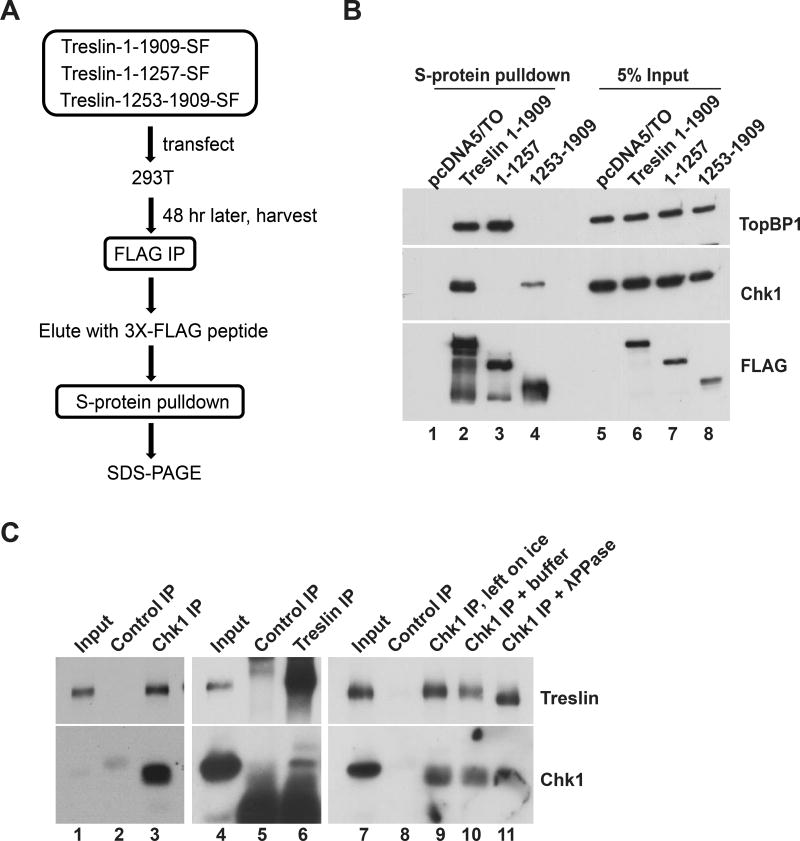
To search for Treslin-interacting proteins, we expressed various tagged versions of Treslin in human 293T cells, re-isolated these polypeptides, and then analyzed associated proteins by mass spectrometry (Figure 1A). For these experiments, we produced recombinant full-length human Treslin with both S-peptide and 3X-FLAG tags at the C-terminal end (designated Treslin-SF). We also prepared tagged fragments corresponding to residues 1-1257 and 1253-1909 of the protein. The 1-1257 fragment can restore DNA replication to Treslin-depleted cells (Kumagai et al., 2011). Thus, the remaining C-terminal domain of Treslin may have some regulatory role. For this study, we focused on proteins that might bind selectively to this area.
Figure 1. Identification of Chk1 as a Treslin-interacting Protein in Human Cells.
(A) Procedure for isolation of Treslin-interacting proteins.
(B) Nuclear lysates from 293T cells expressing tag only (lanes 1 and 5) or indicated forms of Treslin-SF (lanes 2–4 and 6–8) were incubated with S-protein agarose (lanes 1–4). Beads were immunoblotted with anti-TopBP1 (top), anti-Chk1 (middle), and anti-FLAG (bottom). Input lysates, lanes 5–8.
(C) Nuclear lysates from 293T cells were incubated with control IgG (lanes 2, 5, and 8) or antibodies against Chk1 (lanes 3, 9, 10, and 11) or Treslin (lane 6) bound to magnetic beads. In right panel, bead-bound anti-Chk1 immunoprecipitates (lanes 9–11) were either left on ice (lane 9) or incubated without (lane 10) or with 20 U/μl lambda phosphatase (lane 11) for 30 min at room temperature. Immunoprecipitated proteins and input lysates were immunoblotted for Treslin (top) and Chk1 (bottom). See also Figure S1 and Table S1.
We identified Chk1 as a protein that associated with full-length Treslin and the C-terminal 1253-1909 fragment, but not the N-terminal 1-1257 fragment (see Experimental Procedures and Table S1). To validate these findings, we subjected S-protein pulldowns from cells to immunoblotting with anti-Chk1 antibodies (Figure 1B). We likewise observed binding of Chk1 to both the full-length protein and C-terminal fragment. Conversely, as expected from previous studies, TopBP1 associated with the N-terminal but not C-terminal fragment (Kumagai et al., 2011). We also performed reciprocal immunoprecipitation experiments in human cells; we detected the presence of Treslin in anti-Chk1 immunoprecipitates and Chk1 in anti-Treslin immunoprecipitates, respectively (Figure 1C). Treatment with ethidium bromide or Benzonase did not inhibit co-immunoprecipitation of Treslin and Chk1, which rules out bridging of these proteins by DNA (Figure S1). Finally, to address whether this binding required phosphorylation, we treated the anti-Chk1 immunoprecipitates with lambda phosphatase. Although the phosphatase treatment appeared to be effective, as indicated by increased electrophoretic mobility of Treslin, we observed no decrease in binding of Chk1 to Treslin (Figure 1C). Overall, we conclude that Chk1 associates specifically with Treslin in human cells.
Mapping of the Chk1-interacting Domain in Treslin
To investigate the molecular basis of this interaction, we set out to map a Chk1-interacting region in Treslin. We engineered various sub-fragments of the 1253-1909 fragment, expressed them in human cells, performed S-protein pulldowns, and immunoblotted for Chk1. We found that approximately 100 amino acids at the C-terminal end of the protein were necessary for binding to Chk1 (Figures 2A and 2B). We proceeded to show that a 100 amino acid fragment from the C-terminal end (residues 1810-1909) was also sufficient for binding. The sequence of this region is well conserved in the Xenopus and zebrafish homologues of Treslin (Figure 2C). Accordingly, we named this region the TRCT (Treslin C-terminal) domain.
Figure 2. Mapping of the Region in Treslin that Associates with Chk1.
(A) Indicated deletion mutants of Treslin were expressed in 293T cells. Nuclear lysates were incubated with S-protein agarose. Bound proteins and nuclear lysates were immunoblotted with anti-Chk1 (top) and anti-FLAG (bottom).
(B) Abilities of various fragments of Treslin to interact with Chk1. Data from panels A and D; interacting fragments shown in bold.
(C) Amino acids 1810-1909 of human Treslin and corresponding portions of the X. laevis and zebrafish proteins were aligned with the Clustal Omega program. Amino acids with asterisks (S1887, S1893, and T1897) or a line (1846-LTQSPLL-1852) were subjected to mutagenesis.
(D) GST only (lane 2), GST-tagged TRCT (lane 3), TRCT containing mutations S1887A (lane 4), S1893A (lane 5), T1897A (lane 6), or 7A (lane 7), and GST-tagged forms of residues 1810-1872 (lane 8) and 1870-1909 from Treslin (lane 9) were isolated from bacteria with glutathione agarose. Bead-bound fragments were incubated with 293T nuclear lysates. Beads were processed for immunoblotting with anti-Chk1 (top) and staining with Coomassie blue (bottom). Lane 1, input nuclear lysate.
(E) Nuclear lysates from 293T cells expressing SF tag only (lanes 1 and 5) or indicated forms of Treslin-SF (lanes 2–4 and 6–8) were incubated with S-protein agarose. Input lysates (lanes 1–4) and retrieved bead fractions (lanes 5–8) were immunoblotted as indicated. See also Figure S2.
To characterize the TRCT, we prepared this domain as a GST fusion protein (Figure 2D). The purified GST-TRCT bound well to Chk1 in human cell lysates. By contrast, GST-tagged sub-fragments of this region (e.g., residues 1810-1872 and 1870-1909) did not associate with Chk1 significantly, which suggests that the whole domain is necessary for binding. The most conserved stretch within the human TRCT corresponds to the sequence LTQSPLL at positions 1846-1852. This sequence is identical in Xenopus and zebrafish homologues of Treslin, but is not present in budding yeast Sld3. We mutated each residue in this sequence to alanine (to create the 7A mutant) in the context of both the GST-TRCT construct and the full-length Treslin-SF protein. We found that 7A-mutant versions of these polypeptides were completely defective for binding to Chk1 (Figures 2D and 2E). Notably, the 7A mutant of full-length Treslin bound TopBP1 normally. We also identified three residues within the TRCT (S1887, S1893, and T1987) that sit in consensus sequences for phosphorylation by Chk1 (RXXS/T). However, mutation of any one of these residues to alanine had no effect on the binding to Chk1 (Figure 2D). Finally, we assessed whether Treslin associates with Chk1 directly. For this purpose, we incubated the TRCT domain with purified, recombinant Chk1 (Figure S2). We observed that the WT TRCT bound to isolated Chk1 very efficiently, whereas there was virtually no binding of the 7A mutant. Since no phosphorylation could occur under these conditions, this observation reinforces the concept that phosphorylation of the TRCT is not necessary for binding to Chk1. Overall, these results indicate that Chk1 associates directly in a highly specific manner with sequences in the C-terminal region of Treslin.
The TRCT Domain Promotes Chk1-catalyzed Phosphorylation of Treslin
One explanation for the binding of Chk1 to the TRCT would be that it facilitates phosphorylation of Treslin by Chk1. To address this possibility, we first examined whether Treslin could serve as a substrate of Chk1. We initially tested the GST-TRCT construct and found wild-type (WT) recombinant Chk1 but not kinase-dead (KD) Chk1 could phosphorylate this fragment well (Figure 3A). By contrast, there was no phosphorylation of the mutant GST-TRCT-7A fragment by Chk1 above background levels. The S1893A and T1897A mutants of the TRCT domain showed reduced phosphorylation by Chk1, while the S1887A mutant was still an equally good substrate. A combined S1893A/T1897A mutant displayed near background levels of phosphorylation by Chk1 (Figure S3A), which suggests that these positions are the two main in vitro phosphorylation sites within this domain. As anticipated, this mutant also still bound normally to Chk1 (Figure S3B). Phosphorylation of the TRCT domain by Chk1 appears to be quite efficient. For comparison, phosphorylation of a GST-tagged peptide from Cdc25 that contains a single well-documented site for Chk1 (Kumagai et al., 1998) was about 2-fold lower (Figure S3C). Finally, we likewise examined full-length Treslin and found that the WT but not 7A-mutant protein could serve as good substrate for Chk1 (Figures 3B and 3C). Taken together, these results indicate that docking of Chk1 onto the TRCT domain strongly stimulates phosphorylation of Treslin by Chk1.
Figure 3. The TRCT Domain Promotes the Phosphorylation of Treslin by Chk1.
(A) GST (lanes 1–2), GST-TRCT (lanes 3–4), and versions of this fragment containing the S1887A (lanes 5–6), S1893A (lanes 7–8), T1897A (lanes 9–10), or 7A mutations (lanes 11–12) were incubated with recombinant WT Chk1 (lanes 1, 3, 5, 7, 9, and 11) or KD Chk1 (lanes 2, 4, 6, 8, 10, and 12). See Supplemental Experimental Procedures for more details. Reactions were processed for phosphorimaging (top) and Coomassie blue staining (bottom).
(B) Nuclear lysates from human 293T cells expressing SF tag only (lane 1), full-length Treslin-SF WT (lane 2), or Treslin-SF 7A (lane 3) were processed for purification with anti-FLAG beads. Beads were stained with Coomassie blue.
(C) Control (lanes 1–2), Treslin-SF WT (lanes 3–4), and Treslin-SF 7A (lanes 5–6) were incubated with WT Chk1 (lanes 1, 3, and 5) or KD Chk1 (lanes 2, 4, and 6) in kinase buffer. Reactions were processed for phosphorimaging.
(D) Parental U2OS T-REx cells and T-REx cells harboring either WT or 7A siRNA-resistant Treslin were cultured with doxycycline. Cells were treated with either control or Treslin siRNA. At 72 hr, cells were labeled with 10 μM EdU for 1 hr. EdU incorporation was determined with the Click-iT reaction and Alexa 488 dye (Kumagai et al., 2010). Error bars, mean ± S.E.M. (n=3).
(E) T-REx cells (lanes 1–4) and T-REx cells harboring siRNA-resistant WT (lanes 5–8) or 7A Treslin (lanes 9–12) were cultured with doxycycline. Cells were also treated with either control siRNA (lanes 1–2, 5–6, and 9–10) or Treslin siRNA (lanes 3–4, 7–8, and 11–12). At 72 hr, cells were incubated in the absence (lanes 1, 3, 5, 7, 9, and 11) or presence of 10 μg/ml APH (lanes 2, 4, 6, 8, 10, and 12) for 30 min. Cell lysates were immunoblotted as indicated. See also Figure S3.
We employed mass spectrometry to identify in vitro phosphorylation sites for Chk1 on full-length Treslin. This analysis resulted in the identification of numerous sites throughout much of the protein (not shown). For these studies, we decided to focus on using the 7A mutant to investigate the functional significance of the Treslin-Chk1 interaction. This mutant is not an effective substrate for Chk1 in vitro and most likely in vivo. Furthermore, this approach would also address the possibility that the physical association of Chk1 with Treslin may also have a regulatory impact apart from phosphorylation.
Binding of Treslin Is Not Essential for Activation of Chk1
We previously demonstrated that ablation of Treslin from human cells compromises both DNA replication and phosphorylation of Chk1 upon treatment with aphidicolin (APH) (Kumagai et al., 2010). In principle, the latter defect could reflect a direct role for Treslin in the activation of Chk1. Alternatively, this effect could be indirectly due to the absence of replication forks in cells without Treslin. Because Treslin associates with Chk1, we asked whether Treslin is directly necessary for activation of Chk1 in APH-treated cells. For this purpose, we utilized lines of U2OS cells in which expression of siRNA-resistant versions of WT and 7A Treslin was under the control of a doxycycline-inducible promoter (see Experimental Procedures). To characterize these cell lines, we first used labeling with EdU to assess whether the 7A mutant could rescue DNA replication in Treslin siRNA-treated cells. We found that the percentages of EdU-positive nuclei were similar for Treslin-ablated cells expressing WT or 7A Treslin (Figure 3D).
Next, we examined phosphorylation of Chk1 in cells treated with APH. As expected, treatment of the parental U2OS cell line with APH in the presence of control siRNA efficiently induced the phosphorylation of Chk1 on S345 (Figure 3E). As described previously, treatment with Treslin siRNA resulted in markedly reduced phosphorylation of Chk1. We proceeded to show that expression of either WT or 7A siRNA-resistant Treslin could efficiently rescue phosphorylation of Chk1 in APH-treated cells that had been treated with the Treslin siRNA. These observations indicate that the binding of Chk1 to the TRCT domain is not essential for activation of Chk1 in response to APH.
The Isolated TRCT Domain of Treslin Stimulates DNA Replication in Xenopus Egg Extracts
We next considered the possibility that Chk1 regulates DNA replication by associating with the TRCT domain. As one approach to examine this question, we utilized various types of extracts from Xenopus eggs that recapitulate DNA replication in a cell-free reaction. In particular, we utilized the nucleoplasmic extract (NPE) system, in which replication occurs in a soluble nuclear fraction lacking membranes (Walter and Newport, 2000). We also used whole egg extracts in which DNA replication takes place in reconstituted nuclei.
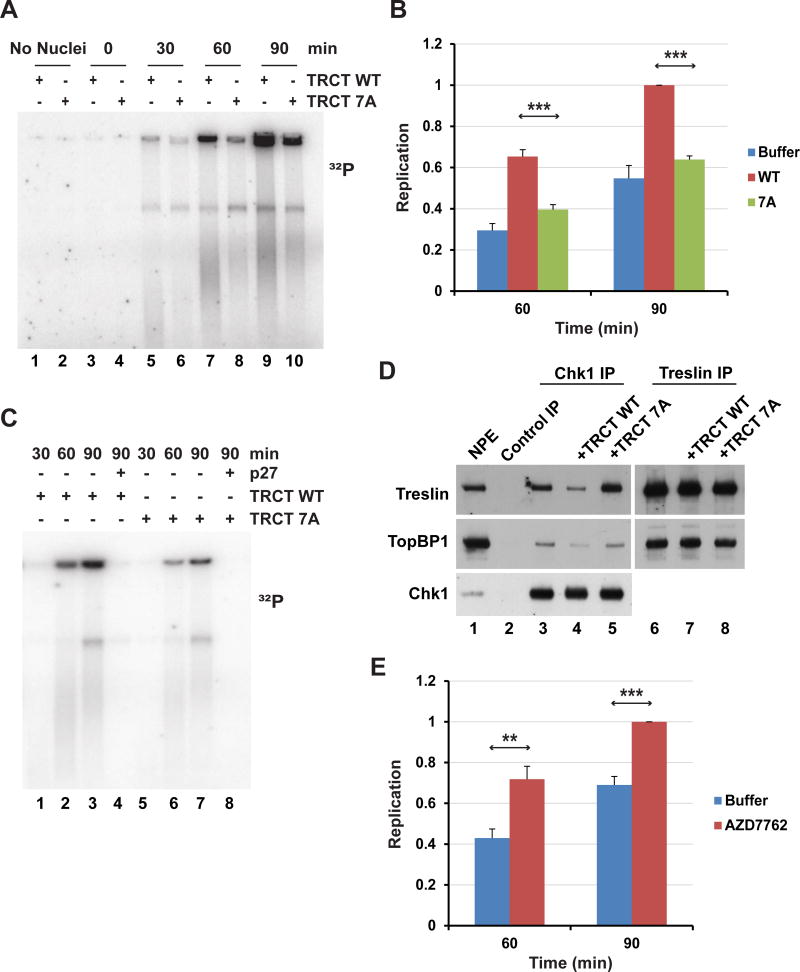
We reasoned that the isolated TRCT domain might act as a competitor of the interaction between endogenous Treslin and Chk1 in egg extracts. To explore this possibility, we added the GST-TRCT to the NPE system and then monitored the time-course of DNA replication. For this purpose, we assessed incorporation of radioactive phosphate from [α-32P]dATP into chromosomal DNA. We observed that addition of the TRCT fragment elicited a significant increase in DNA replication in comparison with samples treated with control buffer alone or the mutant TRCT-7A fragment (Figures 4A and 4B).
Figure 4. The Isolated TRCT Domain Stimulates DNA Replication in the Xenopus NPE System.
(A) HSS was incubated without (lanes 1–2) or with sperm nuclei (lanes 3–10) for 30 min. NPE and either WT (lanes 1, 3, 5, 7, and 9) or 7A TRCT (lanes 2, 4, 6, 8, and 10) were added to HSS. Final concentration of TRCT was 60 ng/μl. Replication was assessed by incorporation of 32P from [α-32P]dATP at indicated times.
(B) Quantitation of the data from panel A and similar experiments (4–6 total). Results for TRCT WT and 7A were compiled from six independent experiments. Data was normalized to replication in the presence of TRCT WT at 90 min (***, p < 0.001). Error bars, S.E.M.
(C) Similar as in (A), except in some cases NPE was pre-treated with 1 μM GST-p27 (lanes 4 and 8).
(D) NPE incubated for 30 min with buffer only (lanes 2, 3, and 6) or 60 ng/μl WT (lanes 4 and 7) or 7A TRCT (lanes 5 and 8). Samples were immunoprecipitated with control IgG (lane 2), anti-Chk1 (lanes 3–5), or anti-Treslin antibodies (lanes 6–8) bound to protein A magnetic beads. Beads were processed for immunoblotting with anti-Treslin (top), anti-TopBP1 (middle), and anti-Chk1 (bottom). NPE (0.5 μl) was loaded in lane 1.
(E) Quantitation of data from seven independent experiments on replication in the NPE system with added buffer alone or 0.5 μM AZD7762. Data was normalized to replication in the presence of AZD7762 at 90 min (**, p < 0.01; ***, p < 0.001). Error bars, S.E.M. See also Figure S4.
To assess whether this replication occurs by the normal CDK-mediated mechanism, we utilized the CDK inhibitor p27 (Figure 4C). We noted that there was no DNA replication in p27-treated extracts in either the presence or absence of the TRCT domain. To gauge whether the TRCT does actually prevent the binding of Chk1 to endogenous Treslin, we performed immunoprecipitation experiments with anti-Chk1 antibodies (Figure 4D). We could readily detect Treslin in anti-Chk1 immunoprecipitates from NPE fractions. Association of Treslin with Chk1 was not inhibited by treatment with ethidium bromide or Benzonase, agents that would prohibit bridging by DNA (Figure S4A). We observed that addition of the WT TRCT fragment to NPE fractions caused a severe reduction in the binding of Treslin to Chk1, whereas the 7A mutant fragment had no effect. By contrast, the TRCT domain had no effect on the binding of Treslin to TopBP1. Overall, these results indicated that blockage of the binding of Chk1 to Treslin results in elevated DNA replication.
Chk1 Regulates DNA Replication in Xenopus Egg Extracts
As another means to investigate the regulation of DNA replication by Chk1, we utilized the Chk1 inhibitor AZD7762. We found that this compound also elicited an increase in DNA replication comparable to that induced by the TRCT-WT fragment (Figure 4E and Figure S4B). We also attempted to immunodeplete Chk1 from the NPE system, which entails separate immunodepletion from the HSS and NPE fractions that are necessary for these experiments. However, these immunodepletion procedures resulted in a non-specific reduction of replication, which confounded this approach.
As an alternative, we attempted to deplete Chk1 from whole egg extracts (Kumagai et al., 1998). We first needed to assess whether the TRCT affects replication in whole egg extracts, which would require transport of the TRCT into reconstituted nuclei in these extracts. Since our initial GST construct lacked a nuclear localization sequence (NLS), we prepared a new construct with this sequence (GST-TRCT-NLS) (Figure 5A). We verified that both WT and 7A versions of the TRCT-NLS could be found in lysates of reconstituted nuclei from whole egg extracts. Unexpectedly, the WT but not 7A version of the TRCT-NLS also caused a dramatic accumulation of Chk1 in the nuclei (Figure 5B). A plausible explanation is that the TRCT-NLS, because of its robust binding to Chk1, may promote nuclear entry of Chk1.
Figure 5. Depletion of Chk1 from Egg Extracts Also Stimulates DNA Replication.
(A) Coomassie blue staining of purified GST-TRCT-NLS WT (lane 1) and 7A (lane 2).
(B) Egg extracts containing added buffer only (lane 1) or 75 ng/μl of either WT TRCT-NLS (lane 2) or 7A TRCT-NLS (lane 3) were incubated with sperm nuclei for 90 min. Nuclear lysates were immunoblotted for Treslin (top), Chk1 (middle), and GST (bottom).
(C) Egg extracts were supplemented with buffer only (lanes 1–3), 75 ng/μl WT TRCT-NLS (lanes 4–6), or 7A TRCT-NLS (lanes 7–9). DNA replication was measured at indicated times.
(D) Quantitation of the data from (C) and two additional experiments (mean ± S.E.M.). Paired Student’s t-tests for extracts treated with WT TRCT-NLS versus extracts treated with buffer only or 7A TRCT-NLS yielded p values < 0.001.
(E) Egg extracts were either mock-depleted with control IgG (lane 1) or depleted with anti-Xenopus Chk1 antibodies (lanes 2 and 3). His6-Chk1 was added back to Chk1-depleted extract (lane 3).
(F) The extracts from panel E were assayed for chromosomal DNA replication.
(G) Quantitation of the data from (F) and two additional experiments (mean ± S.E.M.). Paired Student’s t-tests for Chk1-depleted extracts versus mock-depleted and Chk1-depleted extracts containing recombinant Chk1 yielded p values < 0.05.
Next, we added the WT TRCT-NLS to whole egg extracts containing reconstituted nuclei and found that this fragment also elicited an increase in DNA replication relative to incubations containing control buffer or a 7A mutant version of the TRCT-NLS (Figures 5C and 5D). Finally, we removed Chk1 from whole egg extracts with anti-Chk1 antibodies (Figure 5E). We observed a significant acceleration of DNA replication in Chk1-depleted extracts (Figures 5F and 5G). Furthermore, addition of recombinant Chk1 to the depleted extracts restored replication to the lower level found in mock-depleted extracts. Taken together, these experiments provide multiple lines of evidence that Chk1 regulates DNA replication in the Xenopus system.
The Isolated TRCT Domain Promotes Initiation of Replication in Egg Extracts
A key function of Treslin involves the loading of Cdc45 onto chromatin (Kumagai et al., 2010). It has been well established that the loading of Cdc45 is critical for the initiation of DNA replication in both yeast and vertebrates (Sclafani and Holzen, 2007; Siddiqui et al., 2013; Tanaka and Araki, 2013). To explore the basis of the TRCT-mediated stimulation of replication, we examined the loading of Cdc45 onto chromatin in the NPE system in the presence of this fragment. We observed that addition of the TRCT-WT protein stimulated a large increase in the loading of Cdc45 onto chromatin in comparison with samples containing the TRCT-7A protein (Figure 6A). There was a similar increase in the loading of Sld5 (a component of GINS), PCNA, and DNA polymerase epsilon (Figure S5). The TRCT domain typically did not affect the binding of the ORC and MCM complexes to the DNA. These experiments suggest that the isolated TRCT domain promotes initiation by stimulating the loading of the helicase activators Cdc45 and GINS onto chromatin. Furthermore, we also observed an increase other replication-fork proteins, such as PCNA and DNA polymerase epsilon, which participate directly in the ensuing DNA synthesis.
Figure 6. The TRCT Domain Elicits Increased Loading of Cdc45 and Dysregulated Activation of Chk1.
(A) HSS was incubated without (lanes 1–2) or with sperm nuclei (lanes 3–10) for 30 min. NPE and either WT (lanes 1, 3, 5, 7, and 9) or 7A TRCT (lanes 2, 4, 6, 8, and 10) at a final concentration of 60 ng/μl were added to HSS. Chromatin was isolated at indicated times and immunoblotted with various antibodies. Xenopus ISWI served as loading control. NPE/HSS mixture (0.5 μl) was loaded in lanes 9–10.
(B) Similar as in (A), except NPE was treated with 10 μM actinomycin D as indicated (lanes 5–7).
(C) Distribution of inter-origin distances in Xenopus egg extracts containing added buffer alone, TRCT-NLS WT (75 ng/μl), or TRCT-NLS 7A (75 ng/μl). Results are from three independent experiments (mean ± S.E.M). p < 0.05 for Buffer versus WT and for WT versus 7A in the category of 10–15 kb using Student’s t-test. p < 0.05 for Buffer versus WT and p < 0.001 for WT versus 7A in the category of > 30 kb.
(D) HSS was incubated in the absence (lanes 1–6) or presence of sperm nuclei (lanes 7–14) for 30 min. NPE lacking (lanes 1–3, 7–9, and 13) or containing 50 μg/ml APH (lanes 4–6, 10–12, and 14) and buffer (lanes 1, 4, 7, and 10), TRCT WT (lanes 2, 5, 8, 11, 13, and 14), or TRCT 7A (lanes 3, 6, 9, and 12) were added to HSS. Final concentration of TRCT was 60 ng/μl. Mixtures were incubated for 90 min in the absence (lanes 1–12) or presence of 5 mM caffeine (lanes 13–14). Reactions were immunoblotted with anti-P-Chk1 (top), anti-Chk1 (middle) and anti-GST (bottom). See also Figures S5 and S6.
As another method to characterize this phenomenon, we attempted to block replication just after initiation. Actinomycin D blocks replication in the NPE system shortly after initiation but before significant unwinding of the DNA (Pacek and Walter, 2004). Accordingly, we added actinomycin D to the NPE system in the absence and presence of the TRCT domain. We found that actinomycin D caused a pronounced further accumulation of Cdc45 on the DNA in extracts containing the WT TRCT domain (Figure 6B). There was no effect in extracts containing the TRCT-7A mutant. Taken together, these experiments suggest that the TRCT domain promotes the initiation of replication. The fact that the TRCT domain blocks the binding of Chk1 to Treslin suggests that Chk1 suppresses the initiating function of Treslin.
To obtain further support for this concept, we performed DNA fiber studies in whole egg extracts following addition of the TRCT domain. For this purpose, we incubated extracts sequentially with digoxigenin-dUTP and biotin-16-dUTP, prepared DNA fibers from nuclear fractions, and then measured inter-origin distances (Bellelli et al., 2014; Marheineke et al., 2009). We observed that the WT TRCT fragment caused a significant increase in shorter inter-origin distances (e.g., 10–15 kb) relative to incubations containing added buffer alone or the 7A fragment (Figure 6C; Figures S6A and S6B). Concomitantly, there was a decrease in larger inter-origin distances (e.g., >30 kb) in incubations containing the WT fragment. These findings directly support an increase in the firing of origins, which fits well with the observations that the TRCT domain enhances formation of the activated replicative helicase.
Dysregulated Origin Firing in the Absence of the Treslin-Chk1 Interaction Induces Activation of Chk1
The elevated loading of Cdc45 onto chromatin and the ensuing increase in replication in the presence of the TRCT domain raised the possibility that this fragment might elicit replication stress. To address this issue, we examined phosphorylation of Xenopus Chk1 on S344 (Kumagai et al., 1998). We observed that the WT TRCT domain induced phosphorylation of Chk1 in NPE fractions containing sperm chromatin even in the absence of APH (Figure 6D). The TRCT domain also caused a substantial further increase in phosphorylation of Chk1 in extracts containing APH. By contrast, there was no increase in extracts treated with control buffer or the 7A mutant TRCT domain. Furthermore, the increase in the presence of the WT TRCT domain was abolished by caffeine, an inhibitor of the ATR-catalyzed phosphorylation of Chk1. Therefore, the TRCT-stimulated activation of Chk1 involves the ATR-mediated pathway. This increase also depended on the presence of sperm chromatin as a template for replication, which suggests that the TRCT domain does not somehow directly activate Chk1 by a DNA-independent mechanism. In further support of this concept, we found that inhibition of DNA replication with p27 abolished the TRCT-stimulated increase in the phosphorylation of Chk1 in extracts lacking APH (Figure S6C). Overall, these experiments indicate that the isolated TRCT domain causes a pronounced derangement of DNA replication in the Xenopus egg extract system.
Expression of the Treslin-7A Mutant in Human Cells Leads to Increased Origin Firing
As another strategy to examine the function of the Treslin-Chk1 interaction, we utilized human cells that overexpress the Treslin-7A mutant. In particular, we employed U2OS T-REx cells that express WT and 7A Treslin in a doxycycline-inducible manner. We induced the expression of Treslin in these cells by addition of doxycycline and later examined origin firing by DNA fiber analysis (Jackson and Pombo, 1998; Schlacher et al., 2011). For these studies, we incubated the cells with CldU for 20 min and then with IdU for 20 min (Figure 7A). Next, we prepared DNA fibers from the cells by standard methods and detected incorporation of the modified nucleotides with fluorescently tagged antibodies that detect CldU (green tracks) and IdU (red tracks). Finally, we quantitated the frequency of singly labeled IdU (red) tracks as a measure of new origin firing during the second labeling period. We noted that there was a significant increase in origin firing in cells expressing the full-length Treslin-7A mutant in comparison with cells expressing the WT protein (Figures 7B–7D). The induced levels of the WT and 7A proteins were quite similar in the different cell lines. Moreover, overexpression of WT Treslin did not have a significant effect on origin firing. We also examined replication fork speed in these cells but could not discern a difference between cells expressing WT or 7A Treslin (Figure S7). Overall, these experiments further support our findings with Xenopus egg extracts that disruption of the Treslin-Chk1 interaction leads to increased origin firing. The fact that the Treslin-Chk1 interaction is functionally important in both Xenopus and humans suggests that this regulatory mechanism is a conserved feature of DNA replication in vertebrates.
Figure 7. Overexpression of the Treslin-7A Mutant in Human Cells Elicits Increased Initiation of Replication.
(A) Schematic of DNA fiber analysis. Green tracks, CldU; red tracks, IdU. Examples of various types of tracts are depicted.
(B) U2OS T-Rex cells harboring Treslin WT (lanes 1 and 2) or Treslin 7A (lanes 3 and 4) were incubated for 48 hr in the absence (lanes 1 and 3) or presence of doxycycline (lanes 2 and 4). Cell lysates were immunoblotted as indicated.
(C) U2OS T-Rex cells harboring Treslin WT or Treslin 7A were incubated for 48 hr in the absence or presence of doxycycline. Cells were labeled sequentially with CldU and IdU and processed for preparation of DNA fibers as described in Experimental Procedures. Images of DNA fibers from doxycycline-treated cells expressing WT or 7A Treslin are shown.
(D) Summary of new origins fired during labeling with IdU. Results for induced WT and 7A cells were compiled from four independent experiments with two different clonal isolates for each construct (p < 0.001). Error bars, S.E.M.
(E) Model for the Treslin-Chk1 interaction. See Discussion for details. See also Figure S7.
DISCUSSION
In this report, we have identified an interaction between Treslin and the key checkpoint-effector kinase Chk1. The high specificity of this binding suggested that there is a significant regulatory relationship between these two proteins. Indeed, we have found that Chk1 negatively regulates the replication-initiating function of Treslin (see Figure 7E). To reach this conclusion, we utilized both Xenopus egg extracts and human cells. In one approach, we added the TRCT domain from Treslin to Xenopus egg extracts as a competitor of the Treslin-Chk1 interaction. Strikingly, this peptide elicited a pronounced increase in DNA replication.
In searching for the basis of this phenomenon, we found that the TRCT fragment strongly stimulated the binding of Cdc45 to chromatin. The loading of Cdc45 onto chromatin in egg extracts is rate limiting for origin firing (Mimura et al., 2000; Walter and Newport, 2000). Thus, the TRCT domain appears to act by enhancing initiation. To provide further evidence, we have dissected this process with actinomycin D, a drug that blocks replication in egg extracts just after initiation but before significant unwinding of the DNA (Pacek and Walter, 2004). Treatment with both the TRCT domain and actinomycin D led to a dramatic and sustained accumulation of Cdc45 on chromatin. Finally, we utilized DNA fiber studies to show that the addition of the TRCT domain to egg extracts leads to an overall decrease in inter-origin distances in replicating chromatin.
In a complementary approach, we also employed human cells. In particular, we also used DNA fiber analysis to show that human cells overexpressing the full-length Treslin-7A mutant display increased origin firing during an unperturbed S-phase. This observation is consistent with the fact that human cells with compromised function of Chk1 display elevated firing of replication origins during a normal cell cycle (Maya-Mendoza et al., 2007; McIntosh and Blow, 2012; Miao et al., 2003; Syljuasen et al., 2005). Hence, our studies have revealed a mechanism by which Chk1 controls replication in cells without overtly damaged DNA. It has also been reported that Chk1-deficient cells display decreased replication-fork speed (see Petermann et al., 2010). We have not observed an obvious effect on fork speed in cells expressing the Treslin-7A mutant. This mutant may not cause enough origin firing to disrupt fork progression. Moreover, Chk1 could have distinct targets for initiation versus elongation.
Utilization of replication origins places a number of regulatory demands on cells. The firing of origins depends upon positive regulation of Treslin by S-CDK, but there may also have to be negative regulatory mechanisms that suppress premature or inappropriate action of Treslin at origins. The ability of Chk1 to inhibit the Treslin-mediated loading of Cdc45 would provide such a mechanism. This process could operate generally at all origins or come into play at a subset of origins under certain circumstances.
Typically, the number of loaded MCM complexes on chromatin at the onset of S-phase greatly exceeds the number of origins that actually fire in a given cell cycle. This “MCM paradox” has suggested that there are a large number of dormant origins in the genome (see McIntosh and Blow, 2012). It has been proposed that utilization of these dormant origins would allow cells to cope with replication stress by increasing the likelihood that replication in stressed areas would reach completion. The question arises, however, about how the cell would regulate the firing of such dormant origins under both unstressed and stressed conditions. It has been postulated that these origins might fire stochastically such that passive replication from nearby origins would typically occur under unstressed conditions. However, under stressed conditions of slowed or blocked replication, there would be more time for the dormant origins to undergo firing.
Another type of explanation would incorporate the existence of an inhibitory mechanism that suppresses firing of dormant origins. In principle, inhibition of Treslin-mediated loading of Cdc45 by basally active Chk1 under unstressed conditions could correspond to such a mechanism. Our DNA fiber studies with egg extracts do not suggest that there is rampant firing of dormant origins upon addition of the TRCT domain. However, more limited firing of dormant origins may contribute to the accelerated replication that we have observed under these conditions.
The Treslin-Chk1 interaction might also help to explain the temporal order in firing of origins during S-phase in somatic cells. In eukaryotic cells, there is typically a pattern wherein “early” and “late” origins at distinct chromosomal regions fire at different times (McIntosh and Blow, 2012; Siddiqui et al., 2013). In budding yeast, regulation of the Treslin homologue Sld3 has a role in the distinction between early and late origins (Lopez-Mosqueda et al., 2010; Zegerman and Diffley, 2010). However, the mechanisms that regulate such differential firing in vertebrates are largely unknown. Another potential explanation for the inhibition of Treslin by Chk1 is that this process could be necessary for the suppression of later-firing origins during the earlier parts of S-phase. Further studies will be required to identify the characteristics of origins that are subject to Chk1-dependent inhibition of Treslin.
It is well accepted that the ATR-dependent activation of Chk1 in response to incompletely replicated and damaged DNA leads to inhibition of origin firing at chromosomal regions that are not already engaged in replication (McIntosh and Blow, 2012; Sorensen and Syljuasen, 2012; Yekezare et al., 2013). The activation of Chk1 results in decreased function of Cdc25 and increased function of Wee1 (Perry and Kornbluth, 2007). In turn, these effects promote the inhibitory phosphorylation of Cdk2 (as well as Cdk1) on T14 and Y15. The loading of Cdc45 onto chromatin requires phosphorylation of Treslin by Cdk2, which mediates binding of Treslin to TopBP1 (Boos et al., 2011; Kumagai et al., 2011). Therefore, it might be anticipated that reduced activity of Cdk2 would compromise formation of the Treslin-TopBP1 complex. Indeed, treatment of human cells with hydroxyurea reduces the binding of Treslin to TopBP1, and the Chk1 inhibitor AZD7762 reverses this effect (Boos et al., 2011).
We have not been able to detect an override of the hydroxyurea-induced block to replication in U2OS cells expressing the Treslin-7A mutant (not shown). This mutation would presumably not affect the ability of Chk1 to down-regulate Cdk2. We have also observed that both the WT and 7A forms of Treslin bind equally well to TopBP1. Moreover, the Treslin-7A mutant displays normally regulated binding to TopBP1 (i.e., decreased binding in the presence of hydroxyurea) (not shown). Finally, the TRCT domain does not affect binding of Treslin to TopBP1 in Xenopus NPE fractions. It would be interesting to examine the effect of the Treslin-7A mutant in replication-stressed cells in which the inhibition of Cdk2 had been overridden.
Although inhibition of Cdk2 could account for the effect of Chk1 on DNA replication in stressed cells, it is unclear that this mechanism could explain the role of Chk1 in unstressed cells. The activity of Cdk2 rises substantially at S-phase in unperturbed cells. It is possible that there could be some local inhibition of Cdk2 in certain regions of the genome, but the mechanistic basis for such an effect is unknown. Another explanation is that Chk1 could control replication in some other manner besides regulation of Cdk2 under conditions where there is not a strong, exogenous threat to the DNA. The regulation of Treslin by Chk1 may be such a mechanism. It should also be noted that our studies do not rule out the possibility that Chk1 could have an additional target(s) besides Treslin during an unperturbed S-phase. Overall, inhibition of Treslin by Chk1 could suppress relevant origins during an unperturbed S-phase and perhaps even supplement the inhibition of Cdk2 in replication-stressed cells.
A previous study in the Xenopus system indicated that depletion of Chk1 did not increase DNA replication in either untreated or APH-treated egg extracts (Luciani et al., 2004). For the untreated extracts, these investigators quantitated DNA replication at a single late time point (120 min) when replication would have reached completion. Hence, this analysis would not have detected the early acceleration of replication that we have observed in this study. Another group found that the Chk1 inhibitor UCN-01 did not increase replication in egg extracts, but actually inhibited replication to some extent (Murphy and Michael, 2013). However, UCN-01 also inhibits Cdk2 at the concentrations used in this study (Kawakami et al., 1996), which complicates interpretation of the results.
In principle, phosphorylation of Treslin by Chk1 may alter its conformation or directly affect its interactions with other proteins to preclude helicase activation. Moreover, the physical association of Chk1 might also have such effects on Treslin. Regulation by Chk1 appears not to reduce binding of Treslin to TopBP1, but Chk1 may nonetheless alter the helicase-activating properties of this complex. Chk1 may also inhibit the ability of Treslin and TopBP1 to recognize Cdc45/GINS effectively or deliver Cdc45/GINS to the MCM complex. Finally, Chk1 may influence the interaction of Treslin with some other component(s) of the replication apparatus. We will need to understand better the exact molecular mechanism by which Treslin promotes helicase activation in order to evaluate which, if any, of these possibilities is correct.
In conclusion, we have used both the Xenopus and human systems to provide new perspectives on the function of Chk1 and the regulation of early steps in DNA replication. Further analysis of this process may yield additional insights into how vertebrate cells ensure the faithful propagation of their genomes.
EXPERIMENTAL PROCEDURES
Plasmids
The cDNA for human Treslin (GenBank ADC30133.1) was described before (Kumagai et al., 2010). pcDNA5/TO-Treslin-SF (encoding the SV40 NLS, an S-peptide tag, and a 3X-FLAG tag at the C-terminal end of Treslin) was created from pcDNA5/TO-Treslin-Myc by using PCR to exchange tags (Kumagai et al., 2011).
Human Tissue Culture Cells
293T and U2OS cells were cultured in DMEM containing 10% fetal bovine serum. 293T cells were transfected using FuGENE6. Large-scale transfections as well as preparation of nuclear lysates are described in Supplemental Experimental Procedures.
Antibodies
Anti-Treslin antibodies were previously described (Kumagai et al., 2010). For other antibodies, see Supplemental Experimental Procedures.
Mass spectrometry analysis
Plasmids encoding different versions of Treslin-SF were transfected into 293T cells. Tagged proteins were reisolated and analyzed by mass spectrometry as described in Supplemental Experimental Procedures. Binding of Chk1 to full-length Treslin was established by matching 9 unique peptides that covered 24% of the sequence of Chk1. For binding to the 1253-1909 C-terminal fragment, we detected 10 unique peptides also covering 24% of the sequence. There was no binding of Chk1 to the 1-1257 N-terminal fragment.
Recombinant GST fusion proteins
To produce GST and GST-NLS fusion proteins containing versions of the human TRCT, appropriate DNA was amplified by PCR and cloned into pGEX4T-3 or pGEX-NLS, respectively. Proteins were produced as described in Supplemental Experimental Procedures.
Production of U2OS cell lines expressing Treslin and its mutants
U2OS T-REx cells were maintained, transfected with pcDNA5/TO encoding siRNA-resistant Treslin, and selected as described (Kumagai et al., 2011). Single colonies that expressed the desired protein upon addition of doxycycline were isolated for analysis.
siRNA Experiments
Stealth siRNA specific for Treslin (#2, AGGACACAUUCUGCCUCCUUCUAUU) and control siRNA (low GC) were obtained from Invitrogen. The siRNA (30 nM) was transfected into U2OS cells with Lipofectamine RNAiMAX (Invitrogen). For rescue experiments, pcDNA5/TO-Treslin-SF was rendered resistant to siRNA #2. We obtained more efficient rescue by using siRNA #2 instead of the previous siRNA #1 (Kumagai et al., 2010).
Xenopus Egg Extracts
Whole extracts from Xenopus eggs were prepared as before (Kumagai et al., 2010). Preparation of nuclear lysates, immunodepletion of Chk1, and DNA replication assays were also described previously (Kumagai et al., 1998; Kumagai et al., 2010). Further details are provided in Supplemental Experimental Procedures.
Xenopus NPE System
Nucleoplasmic extract (NPE) and high-speed supernatant (HSS) from Xenopus eggs were prepared as described (Lebofsky et al., 2009). Details on the use of these extracts for assays of DNA replication and binding to chromatin are described in Supplemental Experimental Procedures.
DNA fiber assays
Xenopus egg extracts were incubated sequentially with digoxigenin-11-dUTP and biotin-16-dUTP. Labeled DNA fibers were prepared as described (Bellelli et al., 2014; Marheineke et al., 2009). Human U2OS cells were labeled with 40 μM CldU, washed with phosphate-buffered saline (PBS), and finally labeled with 50 μM IdU as indicated in figures. Preparation of DNA fiber spreads was reported previously (Jackson and Pombo, 1998; Schlacher et al., 2011). Further details on these protocols are provided in Supplemental Experimental Procedures.
Supplementary Material
Figure S1. Treatment with Ethidium Bromide or Benzonase Does Not Inhibit Co-immunoprecipitation of Treslin and Chk1. Related to Figure 1.
293T nuclear lysates were treated with 50 μg/ml ethidium bromide (lanes 4 and 5) or 1 U/μl Benzonase (lanes 6 and 7) for 30 min on ice before immunoprecipitation was performed as in Figure 1C using control IgG (lanes 2, 4, and 6) or antibodies against Chk1 (lanes 3, 5, and 7).
Figure S2. The TRCT Domain Binds Directly to Chk1. Related to Figure 2.
GST only (lane 2), GST-tagged TRCT (lane 3), TRCT containing mutations S1887A (lane 4), S1893A (lane 5), T1897A (lane 6), or 7A (lane 7), and GST-tagged forms of residues 1810-1872 (lane 8) and 1870-1909 from Treslin (lane 9) were isolated from bacteria with glutathione agarose. Bead-bound proteins were incubated with recombinant His6-Chk1 in binding buffer at 4°C for 90 min. Beads were retrieved, washed, and subjected to immunoblotting with anti-Chk1 (top) and staining with Coomassie blue (bottom). Recombinant Chk1 alone was loaded lane 1.
Figure S3. Further Characterization of the Phosphorylation of Treslin by Chk1. Related to Figure 3.
(A) GST only (lanes 1–2), GST-TRCT (lanes 3–4), and versions of this fragment containing the S1887A (lanes 5–6), S1893A (lanes 7–8), T1897A (lanes 9–10), or S1893A and T1897A mutations (lanes 11–12) were incubated with recombinant WT Chk1 (lanes 1, 3, 5, 7, 9, and 11) or KD Chk1 (lanes 2, 4, 6, 8, 10, and 12). Reactions were processed for phosphorimaging (top) and Coomassie blue staining (bottom).
(B) GST only (lane 2), GST-tagged form of wild-type TRCT (lane 3), and a version of this fragment containing both the S1893A and T1897A mutations (lane 4) were expressed in bacteria and isolated with glutathione agarose. Bead-bound fragments were incubated with 293T nuclear lysates. Beads were retrieved, washed, and processed for immunoblotting with anti-Chk1 (top) and Coomassie blue staining (bottom). 293T nuclear lysate was loaded in lane 1 as input.
(C) GST only (lane 1), GST-TRCT plus GST (lanes 2–3), GST-Cdc25(254-316) plus GST (lanes 4–5), and GST-TRCT plus GST-Cdc25(254-316) (lanes 6–7) were incubated with recombinant WT Chk1 (lanes 1, 2, 4, and 6) or KD Chk1 (lanes 3, 5, and 7). Reactions were processed for phosphorimaging (top) and Coomassie blue staining (bottom). Relative levels of phosphorylation normalized to protein amount are shown. Phosphorylation of Cdc25(254-316) in lane 4 was denoted as 1.
Figure S4. Further Characterization of the Effect of Chk1 on DNA Replication. Related to Figure 4.
(A) NPE was treated with 1 U/μl Benzonase (lane 4) or 50 μg/ml ethidium bromide (lane 5) for 30 min on ice before immunoprecipitation was performed as in Figure 4D.
(B) HSS was incubated for 30 min at room temperature. NPE containing buffer (lanes 1–3), or AZD7762 (lanes 4–6) was added to HSS. The final concentration of AZD7762 was 0.5 μM. Samples were incubated in the presence of [α-32P]dATP to determine chromosomal DNA replication. Quantitation of the results is presented in Figure 4E.
Figure S5. The TRCT Domain Stimulates the Loading of Numerous Replication Proteins onto Chromatin. Related to Figure 6.
HSS was incubated without (lanes 3–4) or with sperm nuclei (lanes 5–9) for 30 min. NPE and either WT (lanes 1, 3, 6, and 8) or 7A TRCT (lanes 2, 4, 7, and 9) at a final concentration of 60 ng/μl were added to HSS. Chromatin was isolated at indicated times and immunoblotted with the indicated antibodies. NPE/HSS mixture was loaded in lanes 1–2.
Figure S6. Determination of Inter-origin Distances in Egg Extracts Treated with the TRCT Domain. Related to Figure 6.
(A) Schematic of DNA fiber assay in Xenopus egg extracts and fiber visualization by immunofluorescence.
(B) Representative images of fibers from egg extracts containing buffer alone, TRCT WT (75 ng/μl), or TRCT 7A (75 ng/μl) are shown. Distributions of inter-origin distances are shown in Figure 6C.
(C) HSS was incubated in the presence of sperm nuclei (lanes 1–6) for 30 min. NPE either lacking (lanes 1–3) or containing 1 μM GST-p27 (lanes 4–6) and buffer (lanes 1 and 4), TRCT WT (lanes 2 and 5), or TRCT 7A (lanes 3 and 6) were added to HSS. Final concentration of TRCT was 60 ng/μl. Mixtures were incubated for 90 min and immunoblotted with anti-P-Chk1 (top), anti-Chk1 (middle), and anti-GST (bottom).
Figure S7. Replication-fork Speed in Cells Expressing WT or 7A Treslin. Related to Figure 7.
U2OS T-Rex cells harboring Treslin WT or 7A were cultured in the presence of doxycycline for 48 hr. DNA fibers were prepared and replication-fork speed was determined.
Table S1. Full list of proteins purified specifically with the Treslin 1253-1909 C-terminal fragment. Related to Figure 1.
Acknowledgments
We are grateful to laboratory members for comments on the manuscript. We also thank Juan Ramírez-Lugo for anti-ISWI antibodies. This work was supported by NIH grants GM043974 and GM070891 to W.G.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellelli R, Castellone MD, Guida T, Limongello R, Dathan NA, Merolla F, Cirafici AM, Affuso A, Masai H, Costanzo V, et al. NCOA4 transcriptional coactivator inhibits activation of DNA replication origins. Mol Cell. 2014;55:123–137. doi: 10.1016/j.molcel.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Boos D, Sanchez-Pulido L, Rappas M, Pearl LH, Oliver AW, Ponting CP, Diffley JF. Regulation of DNA replication through Sld3-Dpb11 interaction is conserved from yeast to humans. Curr Biol. 2011;21:1152–1157. doi: 10.1016/j.cub.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Jackson DA, Pombo A. Replicon clusters are stable units of chromosome structure: evidence that nuclear organization contributes to the efficient activation and propagation of S phase in human cells. J Cell Biol. 1998;140:1285–1295. doi: 10.1083/jcb.140.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Futami H, Takahara J, Yamaguchi K. UCN-01, 7-hydroxyl-staurosporine, inhibits kinase activity of cyclin-dependent kinases and reduces the phosphorylation of the retinoblastoma susceptibility gene product in A549 human lung cancer cell line. Biochem Biophys Res Comm. 1996;219:778–783. doi: 10.1006/bbrc.1996.0310. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. Treslin collaborates with TopBP1 in triggering the initiation of DNA replication. Cell. 2010;140:349–359. doi: 10.1016/j.cell.2009.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. Direct regulation of Treslin by cyclin-dependent kinase is essential for the onset of DNA replication. J Cell Biol. 2011;193:995–1007. doi: 10.1083/jcb.201102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev. 2010;24:1208–1219. doi: 10.1101/gad.1933010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebofsky R, Takahashi T, Walter JC. DNA replication in nucleus-free Xenopus egg extracts. Methods Mol Biol. 2009;521:229–252. doi: 10.1007/978-1-60327-815-7_13. [DOI] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Mosqueda J, Maas NL, Jonsson ZO, Defazio-Eli LG, Wohlschlegel J, Toczyski DP. Damage-induced phosphorylation of Sld3 is important to block late origin firing. Nature. 2010;467:479–483. doi: 10.1038/nature09377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani MG, Oehlmann M, Blow JJ. Characterization of a novel ATR-dependent, Chk1-independent, intra-S-phase checkpoint that suppresses initiation of replication in Xenopus. J Cell Sci. 2004;117:6019–6030. doi: 10.1242/jcs.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marheineke K, Goldar A, Krude T, Hyrien O. Use of DNA combing to study DNA replication in Xenopus and human cell-free systems. Methods Mol Biol. 2009;521:575–603. doi: 10.1007/978-1-60327-815-7_33. [DOI] [PubMed] [Google Scholar]
- Maya-Mendoza A, Petermann E, Gillespie DA, Caldecott KW, Jackson DA. Chk1 regulates the density of active replication origins during the vertebrate S phase. EMBO J. 2007;26:2719–2731. doi: 10.1038/sj.emboj.7601714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh D, Blow JJ. Dormant origins, the licensing checkpoint, and the response to replicative stresses. Cold Spring Harb Perspect Biol. 2012;4:a012955. doi: 10.1101/cshperspect.a012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Seiler JA, Burhans WC. Regulation of cellular and SV40 virus origins of replication by Chk1-dependent intrinsic and UVC radiation-induced checkpoints. J Biol Chem. 2003;278:4295–4304. doi: 10.1074/jbc.M204264200. [DOI] [PubMed] [Google Scholar]
- Mimura S, Masuda T, Matsui T, Takisawa H. Central role for Cdc45 in establishing an initiation complex of DNA replication in Xenopus egg extracts. Genes Cells. 2000;5:439–452. doi: 10.1046/j.1365-2443.2000.00340.x. [DOI] [PubMed] [Google Scholar]
- Murphy CM, Michael WM. Control of DNA replication by the nucleus/cytoplasm ratio in Xenopus. J Biol Chem. 2013;288:29382–29393. doi: 10.1074/jbc.M113.499012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek M, Walter JC. A requirement for MCM7 and Cdc45 in chromosome unwinding during eukaryotic DNA replication. EMBO J. 2004;23:3667–3676. doi: 10.1038/sj.emboj.7600369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JA, Kornbluth S. Cdc25 and Wee1: analogous opposites? Cell Div. 2007;2:12. doi: 10.1186/1747-1028-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann E, Woodcock M, Helleday T. Chk1 promotes replication fork progression by controlling replication initiation. Proc Nat Acad Sci USA. 2010;107:16090–16095. doi: 10.1073/pnas.1005031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansam CL, Cruz NM, Danielian PS, Amsterdam A, Lau ML, Hopkins N, Lees JA. A vertebrate gene, ticrr, is an essential checkpoint and replication regulator. Genes Dev. 2010;24:183–194. doi: 10.1101/gad.1860310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlacher K, Christ N, Siaud N, Egashira A, Wu H, Jasin M. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell. 2011;145:529–542. doi: 10.1016/j.cell.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani RA, Holzen TM. Cell cycle regulation of DNA replication. Annu Rev Genet. 2007;41:237–280. doi: 10.1146/annurev.genet.41.110306.130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui K, On KF, Diffley JF. Regulating DNA replication in eukarya. Cold Spring Harb Perspect Biol. 2013;5:a012930. doi: 10.1101/cshperspect.a012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen CS, Syljuasen RG. Safeguarding genome integrity: the checkpoint kinases ATR, CHK1 and WEE1 restrain CDK activity during normal DNA replication. Nucleic Acids Res. 2012;40:477–486. doi: 10.1093/nar/gkr697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, Helleday T, Sehested M, Lukas J, Bartek J. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Araki H. Helicase activation and establishment of replication forks at chromosomal origins of replication. Cold Spring Harb Perspect Biol. 2013;5:a010371. doi: 10.1101/cshperspect.a010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo LI, Murga M, Fernandez-Capetillo O. Targeting ATR and Chk1 kinases for cancer treatment: a new model for new (and old) drugs. Mol Oncol. 2011;5:368–373. doi: 10.1016/j.molonc.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah Z, de Renty C, DePamphilis ML. Checkpoint kinase 1 prevents cell cycle exit linked to terminal cell differentiation. Mol Cell Biol. 2011;31:4129–4143. doi: 10.1128/MCB.05723-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Newport J. Initiation of eukaryotic DNA replication: origin unwinding and sequential chromatin association of Cdc45, RPA, and DNA polymerase alpha. Mol Cell. 2000;5:617–627. doi: 10.1016/s1097-2765(00)80241-5. [DOI] [PubMed] [Google Scholar]
- Yekezare M, Gomez-Gonzalez B, Diffley JF. Controlling DNA replication origins in response to DNA damage - inhibit globally, activate locally. J Cell Sci. 2013;126:1297–1306. doi: 10.1242/jcs.096701. [DOI] [PubMed] [Google Scholar]
- Zachos G, Black EJ, Walker M, Scott MT, Vagnarelli P, Earnshaw WC, Gillespie DA. Chk1 is required for spindle checkpoint function. Dev Cell. 2007;12:247–260. doi: 10.1016/j.devcel.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegerman P, Diffley JF. Checkpoint-dependent inhibition of DNA replication initiation by Sld3 and Dbf4 phosphorylation. Nature. 2010;467:474–478. doi: 10.1038/nature09373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Treatment with Ethidium Bromide or Benzonase Does Not Inhibit Co-immunoprecipitation of Treslin and Chk1. Related to Figure 1.
293T nuclear lysates were treated with 50 μg/ml ethidium bromide (lanes 4 and 5) or 1 U/μl Benzonase (lanes 6 and 7) for 30 min on ice before immunoprecipitation was performed as in Figure 1C using control IgG (lanes 2, 4, and 6) or antibodies against Chk1 (lanes 3, 5, and 7).
Figure S2. The TRCT Domain Binds Directly to Chk1. Related to Figure 2.
GST only (lane 2), GST-tagged TRCT (lane 3), TRCT containing mutations S1887A (lane 4), S1893A (lane 5), T1897A (lane 6), or 7A (lane 7), and GST-tagged forms of residues 1810-1872 (lane 8) and 1870-1909 from Treslin (lane 9) were isolated from bacteria with glutathione agarose. Bead-bound proteins were incubated with recombinant His6-Chk1 in binding buffer at 4°C for 90 min. Beads were retrieved, washed, and subjected to immunoblotting with anti-Chk1 (top) and staining with Coomassie blue (bottom). Recombinant Chk1 alone was loaded lane 1.
Figure S3. Further Characterization of the Phosphorylation of Treslin by Chk1. Related to Figure 3.
(A) GST only (lanes 1–2), GST-TRCT (lanes 3–4), and versions of this fragment containing the S1887A (lanes 5–6), S1893A (lanes 7–8), T1897A (lanes 9–10), or S1893A and T1897A mutations (lanes 11–12) were incubated with recombinant WT Chk1 (lanes 1, 3, 5, 7, 9, and 11) or KD Chk1 (lanes 2, 4, 6, 8, 10, and 12). Reactions were processed for phosphorimaging (top) and Coomassie blue staining (bottom).
(B) GST only (lane 2), GST-tagged form of wild-type TRCT (lane 3), and a version of this fragment containing both the S1893A and T1897A mutations (lane 4) were expressed in bacteria and isolated with glutathione agarose. Bead-bound fragments were incubated with 293T nuclear lysates. Beads were retrieved, washed, and processed for immunoblotting with anti-Chk1 (top) and Coomassie blue staining (bottom). 293T nuclear lysate was loaded in lane 1 as input.
(C) GST only (lane 1), GST-TRCT plus GST (lanes 2–3), GST-Cdc25(254-316) plus GST (lanes 4–5), and GST-TRCT plus GST-Cdc25(254-316) (lanes 6–7) were incubated with recombinant WT Chk1 (lanes 1, 2, 4, and 6) or KD Chk1 (lanes 3, 5, and 7). Reactions were processed for phosphorimaging (top) and Coomassie blue staining (bottom). Relative levels of phosphorylation normalized to protein amount are shown. Phosphorylation of Cdc25(254-316) in lane 4 was denoted as 1.
Figure S4. Further Characterization of the Effect of Chk1 on DNA Replication. Related to Figure 4.
(A) NPE was treated with 1 U/μl Benzonase (lane 4) or 50 μg/ml ethidium bromide (lane 5) for 30 min on ice before immunoprecipitation was performed as in Figure 4D.
(B) HSS was incubated for 30 min at room temperature. NPE containing buffer (lanes 1–3), or AZD7762 (lanes 4–6) was added to HSS. The final concentration of AZD7762 was 0.5 μM. Samples were incubated in the presence of [α-32P]dATP to determine chromosomal DNA replication. Quantitation of the results is presented in Figure 4E.
Figure S5. The TRCT Domain Stimulates the Loading of Numerous Replication Proteins onto Chromatin. Related to Figure 6.
HSS was incubated without (lanes 3–4) or with sperm nuclei (lanes 5–9) for 30 min. NPE and either WT (lanes 1, 3, 6, and 8) or 7A TRCT (lanes 2, 4, 7, and 9) at a final concentration of 60 ng/μl were added to HSS. Chromatin was isolated at indicated times and immunoblotted with the indicated antibodies. NPE/HSS mixture was loaded in lanes 1–2.
Figure S6. Determination of Inter-origin Distances in Egg Extracts Treated with the TRCT Domain. Related to Figure 6.
(A) Schematic of DNA fiber assay in Xenopus egg extracts and fiber visualization by immunofluorescence.
(B) Representative images of fibers from egg extracts containing buffer alone, TRCT WT (75 ng/μl), or TRCT 7A (75 ng/μl) are shown. Distributions of inter-origin distances are shown in Figure 6C.
(C) HSS was incubated in the presence of sperm nuclei (lanes 1–6) for 30 min. NPE either lacking (lanes 1–3) or containing 1 μM GST-p27 (lanes 4–6) and buffer (lanes 1 and 4), TRCT WT (lanes 2 and 5), or TRCT 7A (lanes 3 and 6) were added to HSS. Final concentration of TRCT was 60 ng/μl. Mixtures were incubated for 90 min and immunoblotted with anti-P-Chk1 (top), anti-Chk1 (middle), and anti-GST (bottom).
Figure S7. Replication-fork Speed in Cells Expressing WT or 7A Treslin. Related to Figure 7.
U2OS T-Rex cells harboring Treslin WT or 7A were cultured in the presence of doxycycline for 48 hr. DNA fibers were prepared and replication-fork speed was determined.
Table S1. Full list of proteins purified specifically with the Treslin 1253-1909 C-terminal fragment. Related to Figure 1.