Abstract
Context
Symptoms and subsequent functional impairment have been associated with the biological processes of a disease, including the interaction between the disease and treatment in a measurement model of symptoms. However, hitherto cluster analysis has primarily focused on symptoms.
Objectives
This study among patients within 100 days of diagnosis with advanced cancer explored whether self-reported physical symptoms and functional impairments formed clusters at the time of diagnosis.
Methods
We applied the cluster analysis to self-reported symptoms and activities of daily living of 111 patients newly diagnosed with advanced gastrointestinal (GI), gynecological, head and neck, and lung cancers. Based on the content, expert evaluations, the best techniques, variables were identified, yielding the best solution.
Results
The best cluster solution used a K-means algorithm and cosine similarity and yielded five clusters of physical as well as emotional symptoms and functional impairments. Cancer site formed the predominant organizing principle of composition for each cluster. The top five symptoms and functional impairments in each cluster were Cluster 1 (GI): outlook, insomnia, appearance, concentration, and eating/feeding; Cluster 2 (GI): appetite, bowel, insomnia, eating/feeding, and appearance; Cluster 3 (gynecological): nausea, insomnia, eating/feeding, concentration, and pain; Cluster 4 (head and neck): dressing, eating/feeding, bathing, toileting, and walking; and Cluster 5 (lung): cough, walking, eating/feeding, breathing, and insomnia.
Conclusion
Functional impairments in patients newly diagnosed with late-stage cancers behave as symptoms during the diagnostic phase. Health care providers need to expand their assessments to include both symptoms and functional impairments. Early recognition of the functional changes may accelerate diagnosis at an earlier cancer stage.
Keywords: Cluster analysis, symptom, function, activities of daily living, cancer, newly diagnosed, advanced cancer
Introduction
In the late 1960s and early 1970s, the dedication and commitment of clinicians such as Twycross,1 McCaffery,2 and Foley3 led to an international movement that recognized that symptoms have to be assessed through patients' own accounts. Initially, clinical research focused on single symptoms. This focus, however, neglected the multiplicity of symptoms that patients experience. More recently, researchers explored the multiplicity and concurrence of patients' self-report of symptoms.4 Blesch et al.,5 for example, documented that increases in fatigue were associated with higher levels of pain and depression. Miaskowski and Lee,6 in a study among patients with osseous metastases undergoing palliative radiation therapy, found the troika of insomnia, pain, and fatigue to be concurrent and related. The findings of these and other seminal studies7–13 prompted Dodd et al.14 to offer the term “symptom cluster” to describe a group of three or more concurrent and related symptoms.
Since the addition of this term to the lexicon, several studies have focused on the identification of symptom clusters. Reyes-Gibby et al.15 have identified fatigue, pain, and depressed mood as a singular common cluster. Francouer,16 in a study of 268 patients who received radiation therapy for osseous metastases and home-based palliative care, identified four clusters: pain and fatigue, pain and weight loss, pain and fever, and sleep and fever. Each cluster was associated with depressed mood. In a sample of 1366 outpatients with different advanced cancers receiving palliative care, Cheung et al.17 identified two clusters. The first included fatigue, drowsiness, nausea, decreased appetite, and dyspnea and the second included anxiety and depression.
Symptom cluster research carries with it methodological challenges. For example, symptom clusters may differ depending on the site and stage of the disease and on the treatment,18,19 hence samples need to be limited to homogeneous characteristics, such as common stages, and participants need to be assessed in specific time frames, such as during active treatment or at the same time since diagnosis.20
Another challenge to the cluster research is that the symptoms may affect the patients' functional abilities.21 Symptom cluster research needs to account for this relationship. Miaskowski and Lee,6 for example, found that the symptom cluster of insomnia, pain, and fatigue had a consistent effect on the patients' function. Specifically, the relationship of symptom clusters with the stage of disease and the function is important. Kurtz et al.,22 in an investigation on the effect of symptoms on the physical functioning of patients with advanced cancer, found that the levels of symptoms increased with advancing disease. More importantly, they found that the levels of symptoms predicted (P = 0.002) the patients' dependency on others for activities of daily living. In addition, the levels of symptoms and the more advanced stage of disease predicted (P = 0.003) the patients' immobility.
Impairment in activities of daily living may play an important role in the constellation of diagnostic symptomatology. For some patients, physical symptoms may be the first sign that an additional evaluation is needed to uncover a health care problem and determine a diagnosis. However, this is not always the case. Some people newly diagnosed with advanced cancer do not experience the common physical symptoms relied on to indicate further evaluation. Their first indication may be impairment in their ability to perform daily activities. McCorkle23 has examined factors other than symptoms that may indicate a need for further evaluation and diagnostic work-up, especially patients' functional abilities.24 Similarly, Cleeland and Sloan23 conceptualized that symptoms and subsequent functional impairment are most directly associated with the biological processes of a disease, including the interaction between the disease and treatment in a measurement model of symptoms.
Supported by the results of the previous work by McCorkle, we proposed to explore whether impairments in activities of daily living were, along with physical symptoms, present at the time of diagnosis among patients newly diagnosed with advanced cancer. Following the methodological recommendations of Barsevick et al.,20 we selected a sample of patients homogeneous with regard to the stage of the disease and at the same time since diagnosis. The specific aim of this study, which was conducted among patients within 100 days of diagnosis with late-stage cancer, was to explore whether self-reported symptoms and impairments in the activities of daily living form clusters based on the site of cancer. We hypothesized that people who present with late-stage cancers near the time of diagnosis experience clusters of symptoms and impairments of activities of daily living one would expect from the site of cancer with which they are diagnosed.
Methods
Design
This study was a cross-sectional analysis of data from the parent study in which 111 patients with late-stage cancer were recruited from the gastrointestinal (GI), gynecological, head and neck, and lung disease-specific oncology clinics of Smilow Cancer Hospital at Yale, New Haven. Criteria for the entry of patients into the parent study included: 1) within 100 days of a definitive primary diagnosis of Stage 3 or 4 GI (including pancreatic and esophageal), gynecological, head and neck, or lung cancers; 2) postsurgical (including biopsies) with a physician's order for ongoing oncologic treatment; 3) life expectancy of at least six months as confirmed by a medical oncologist; 4) age of 21 years or older; and 5) living within the state of Connecticut. The parent study was designed as a translational study of an advanced practice nursing intervention to improve patient outcomes, including symptoms, which we have previously developed and reported on.25
Data Collection
Outcome data in the parent study were collected at three points, the first (baseline) within the first 100 days after diagnosis, the second at one month after surgery and the third, three months after surgery. Only data collected at the baseline were included in this secondary analysis. The Yale School of Medicine, Human Subjects Institutional Review Board approved the present study. Informed consent was obtained from all patients and study identification numbers were used in place of names or personal identifying data to protect their rights.
Measures
Patient History and Clinical Treatment Form
An investigator-developed form used to obtain data related to sociodemographics, health history, insurance, cancer treatment, and clinical information was administered at the baseline. Nonparticipants were asked their reasons for nonparticipation. There were no differences in the demographic information between those who participated and those who declined.
Emotional Distress
It was measured by the emotional distress thermometer, which is a rapid method to evaluate whether the patients indicate that they have distress on a scale of 0 to 10, with a mark of four or above indicating a need for further evaluation.
Comorbidity
It was assessed as the number of other medical diagnoses adapted from the co-morbidity checklist used by Satariano et al.27 and by the Human Population Laboratory in the Alameda County Survey.28,29
Symptom Distress
It was measured by the Symptom Distress Scale (SDS). The SDS has been translated into multiple languages and is used internationally as a cancer-specific tool for assessing symptoms. It comprises 13 cancer-specific symptoms: nausea (presence and intensity), mood, appetite, insomnia, fatigue, pain (presence and intensity), mobility, bowel patterns, concentration, and appearance.30 Each symptom is placed on a 5 × 7 card with a five-point Likert-type format ranging from 1 (normal or no distress) to 5 (extensive distress). Total symptom distress is obtained as the unweighted sum of the 13 scales, a value that could range from 13 to 65. Both internal consistency and test-retest reliability estimates have indicated the scale's reliability.31,32
Activities of Daily Living
They were measured by the personal competence component of the Enforced Social Dependency Scale (ESDS). The ESDS, developed to measure the social and personal functional abilities of patients with cancer, consists of two components: personal and social competence. Personal competence includes six daily living activities: eating/feeding, dressing, walking, traveling, bathing, and toileting. Dependency in each activity was reported by the patient and rated by the interviewer on a six-point scale. Scores for personal competence were summed and ranged from 6 to 36, with higher scores reflecting greater dependency. The ESDS has demonstrated reliability (Cronbach's alpha = 0.72–0.96) and both concurrent and predictive validities.33
Statistical Analysis
We conducted a cluster analysis using the Matlab v. 7.11.1.866 provided by Mathworks (Natick, MA). This tool allowed for fitting different clustering algorithms with a variety of similarity measures. To determine which clustering algorithm best fits the data, we applied the agglomerative, spectral clustering and K-means clustering algorithms to the data. We evaluated similarity measures based on the Euclidean distance (the distance between two persons as computed as the sum of the squared differences between their corresponding characteristics) and the cosine function (computed as the dot product between their vectors of normalized characteristics).
We integrated the SDS and ESDS variables into one set to examine their combined contribution to forming clusters. We chose to use all six individual personal competence activities of daily living instead of the combined score of the personal competence component of the ESDS because we wanted to see whether the individual symptoms and impairments in activities of daily living combined to form a specific cluster, and whether that specific cluster cohered with the symptomatology characteristic of the predominant cancer site represented by the patients in that cluster. Although the sample is homogeneous regarding the stage of the disease at diagnosis (i.e., all participants had advanced disease at diagnosis), we hypothesized that the clusters of specific symptoms and activities of daily living would differ by the site of the disease (GI, gynecologic, head and neck, and lung).
Examination of initial clusters showed that four variables did not contribute to the cluster formation; rather, they were highly correlated with the remaining variables. These four were fatigue, nausea intensity, and pain intensity, as measured by the SDS, and traveling, as measured by the ESDS; hence, they were dropped from further analysis. The following 15 variables remained and were normalized: the SDS-measured symptoms of nausea, appetite, insomnia, pain, bowel patterns, concentration, appearance, breathing, outlook, and cough and the ESDS-measured activities of daily living of eating/feeding, dressing, walking, bathing, and toileting. From this set, we observed that, from the other possible clustering algorithms, the K-means algorithm, in combination with cosine similarity, formed the most cohesive and interpretable clusters; thus, we used the K-means algorithm for analysis.
The K-means algorithm is a partitioning approach that generates disjointed clusters of participants; K is the presumed number of clusters in the data. The K-means algorithm randomly selected a study participant to represent the center of each cluster. All remaining participants were then assigned to the cluster to which the cosine function determined they had the highest similarity. This iterative process was performed until all participants were placed in the group of clusters that maximized overall similarity.
We had previous knowledge that the data set contained four groups of patients based on the site of cancer (GI, gynecological, head and neck, and lung cancers). To allow for the possibilities of fewer and more clusters than the number of sites of cancer, we computed cluster solutions for two to six clusters. Each cluster in the different initial solutions was annotated with a number. We selected the final numbers of clusters based on the similarity of cosine dot product for each cluster (the shortest distance created a cluster), average normalized values (>0.05), and the clinical content.
To validate the cohesiveness of clusters of patients by the site of cancer, we calculated the conditional probability of a symptom and an activity of daily living by cluster. To do this, we first transformed the averages of symptoms and activities of daily living across clusters into a joint probability distribution. From this distribution, we computed marginal probability distributions for all symptoms and activities of daily living and for the clusters. Subsequently, the joint and marginal distributions were used to compute the desired conditional probabilities of symptoms and activities of daily living given the clusters.
Results
The demographic characteristics of 111 patients enrolled in the trial as measured at the baseline are presented in Table 1. The average age of the sample was 59.6 (12.7) years, with a range of 27.8–86.7 years. The sample was predominately white, highly educated, professional, and living with others. The sample included almost twice as many patients with GI cancers as gynecological, head and neck, and lung cancer. Diagnosis was made through biopsy for 41% of the participants (n = 45); the remaining participants were diagnosed through surgery. Eighty-five percentage of participants (n = 94) were receiving anticancer treatment after diagnosis. Of these, 70% were being treated with chemotherapy alone (n = 78) and 15% (n = 16) were being treated with combined chemotherapy and radiation.
Table 1. Demographic and Clinical Characteristics (N = 111).
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 46 (41) |
| Female | 65 (59) |
| Race | |
| White | 98 (88) |
| Black | 10 (9) |
| Native American | 1 (1) |
| Not stated | 2 (2) |
| Ethnicity | |
| Latino | 8 (7) |
| Non-Latino | 103 (93) |
| Relationship status | |
| Married or partnered | 60 (54) |
| Never married | 18 (16) |
| Divorced or separated | 19 (17) |
| Widowed | 11 (10) |
| Not stated | 3 (3) |
| Living arrangements | |
| Lives with others | 92 (83) |
| Lives alone | 19 (17) |
| Education level completed | |
| Primary school | 7 (6) |
| Secondary school graduate | 25 (22) |
| Some university or trade school | 33 (31) |
| University graduate | 30 (27) |
| Graduate school | 16 (14) |
| Occupation | |
| Professional/management/technical | 30 (27) |
| White collar/clerical/sales | 10 (9) |
| Blue collar | 14 (12) |
| Homemaker | 3 (3) |
| Student | 2 (2) |
| Retired | 4 (4) |
| Not stated | 48 (43) |
| Cancer site | |
| Gastrointestinal | 48 (43) |
| Gynecological | 21 (19) |
| Head and neck | 20 (18) |
| Lung | 22 (20) |
| Diagnostic modality | |
| Surgery | 66 (59) |
| Biopsy only | 45 (41) |
| Postdiagnosis anticancer treatment | |
| Chemotherapy alone | 78 (70) |
| Chemoradiation | 16 (15) |
| Radiation alone | 4 (3) |
| None | 13 (12) |
Patients' emotional distress, comorbidities, symptom distress, and ability to perform activities of daily living as measured at the baseline in the parent study are reported in Table 2. Overall in the sample, patients had two or more comorbidities. Patients with lung cancer had the highest average of numbers of comorbidities. The overall sample had moderate symptom distress; the head and neck patients had the highest average of symptom distress. Similarly, patients with head and neck cancer reported the most limitations in their activities of daily living.
Table 2. Emotional Distress, Cormorbidities, Symptom Distress, and the Ability to Perform Activities of Daily Living by the Total Sample and Cancer Sites.
| Total (N = 111) | GI Cancers (n = 48) | Gynecological Cancers (n = 21) | Head and Neck Cancers (n = 20) | Lung Cancers (n = 22) | |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| Symptoms | Mean (SD) [Range] | Mean (SD) [Range] | Mean (SD) [Range] | Mean (SD) [Range] | Mean (SD) [Range] |
| Emotional Distress Scale (0 = no distress, 10 = highest level of distress) | 3.9 (2.7) | 3.4 (2.6) | 4.5 (2.6) | 4.7 (2.7) | 3.6 (2.6) |
| Comorbidity checklist (Number of comorbidities reported) | 2.6 (2.1) [0–9] | 2.4 (2.3) [0–9] | 2.6 (1.7) [0–6] | 2.2 (1.8) [0–5] | 3.4 (2.4) [0–8] |
| Symptom Distress Scale (13 = lowest symptom distress, 45 = highest symptom distress) | 23.6 (6.8) [13–44] | 21.9 (6.3) [13–40] | 24.3 (6.8) [15–39] | 26.0 (5.6) [15–38] | 24.3 (8.3) [14–44] |
| Nausea occurrence (seldom [1] –continually [5]) | 1.6 (0.9) | 1.4 (0.9) | 1.7 (1.1) | 1.9 (1.0) | 1.6 (0.8) |
| Nausea intensity (very mild [1]–sick as possibly could be [5]) | 1.4 (0.8) | 1.2 (0.6) | 1.7 (1.2) | 1.5 (0.6) | 1.5 (0.9) |
| Appetite (normal [1]–cannot stand the thought of food [5]) | 1.6 (1.1) | 1.8 (1.1) | 1.7 (0.8) | 2.5 (1.4) | 1.6 (0.8) |
| Insomnia (sleep as always [1]–impossible to sleep [5]) | 2.3 (1.3) | 2.2 (1.1) | 2.4 (1.3) | 2.7 (1.5) | 2.1 (1.4) |
| Pain occurrence (never [1]–constantly [5]) | 2.1 (1.3) | 1.9 (1.3) | 2.0 (1.2) | 2.5 (1.4) | 2.2 (1.5) |
| Pain intensity (very mild [1]–unbearable [5]) | 1.6 (0.9) | 1.5 (0.9) | 1.7 (0.9) | 1.9 (0.8) | 1.6 (1.1) |
| Fatigue (seldom [1]–exhausted most of the time [5]) | 2.6 (1.2) | 2.5 (1.3) | 2.7 (1.3) | 2.6 (1.0) | 2.5 (1.2) |
| Bowel (normal [1]–constant discomfort [5]) | 1.9 (1.1) | 1.9 (1.2) | 2.1 (1.3) | 1.8 (0.8) | 1.7 (0.9) |
| Concentration (normal ability [1]–cannot concentrate at all [5]) | 1.7 (1.0) | 1.6 (0.9) | 1.9 (1.2) | 1.7 (0.8) | 1.9 (1.2) |
| Appearance (unchanged [1]–constant, preoccupying concern [5]) | 1.7 (1.0) | 1.5 (0.8) | 1.9 (1.0) | 1.9 (1.2) | 2.1 (1.3) |
| Breathing (normal [1]–severe trouble [5]) | 1.3 (0.7) | 1.2 (0.7) | 1.3 (0.5) | 1.2 (0.4) | 1.6 (0.9) |
| Outlook (not worried or frightened about future [1]–terrified by thoughts of future [5]) | 1.9 (0.9) | 1.9 (0.9) | 2.0 (1.1) | 1.8 (0.8) | 1.9 (0.9) |
| Cough (seldom [1]–persistent and severe spells [5]) | 1.6 (0.9) | 1.3 (0.6) | 1.2 (0.5) | 2.2 (1.1) | 2.0 (1.1) |
| Enforced Social Dependency Scale (10 = lowest dependency, 51 = greatest dependency) | 22.2 (10.8) [10–47] | 20.9 (11.2) [10–46] | 21.6 (8.0) [10–38] | 30.5 (11.9) [10–47] | 18.4 (7.8) [10–40] |
| Personal competence subscale (6 = lowest dependency, 36 = greatest dependency) | 13.5 (7.9) [6–36] | 12.7 (8.2) [6–33] | 12.9 (5.5) [6–25] | 19.4 (9.3) [6–34] | 10.4 (5.5) [6–27] |
| Eating/feeding | 2.4 (1.5) | 2.3 (1.5) | 2.3 (1.2) | 3.5 (1.8) | 1.6 (1.2) |
| Dressing | 2.2 (1.7) | 2.0 (1.6) | 1.8 (1.1) | 3.8 (2.4) | 1.6 (0.7) |
| Walking | 2.0 (1.2) | 1.9 (1.3) | 2.0 (1.0) | 2.5 (1.5) | 1.9 (1.0) |
| Traveling | 3 (2.1) | 2.7 (2.1) | 3.4 (1.9) | 4.3 (2.2) | 2.1 (1.5) |
| Bathing | 2.0 (1.4) | 1.9 (1.5) | 1.7 (1.0) | 3.2 (1.6) | 1.6 (0.9) |
| Toileting | 1.9 (1.2) | 2.0 (1.4) | 1.7 (0.9) | 2.2 (1.4) | 1.6 (0.7) |
| Social competence subscale (3 = lowest dependency, 12 = greatest dependency) | 7.7 (3.2) [3–12] | 7.1 (3.4) [3–12] | 7.8 (2.8) [3–12] | 10.1 (3.0) [3–12] | 7.0 (2.7) [3–12] |
| Activities in home | 2.4 (1.2) | 2.2 (1.2) | 2.3 (1.0) | 3.3 (1.1) | 2.1 (1.1) |
| Work activities | 2.9 (1.2) | 2.7 (1.3) | 3.1 (1.1) | 3.5 (1.0) | 2.9 (1.2) |
| Recreational and social activities | 2.4 (1.1) | 2.2 (1.2) | 2.4 (1.0) | 3.4 (1.0) | 2.1 (1.0) |
GI, gastrointestinal.
Five clusters made the most clinical sense, in that the top five symptoms in each of the clusters corresponded to the prevalent symptoms, including impairment of activities of daily living, of patients of the dominant site of cancer for that cluster. The symptoms and impairments in activities of daily living for patients with GI cancer formed two distinct clusters (Clusters 1 and 2) while each other site of cancer appeared in singular clusters: gynecological (Cluster 3), head and neck (Cluster 4), and lung (Cluster 5). Table 3 displays the averages of the normalized index values for the K-means clustering of symptoms and activities of daily living for each of the five clusters.
Table 3. Average of Normalized Index Values and Conditional Probabilities for the K-Means Clustering of the Symptoms and Competence of the Activities of Daily Living.
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Symptoms | Normalized Index Values |
Conditional Probabilities |
Normalized Index Values |
Conditional Probabilities |
Normalized Index Values |
Conditional Probabilities |
Normalized Index Values |
Conditional Probabilities |
Normalized Index Values |
Conditional Probabilities |
| Physical and emotional symptoms | ||||||||||
| Nausea | 0.063 | 0.059 | 0.067 | 0.059 | 0.192 | 0.145 | 0.075 | 0.049 | 0.064 | 0.060 |
| Appetite | 0.066 | 0.062 | 0.124 | 0.110 | 0.092 | 0.069 | 0.079 | 0.051 | 0.066 | 0.062 |
| Insomnia | 0.089 | 0.084 | 0.080 | 0.071 | 0.100 | 0.075 | 0.086 | 0.056 | 0.058 | 0.055 |
| Pain | 0.079 | 0.074 | 0.059 | 0.052 | 0.110 | 0.083 | 0.096 | 0.063 | 0.074 | 0.070 |
| Bowel | 0.059 | 0.055 | 0.114 | 0.101 | 0.087 | 0.066 | 0.098 | 0.064 | 0.063 | 0.059 |
| Concentration | 0.085 | 0.080 | 0.083 | 0.074 | 0.111 | 0.084 | 0.078 | 0.051 | 0.061 | 0.057 |
| Appearance | 0.092 | 0.086 | 0.090 | 0.080 | 0.091 | 0.069 | 0.068 | 0.044 | 0.066 | 0.062 |
| Breathing | 0.077 | 0.072 | 0.076 | 0.067 | 0.076 | 0.057 | 0.088 | 0.057 | 0.110 | 0.104 |
| Outlook | 0.122 | 0.115 | 0.068 | 0.060 | 0.076 | 0.057 | 0.066 | 0.043 | 0.070 | 0.066 |
| Cough | 0.067 | 0.063 | 0.074 | 0.066 | 0.068 | 0.051 | 0.085 | 0.055 | 0.130 | 0.123 |
| Activities of daily living | ||||||||||
| Eating/feeding | 0.053 | 0.050 | 0.069 | 0.061 | 0.088 | 0.066 | 0.134 | 0.087 | 0.056 | 0.053 |
| Dressing | 0.044 | 0.041 | 0.041 | 0.036 | 0.050 | 0.038 | 0.162 | 0.105 | 0.051 | 0.048 |
| Walking | 0.061 | 0.057 | 0.061 | 0.054 | 0.068 | 0.051 | 0.131 | 0.085 | 0.076 | 0.072 |
| Bathing | 0.053 | 0.050 | 0.055 | 0.049 | 0.050 | 0.038 | 0.150 | 0.098 | 0.058 | 0.055 |
| Toileting | 0.054 | 0.051 | 0.066 | 0.059 | 0.068 | 0.051 | 0.140 | 0.091 | 0.058 | 0.055 |
|
| ||||||||||
| Composition by Cancer Sitea | n (%) | n (%) | n (%) | n (%) | n (%) | |||||
|
| ||||||||||
| Gastrointestinal | 16 (47) | 12 (63) | 3 (23) | 11 (41) | 5 (28) | |||||
| Gynecological | 8 (24) | 3 (16) | 6 (46) | 3 (11) | 1 (5) | |||||
| Head and neck | 3 (8) | 3 (16) | 1 (8) | 12 (44) | 3 (17) | |||||
| Lung | 7 (21) | 1 (5) | 3 (23) | 1 (4) | 9 (50) | |||||
| N = 34 | N = 19 | N = 13 | N = 27 | N = 18 | ||||||
Bold cells indicate that the symptom is more closely associated with the corresponding cluster than with any other cluster.
Columns equal numbers in clusters, but rows do not equal numbers of participants with that cancer site as a result of participants loading on more than one cluster.
Table 3 also displays the conditional probabilities of symptoms and activities of daily living in each cluster. It is more likely for patients in Cluster 1 to suffer more insomnia, appearance, and outlook complaints and for patients in Cluster 2 to complain of losing appetite and of bowel pains. Nausea is more likely for patients in Cluster 3. Patients in Cluster 4 are more likely to present with impairments in eating, dressing, bathing, and walking. Finally, it is more likely for patients in Cluster 5 to suffer breathing and coughing symptoms as compared with patients in other clusters.
Fig. 1 presents the clusters with the top five symptoms and impairments in activities of daily living sorted in the descending order of conditional probability. Inability to eat is among the top five in all clusters. The top five for Cluster 4 are all the impairments in activities of daily living (dressing, eating, bathing, toileting, and walking). In addition to eating, Cluster 5 has walking difficulties among its top five.
Fig. 1.
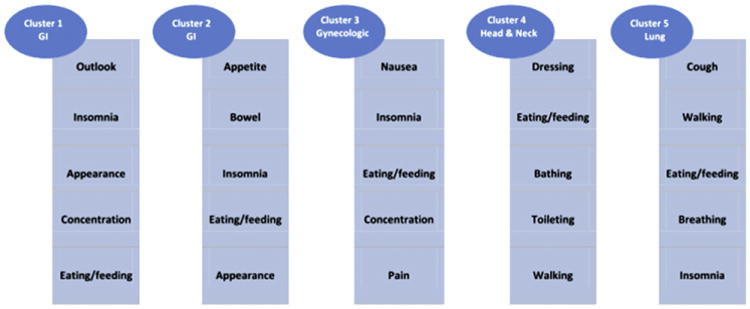
Top five symptoms and impairments in activities of daily living listed in descending order of conditional probability in each cluster.
Discussion
In this study, we sought to explore whether self-reported symptoms and impairments in activities of daily living of patients with late-stage cancers form clusters by the site of cancer at the same time since diagnosis. This homogeneity of stage and time since diagnosis and heterogeneity of cancer site were central to testing our hypothesis that people who present with late-stage cancers at the time of diagnosis experience clusters of symptoms and impairments of activities of daily living that cohered with the typical symptomatology of that site of cancer. We found five distinct clusters that formed around the site of cancer.
In the first cluster, patients reported symptoms (outlook, insomnia, appearance, and concentration) and changes in activities of daily living (eating and the inability to sleep). Patients in this cluster, more than 70% of whom were patients with GI and gynecologic cancers, may have had a poorer prognosis at the time of diagnosis, accounting for their poor outlook. Also, they may have lost weight or had a radical surgery that required an ostomy, and thus their appearance may have been affected. Whatever the reasons, it is important to recognize that changes in their abilities to eat and sleep form part of the constellation of their self-reported problems that concerned them, and thus should be included in the assessment of their diagnostic signs and symptoms. It is important to note that for Clusters 2, 3, and 5, the inability to eat, an activity of daily living, formed part of the clusters. In addition to eating problems, Cluster 5 had changes in the ability to walk, as one might expect from patients with late-stage lung cancer for whom breathing is also a difficulty. Strikingly, the top five symptoms for patients in Cluster 4, 85% of whom had head and neck and GI cancers, were all changes in their activities of daily living.
The importance of capturing clusters of individual symptoms and impairments in activities of daily living at the time of diagnosis is illustrated in Cluster 4. Patients who presented with Cluster 4 did not present with a cluster of troublesome physical symptoms, typical of a symptom cluster that would prompt further diagnostic testing to rule out cancer. Rather, they presented with a cluster of impairments in their abilities to perform activities of daily living. This impairment in function led them to seek additional diagnostic tests, which in turn led to a confirmation of cancer. These patients may have been unaware—or if aware, unable emotionally—to communicate their symptoms to loved ones or to care providers until their functional impairments became noticeable. This may suggest that the group represented by Cluster 4 was, before and at the time of diagnosis, in the midst of an existential crisis. Indeed, participants in our sample with head and neck cancer, who predominantly made up Cluster 4, reported the highest mean emotional distress. The possible relationship of functional impairment and emotional distress, particularly among patients with head and neck cancer, needs further study.
Unlike physical symptoms, others can often recognize the changes in patients' abilities to perform activities of daily living and report them in an objective manner. In addition, some ethnic groups of patients may find it as a sign of weakness to report their symptoms. In these instances, health care providers need to broaden their assessment of traditional self-reported symptoms to include the symptoms of impaired functioning.34 Our results point to the importance of assessing functional abilities along with assessing symptoms for the symptom management plan of care for the newly diagnosed patients with advanced cancer.
Although our study is not the first to identify that functional abilities are related to clusters of symptoms, we were not able to locate any other work that has included functional impairment as a symptom within the symptom cluster analysis. In our study, the inability to eat is prominent in all five clusters and can commonly be associated with fatigue and weight loss. Chow et al.35 identified broader aspects of functional interference, such as normal work and relations with others, along with personal dimensions of difficulty walking and inability to sleep. These personal dimensions of function (eating, bathing, and dressing) may be the most informative in helping to accelerate diagnosing people with late-stage cancers.
To accomplish our study, we recruited more than a 100 patients, all recently diagnosed (within 100 days of diagnosis) with Stage 3 or 4 cancers at four different sites, who were within the first month of their active cancer treatment. All had been diagnosed through a series of diagnostic tests and scans leading to either a biopsy or a major surgery. Most were receiving subsequent chemotherapy or chemotherapy and radiation combined. Many of these patients had been living with their cancer symptom-free for a significant period before the diagnosis. However, during that time, there were other indicators that finally prompted them to seek help and confirm a diagnosis. One of those nonsymptom-related factors was changes in their ability to engage in daily activities. Previously, McCorkle23 identified that some people only seek help when they can no longer do things they normally do in their lives. Health care providers often support patients' minimization of these presenting changes as symptoms because there is no obvious link to the causation or etiology. Studying a cohort of newly diagnosed patients with advanced solid tumors provided an opportunity to include impairments in activities of daily living as symptoms within the exploration of symptom clusters, particularly at the time for diagnosis. By adding impairments in everyday activities of daily living as a symptom in symptom cluster analysis, we were able to identify additional factors that are amenable to interventions to improve the way patients experience their disease and treatment.
Limitations
Our results show promise for identifying factors that may assist with diagnosing patients with advanced cancer earlier, and our results point to including inabilities to perform activities of daily living in the symptom management assessment of patients with newly diagnosed advanced cancer; however, there are several limitations that must be acknowledged. Our measurement of symptoms was one-dimensional and focused only on the presence of symptoms and not on their intensity or interference with activities. Secondly, although our sample was homogeneous, representing people with newly diagnosed late-stage cancer, there were different types of cancers included. Patients with late-stage lung cancer were treated very differently from late-stage ovarian cancer or pancreatic cancer. In addition, data presented in this secondary analysis were cross-sectional and represented only one data-collection point, baseline, in a longitudinal randomized trial to test a nursing intervention to improve patient outcomes, including symptoms. Finally, our sample size was moderate (N = 111); however, this was an exploratory study.
Conclusions
In this exploratory study, we conducted a cluster analysis of symptoms and impairments of daily activities using K-means clustering algorithms, in combination with cosine similarity, to form the most cohesive and inter-pretable clusters. Five clusters, which formed around the predominant site of cancer represented by each cluster, resulted. Our findings suggest that impairments in activities of daily living, such as eating and walking in patients newly diagnosed with late-stage cancers, behave as signs and symptoms during the diagnostic phase. Although additional research is needed to confirm these findings, our exploratory study suggests that health care providers need to expand their symptom assessments to include impairments in the activities of daily living. Early recognition of changes in functional abilities may help accelerate patients' diagnoses at an earlier cancer stage.
Acknowledgments
Ruth McCorkle, Mei Bai, and Elizabeth Ercolano are partially supported by the NIH/NINR grant R01NR011872. Dr. McCorkle was Principal Investigator.
Footnotes
Disclosures: The authors have no financial or personal relationships that might bias the work to disclose.
References
- 1.Twycross R. Patient care: past, present, and future. OMEGA, Journal of Death and Dying. 2007-2008;56:7–19. doi: 10.2190/om.56.1.b. [DOI] [PubMed] [Google Scholar]
- 2.McCaffery M. Patients in pain: what they say, and what they really mean. Director. 2005;13:104–106. [PubMed] [Google Scholar]
- 3.Foley K. Building the field of cancer pain. J Palliat Med. 2008;11:176–179. doi: 10.1089/jpm.2008.9974. [DOI] [PubMed] [Google Scholar]
- 4.Miaskoswki C, Dodd M, Lee K. Symptom clusters: the new frontier in symptom management research. JNCI Monographs. 2004;32:17–21. doi: 10.1093/jncimonographs/lgh023. [DOI] [PubMed] [Google Scholar]
- 5.Blesch KS, Paice JA, Wickham R, et al. Correlates of fatigue in people with breast or lung cancer. Oncol Nurs Forum. 1991;18:81–87. [PubMed] [Google Scholar]
- 6.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology patients receiving radiation therapy for bone metastases: a pilot study. J Pain Symptom Manage. 1999;17:320–325. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 7.Ciaramella A, Poli P. Assessment of depression among cancer patients: the role of pain, cancer type, and treatment. Psychooncology. 2001;10:156–165. doi: 10.1002/pon.505. [DOI] [PubMed] [Google Scholar]
- 8.Dodd MJ, Miaskowski C, Paul S. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–470. [PubMed] [Google Scholar]
- 9.Gaston-Johansson F, Fall-Dickson JM, Bakos AB, Kennedy MJ. Fatigue, pain, and depression in pre-autotransplant breast cancer patients. Cancer Pract. 1999;7:240–247. doi: 10.1046/j.1523-5394.1999.75008.x. [DOI] [PubMed] [Google Scholar]
- 10.Given B, Given C, Azouz F, Stommel M. Physical functioning of elderly cancer patients prior to diagnosis and following initial assessment. Nurs Res. 2001;50:222–232. doi: 10.1097/00006199-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Hickok JT, Morrow GR, McDonald S, Bellg AJ. Frequency and correlates of fatigue in lung cancer patients receiving radiation therapy: implications for management. J Pain Symptom Manage. 1996;11:370–377. doi: 10.1016/0885-3924(96)00008-5. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel D, Sands S, Koopman C. Pain and depression in patients with cancer. J Cancer. 1994;74:2570–2578. doi: 10.1002/1097-0142(19941101)74:9<2570::aid-cncr2820740927>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Sarna L. Correlates of symptom distress in women with lung cancer. J Cancer Pract. 1993;1:21–28. [PubMed] [Google Scholar]
- 14.Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. JNCI Monographs. 2004;32:76–78. doi: 10.1093/jncimonographs/lgh008. [DOI] [PubMed] [Google Scholar]
- 15.Reyes-Gibby CC, Aday LA, Anderson KO, Mendoza TR, Cleeland CS. Pain, depression, and fatigue in community-dwelling adults with and without a history of cancer. J Pain Symptom Manage. 2006;32:118. doi: 10.1016/j.jpainsymman.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francouer RB. The relationship of cancer symptom clusters to depressive affect in the initial phase of palliative radiation. J Pain Symptom Manage. 2005;29:130–155. doi: 10.1016/j.jpainsymman.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung WY, Le LW, Zimmermann C. Symptom clusters in patients with advanced cancers. Support Care Cancer. 2009;17:1223–1230. doi: 10.1007/s00520-009-0577-7. [DOI] [PubMed] [Google Scholar]
- 18.Given C, Sikorskii A, Tamkus D, et al. Managing symptoms among patients with breast cancer during chemotherapy: results of a two-arm behavioral trial. J Clin Oncol. 2008;26:5855–5862. doi: 10.1200/JCO.2008.16.8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miaskowski C. Symptom clusters: establishing the link between clinical practice and symptom management research. Support Care Cancer. 2006;14:792–794. doi: 10.1007/s00520-006-0038-5. [DOI] [PubMed] [Google Scholar]
- 20.Barsevick AM, Whitmer K, Nail LM, et al. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31:85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Gilbertson-White S, Aouizerat BE, Jahan T, Miaskowski C. A review of the literature on multiple symptoms, their predictors, and associated outcomes in patients with advanced cancer. Palliat Support Care. 2011;9:81–102. doi: 10.1017/S147895151000057X. [DOI] [PubMed] [Google Scholar]
- 22.Kurtz ME, Given B, Kurtz JC, Given C. The interaction of age, symptoms, and survival status on physical and mental health of patients with cancer and their families. Cancer. 1994;74:2071–2078. doi: 10.1002/1097-0142(19941001)74:7+<2071::aid-cncr2820741715>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Cleeland CS, Sloan JA. Assessing the symptoms of cancer using patient-reported outcomes (ASC-PRO): searching for standards. J Pain Symptom Manage. 2000;39:1077–1085. doi: 10.1016/j.jpainsymman.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson G, McCorkle R, Georgiadou F, Benoliel JQ. Distress, dependency, and threat in newly-diagnosed cancer and heart patients. Multi-variate Behav Res Monogr. 1986;21:267–298. doi: 10.1207/s15327906mbr2103_1. [DOI] [PubMed] [Google Scholar]
- 25.McCorkle R, Dodd M, Ercolaneo E, et al. Effects of a nursing intervention on quality of life outcomes in post-surgical women with gynecological cancers. Psychooncology. 2009;18:62–70. doi: 10.1002/pon.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth AJ, Kornblith AB, Batel-Copel L, Holland J. Rapid screening for psychologic distress in men with prostate carcinoma. Cancer. 1998;82:1904–1908. doi: 10.1002/(sici)1097-0142(19980515)82:10<1904::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Satariano WA, Ragheb NE, Depuis MH. Comorbidity in older women with breast cancer: an epidemiologic approach. In: Yancik R, Yates J, editors. Cancer in the elderly: Approaches to early detection and treatment. New York: Springer; 1998. pp. 71–107. [Google Scholar]
- 28.Berkman LF, Breslow L. Health and ways of living: The Alameda County Study. New York: Oxford University Press; 1983. [Google Scholar]
- 29.Kaplan G, Kotler P. Self-reports predictive of mortality from ischemic heart disease: a nine-year follow-up of the Human Population Laboratory cohort. J Chronic Dis. 1985;38:195–201. doi: 10.1016/0021-9681(85)90092-x. [DOI] [PubMed] [Google Scholar]
- 30.McCorkle R, Benoliel JQ. Symptom distress, current concerns, and mood disturbance after a diagnosis of life threatening disease. Soc Sci Med. 1983;17:431–438. doi: 10.1016/0277-9536(83)90348-9. [DOI] [PubMed] [Google Scholar]
- 31.McCorkle R, Cooley ME, Shea J. Unpublished manuscript. New Haven, CT: Yale School of Nursing; 1998. A user's manual for the Symptom Distress Scale. [Google Scholar]
- 32.Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12:694–708. doi: 10.1002/pon.694. [DOI] [PubMed] [Google Scholar]
- 33.Tang S, McCorkle R. Unpublished manuscript. New Haven, CT: Yale School of Nursing; 2002. A user's manual for the Enforced Social Dependency Scale. [Google Scholar]
- 34.Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;4:270–282. doi: 10.1097/00002820-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Chow E, James J, Barsevick A, et al. Functional interference clusters in cancer patients with bone metastases: a secondary analysis of RTOG9714. Int J Radiat Oncol Biol Phys. 2010;76:1507–1511. doi: 10.1016/j.ijrobp.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


