Abstract
Interest in the value of Omega—3 (n—3) fatty acid supplementation for treatment of ADHD remains high. No prior meta-analysis has examined whether ADHD is associated with alterations in blood lipid levels and meta-analyses of supplementation have reached conflicting conclusions.
Methods
We report two new meta-analyses. Study 1 examined blood levels of Omega—3 fatty acids in relation to ADHD. Study 2 examined a larger sample of randomized intervention trials than previously reported.
Results
Study 1 included 9 studies (n = 586) and found lower overall blood levels of n—3 in individuals with ADHD versus controls (g = 0.42, 95% CI = 0.26–0.59; p < .001). Study 2 included 16 studies (n = 1408) and found that n—3 supplementation improved ADHD composite symptoms; using the best available rating and reporter (g = 0.26, 95% CI = 0.15–0.37; p < .001). Supplementation showed reliable effects on hyperactivity by parent and teacher report, but reliable effects for inattention only by parent report.
Conclusions
Omega—3 levels are reduced in children with ADHD. Dietary supplementation appears to create modest improvements in symptoms. There is sufficient evidence to consider Omega—3 fatty acids as a possible supplement to established therapies. However it remains unclear whether such intervention should be confined to children with below normal blood levels.
Keywords: ADHD, Omega—3, Polyunsaturated fatty acids, Supplementation, Intervention, Meta-analysis
1. Introduction
Whether ADHD may be related to inadequate bioavailability of Omega—3 fatty acids, and whether it may be improved by dietary supplementation, has drawn increasing interest in part due to the growing awareness of the role of nutrition in neural development (Janssen & Kiliaan, 2013) and, potentially, in ADHD (Arnold, Lofthouse, & Hurt, 2012; Bloch & Qawasmi, 2011; Nigg, Lewis, Edinger, & Falk, 2012; Stevenson et al., 2014). Furthermore, the importance of long chain polyunsaturated fatty acids (LC-PUFAs), in particular Omega—3 long chain fatty acids, has been highlighted as a promising area of research interest to physical as well as mental health (Milte, Sinn, & Howe, 2009; Sinn & Bryan, 2007; Vaisman et al., 2008; Voigt et al., 2001). It is increasingly recognized that like other mental disorders, ADHD, while heritable, is probably a product of interplay of genetic liability and environmental stressors (Nigg, Nikolas, & Burt, 2010), of which nutrition may be a component. However, recent reviews and meta-analyses have reached conflicting conclusions with regard to Omega—3 supplementation, as detailed below.
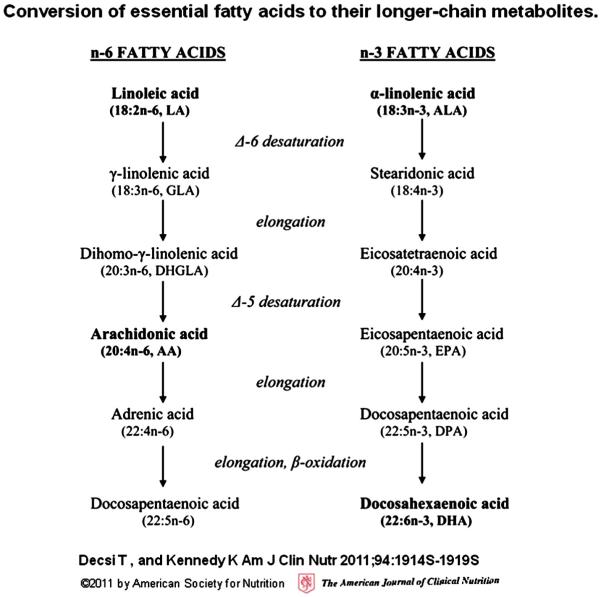
Polyunsaturated fatty acids (PUFAs) exist in two classes: Omega—3 (n—3) and omega—6 (n—6) and humans cannot synthesize them. Once ingested, short chain PUFAs are converted to long-chain fatty acids (LC-PUFAs); their conversion chains are summarized in Fig. 1. Optimal health and development require a balanced ratio of n—3 to n—6 but the typical western diet provides a much larger share of n—6 as compared to n—3, often resulting in an imbalance and in an insufficient n—3 (Schuchardt, Huss, Stauss-Grabo, & Hahn, 2009). Therefore the n—3 status is of most interest in the present report. In that domain, as Fig. 1 shows, ALA (which must be ingested) is converted to EPA and then DHA through enzymatic elongation, resulting in their designation as LC-PUFA. (Decsi & Kennedy, 2011) Genetically mediated differences in conversion and metabolism may be present if children with ADHD have low blood levels of EPA and DHA despite adequate dietary intake and absorption. A first question, therefore, is whether ADHD is characterized by lower blood levels of these compounds.
Fig. 1.
Fatty acid elongation. Metabolic conversion process of essential fatty acids to long-chain fatty acids.
EPA and DHA are the most well studied and accordingly are the most commonly supplemented fatty acids in over-the-counter fish oils; DHA has been added to many pre-natal vitamins and to infant formula. Breast milk also naturally contains LC-PUFAs, which may be related to better cognitive outcomes in children who were breastfed (Drane & Logemann, 2000), compared to formulas prior to their supplementation with LC-PUFAs (Belfort et al., 2013).
Additionally, recent research has shown inverse correlations between levels of DHA in cord blood and offspring inattention and hyperactivity at age ten years (Kohlboeck et al., 2011).
Links to ADHD are plausible because fatty acids are essential to neural development and signaling. For example, levels of available PUFA and LC-PUFA influence cell membrane fluidity, affecting absorption or release of neurotransmitters (Janssen & Kiliaan, 2013). and contribute to cortical organization and connectivity (Grayson, Kroenke, Neuringer & Fair, 2014). Adding to the importance of the current study is that consumers may in fact frequently be supplementing children’s diets in an effort to control ADHD symptoms (Millichap & Yee, 2012). This makes evidence as to their effectiveness—or not—quite timely.
Multiple studies, reviews and three prior meta-analyses have examined the efficacy of fatty acid supplementation on ADHD symptoms. The following summary of their findings notes relevant limitations of each study’s methodology. Bloch and Qawasmi (2011) examined 10 intervention studies of ADHD and fish oil (a major source of PUFAs) or Omega—3 supplementation, selected either a parent or a teacher rating from each study but did not compare them, and concluded that there was a benefit, with a standardized mean effect size (SMD) of 0.31 (95% CI = 0.16 to 0.47, p < 0.001). Gillies, Sinn, Lad, Leach, and Ross (2012) included studies using any combination of Omega—3 and omega—6 acids but pooled results separately for different study designs, leading to small sample sizes for particular comparisons. Although they noted a higher likelihood of improvement in the Omega—3/6 group compared to placebo (risk ratio = 2.19, 95% CI = 1.04–4.62), this pertained only to two studies. Their primary conclusion was that when they pooled 5 studies of ADHD symptoms (n = 413) there was no difference in ADHD symptoms by parent-rating (SMD (standard mean difference) = 0.17, 95% CI = 0.03–0.38) or teacher rating (SMD = 0.05, 95% CI = −0.18–0.27). Sonuga-Barke et al. (2013) examined 11 intervention studies (7 studies included in Bloch & Qawasmi, 2011 and 2 studies supplementing with only n—6) and selected the “most proximal rater.” They found a reliable but modest effect of SMD = 0.21 (95% CI = 0.05–0.36, p = 0.007), which weakened to SMD = 0.16 (95% CI = 0.01–0.31, p = 0.04) when only “probably blinded” trials were included. Stevenson et al. (2014) reviewed and critiqued these meta-analyses as well as newer studies, and concluded that LCPUFA’s have a modest effect on ADHD symptoms. We sought to expand and clarify the previous work in three ways.
First, it is unclear whether children with ADHD have inadequate baseline Omega—3 levels. This is relevant to theories of etiology and to directing future work to determine whether all or only some children with ADHD may be appropriate clinical targets for supplementation. No prior meta-analysis had addressed this critical point, to the best of our knowledge, although some studies have shown reduced levels in participants with ADHD in comparison to the reference samples (Antalis et al., 2006; Stevens et al., 2003).
Second, with regard to results of supplementation trials, it is unclear whether ADHD symptom domains of (a) inattention/disorganization versus (b) hyperactivity/impulsivity respond differentially to supplementation, which will be important to interpreting future studies as well as aiming toward individualized clinical application of dietary supplementation. Gillies et al. (2012) looked at this but only across 5 studies, and we sought here to do so more comprehensively.
Third, previous meta-analyses have been inconsistent when examining the potential effects the rater may have on the results. We were interested in rater effects as a sign of replication or non-replication of results across rater and setting, important to fully evaluating the strength and generalizability of effects—particularly in light of questions about adequacy of observer blinding in these studies. No prior meta-analysis directly examined this.
Finally, each prior meta-analysis excluded some available studies for various reasons. We added 5 studies that none of the other meta-analyses used. Three of these are relatively new reports that met our criteria for inclusion (Milte et al., 2012; Perara, Jeewandara, Seneviratne, & Guruge, 2012; Richardson, Burton, Sewell, Spreckelsen, & Montgomery, 2012). As far as we can tell, should they have been published at the time, they would have met the inclusion criteria for one or more of the prior meta-analyses. We also included Kirby, Woodward, Jackson, Wang, and Crawford (2010). It was not mentioned by the prior reviews but it appeared to us that it would have met the inclusion criteria for one or more of them because it was a randomized controlled trial using a validated ADHD rating scale. We added one another study: Itomura et al. (2005). It was considered only by Bloch and Qawasmi (2011), who excluded the study because it was not clear what parent-rating scale of DSM symptoms was used or whether it was done with a validated rating scale. We did not consider this a reason to exclude the study, because counts of ADHD symptoms by parent rating tend to be highly correlated across types of measures, and number of DSM symptoms (albeit assessed with a known rating scale) has been used in other important ADHD treatment studies including the MTA study. We excluded 4 studies that were used in at least one previous analysis. Aman, Mitchell, and Turbott (1987) and Arnold et al. (1989) were excluded for using only omega—6 as the intervention. Although Hirayama, Hamazaki, and Terasawa (2004) met our inclusion criteria, insufficient data were published in the paper to enable an effect size to be computed and attempts to reach the author were unsuccessful. Finally, Brue, Oakland, and Evans (2001) study was excluded for using a supplement with various active compounds so that effects could not be attributed to n—3. All of these resulted in an updated and larger data base than any prior meta-analysis with which to estimate population effects, focused specifically on n—3.
We organize this report into two distinct meta-analyses. Study 1 aims to examine blood lipid profiles of children with and without ADHD. Study 2 aims to replicate and extend meta-analytic results for intervention studies of Omega—3 fatty acids and fish oil.
Both meta-analyses included studies that reported n—3 data; report of n—6 data was not used herein. We made this decision because n—6 PUFA levels are typically higher than n—3 levels; therefore increases in dietary intake produce only modest increases in the linoleic acid (LA) and arachidonic acid (AA) content of the plasma. However, even small increases in dietary n—3 PUFA intake can produce relatively large increases in plasma n—3 PUFA. Additionally, n—6 acids have pro-inflammatory properties while n—3 acids have anti-inflammatory properties; the latter are more heavily theorized to be beneficial for ADHD and brain development under Western diets (Schuchardt et al., 2009).
2. Study 1: blood lipid profiles of children with and without ADHD
2.1. Search methods
Pubmed and Pyschinfo were used for the article search from January 2001 through March 2013. Search terms included: ADHD, attention deficit hyperactivity disorder, fatty acid, n—3, Omega—3, polyunsaturated fatty acids, PUFA, blood levels, plasma and RBC. In addition we searched reference sections of prior meta-analyses and reviews.
2.2. Inclusion criteria
Inclusion in Study 1 required (a) an operationally defined ADHD group, (b) a control group exhibiting typical development, and (c) reported data of red blood cells (erythrocytes) and/or plasma levels of Omega—3 compounds. Plasma levels reflect more recent fluctuations (days) in phospholipids while erythrocytes reflect more long term changes (months). Studies were excluded for not having a control group (Gow et al., 2009; Sorgi, Hallowell, Hutchins, & Sears, 2007; Vaisman et al., 2008), or if data were not reported or discoverable upon inquiry (Germano et al., 2007; Itomura et al., 2005; Stevens, Zentall, Abate, Kuczek, & Burgess, 1996). Fig. 2 shows the flow diagram of study selection and exclusion on the left hand side. Moderators tested include: age, tissue type, and nation in which the study was conducted.
Fig. 2.
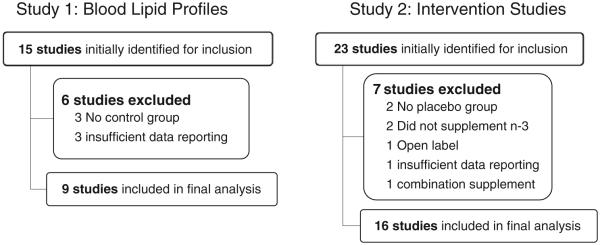
Study selection. Study selection parameters for each meta-analysis.
2.3. Analytic approach
The software Comprehensive Meta-Analysis (Borenstein, Hedges, Higgins, & Rothstein, 2005) was used to calculate effect sizes and analyze pooled results. A random effects model was used and effect size was calculated using Hedges’s g (a bias corrected version of Cohen’s “d”) with a 95% CI. Heterogeneity was quantified using the I2 statistic and statistically tested with the Q statistic (Huedo-Medina, Sánchez-Meca, Marín-Martínez, & Botella, 2006).
2.4. Results
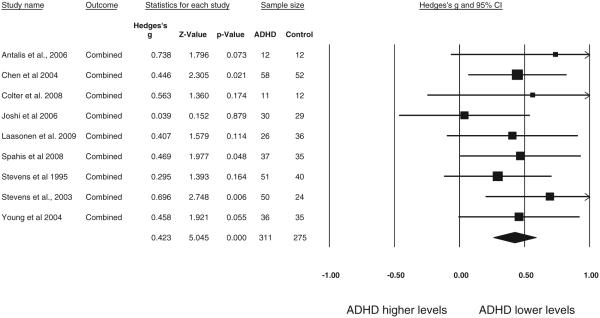
Study 1 included 9 studies (n = 586) with an average age of 16.3 (age range 8–35); these studies are summarized in Table 1. Blood levels were entered for all reported categories of Omega—3’s including: EPA, DHA, ALA and DPA. We first pooled all four values (or all available if less than four reported) within study using the default modified fixed effects model provided in CMA (using a fixed effect model within study), and conducted a meta-analysis of the pooled effect sizes using a random effects model. That result revealed lower overall levels of n—3 in the ADHD subjects (g = 0.42, 95% CI = 0.26–0.59, z = 5.05, p < .001); the results are depicted as a forest plot in Fig. 3. We next filtered for EPA and DHA only (long chain fatty acids), pooling those two values within study. When we did this the effect was somewhat larger (g = 0.51, 95% CI 0.34–0.67, z = 6.00, p < 0.001). We then examined EPA alone (g = 0.41, 95% CI 0.25–0.57, z = 4.91, p < 0.001) and DHA alone, which showed the largest effect (g = 0.59, 95% CI 0.40–0.77, z = 6.34, p < 0.001), suggesting that DHA levels were likely “carrying” much of the n—3 effect.
Table 1.
Details of studies included in Study 1: Blood level meta-analysis.
| Author | Year | ADHD N | Control N | Total N | Mean age | Country | Plasmaa | RBCb | Dietary analysis |
Method | Difference in dietary n–3 intake? |
Dietary data reported? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antalis et al. | 2006 | 12 | 12 | 24 | 23.5 | USA | Y | Y | Y | 3 day diet record | N | No; 30% more calories from sat fat in ADHD |
| Chen et al. | 2004 | 58 | 52 | 110 | 8.2 | Taiwan | Y | Y | Y | 3 day diet record | N | Yes: no difference in ALA intake across groups |
| Colter et al. | 2008 | 11 | 12 | 23 | 13.9 | Canada | N | Y | Y | 7 day diet record | N | Yes: no diff in any n–3 intake across groups |
| Joshi et al. | 2006 | 30 | 29 | 59 | 7.75 | India | Y | Y | N | N/A | N/A | N/A |
| Laasonen et al. | 2009 | 26 | 36 | 62 | 35.2 | Finland | Y | Y | y | Self report dietary questionnaire | N | Yes: no diff in intake across groups fatty fish or supplements |
| Spahis et al. | 2008 | 37 | 35 | 72 | 8.8 | Canada | Y | Y | Y | Self report dietary questionnaire | N | No: compared to a population sample |
| Stevens et al. | 1995 | 51 | 41 | 92 | 9.1 | USA | Y | Y | Y | 3 day diet record | N | Yes: total PUFA only (not n–3) sig. (p < .05) but no difference in high vs. low subjects |
| Stevens et al. | 2003 | 50 | 24 | 74 | 9.8 | USA | Y | Y | Y | Parent report | N | Yes: no diff in ALA intake across groups |
| Young et al. | 2004 | 36 | 35 | 71 | 30.3 | Canada | N | Y | N | N/A | N/A | N/A |
Plasma: fluid component of blood.
RBC: red blood cells (erythrocytes).
Fig. 3.
ADHD vs. non-ADHD Omega—3 blood level: All measures pooled. Study 1 forest plot of Omega—3 blood levels (RBC or plasma) in ADHD vs. non-ADHD participants shows significantly lower levels for ADHD participants (g = 0.423). The size of the square indicates the study weight, and the width of the diamond is the 95% confidence interval (CI).
Secondary analyses are reported on the pooled result (that is, across all four n—3’s). For that effect, heterogeneity was not observed (I2 = 0%, Q = 4.5, p = 0.81). When testing for moderators, no significant effects were seen for age, tissue type (RBC vs. plasma) or nation in which the study was conducted (all p > .07). A sensitivity analysis (one study removed) revealed no significant effect from a single study removed; point estimate range of 0.39 to 0.47 (all p < 0.001). To address the possibility of a file drawer effect, Orwin’s fail-safe N indicated that 30 unpublished studies with a zero effect would be necessary to reduce the effect size of Study 1 to a trivial g = 0.10. Investigation of publication bias indicated the presence of bias (i.e., over-representation of large studies); Duval and Tweedie’s (2000) Trim and Fill analysis was applied to address this bias. This procedure examines the funnel plot for asymmetry around the observed mean and fills in (imputes) missing studies of equal size to create a symmetric funnel plot and evaluate the change in estimate. This procedure required adding of three imputed studies (effect sizes) the left side of the funnel plot to create symmetry. This reduced the effect size only slightly (g = 0.36, 95% CI 0.21–0.51, Q = 7.86) suggesting again that publication bias was unlikely to account for findings.
2.5. Summary of Study 1
Children with ADHD had reliably lower blood levels of n—3 fatty acids than typically developing children, with a moderate effect size of one third (pooled across compounds) to one half (for DHA/EPA) of a standard deviation and a 95% confidence interval ranging from 1/4 to 3/4 of a standard deviation.
3. Study 2: intervention studies of Omega—3 fatty acids in ADHD
3.1. Search methods
Pubmed and Psychinfo were used for the article search from January 2001 through March 2013. Search terms included: ADHD, attention deficit hyperactivity disorder, fatty acid, n—3, Omega—3, polyunsaturated fatty acids, PUFAs, supplementation and intervention. Prior reviews were examined to ensure all studies cited by them were considered. When insufficient data were reported attempts were made to contact authors to acquire the data. Fig. 2 shows the flow diagram of study selection and exclusion on the right hand side.
3.2. Inclusion criteria
This was identical to Study 1.
3.3. Analytic approach
Inclusion in the analysis of intervention studies required (a) a randomized, single or double blind trial with an n—3 compound, examining change in ADHD symptoms in children with or without ADHD, (b) use of a validated parent or teacher rating scale of ADHD symptoms or DSM-IV classifications, and (c) reporting of dosage and compound, and (d) duration of intervention. Seven potential studies were excluded for the following reasons: not including a placebo control group (Germano et al., 2007; Joshi et al., 2006), not supplementing with n—3 (Aman et al., 1987; Arnold et al., 1989), being open label (Sorgi et al., 2007), insufficient data reporting to enable calculation of an effect size (Hirayama et al., 2004), with no response from the author when contacted, and for using a supplement with additional active compounds (Brue et al., 2001).
Individual analyses were performed on (a) parent and (b) teacher ratings on three outcome measures: (1) a composite measure of ADHD using the best available summary measure (either Conners' ADHD Index or, if not available, DSM-IV total symptom score) (2) inattention symptom total, and (3) hyperactivity–impulsivity symptom total. Moderators tested using meta-regression include: intervention compound and dosage, length of intervention, reporter, nation, and mean age. Heterogeneity and publication bias were handled as in Study 1. In addition, a replication analysis was performed on Bloch and Qawasmi’s (2011) work using a random effects model, including two new studies that identified an ADHD group by diagnosis or rating scale, following Bloch and Qawasmi’s inclusion criteria.
3.4. Results
16 Intervention Studies (n = 1408) with an average age of 9.7 (age range 6–18) were included in the initial analysis and are summarized in Table 2. The average n—3 dosage was 616 mg and the average length of dose was 14.5 weeks.
Table 2.
Details of studies included in Study 2: Intervention meta-analysis.
| Author | Year | Na | Mean age | Country | Daily n – 3 (mg) | EPA (mg) | DHA (mg) | ALA (mg) | DPA (mg) | Study design | Parent report | Teacher report | Rating scalec | Length (weeks) | Additional interventions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belanger et al. | 2009 | 26 | 9.2 | Canada | 1050 | 750 | 300 | 0 | 0 | One-way crossover | Y | N | CPRS-L | 16 | n/a |
| Gustafsson et al. | 2010 | 92 | NRb | Sweden | 500 | 500 | 0 | 0 | 0 | Parallel | Y | Y | CPRS-S, CTRS-S | 15 | n/a |
| Itomura et al. | 2005 | 85 | 10.3 | Japan | 634 | 120 | 514 | 0 | 0 | Parallel | Y | N | DSM-IV | 12 | n/a |
| Johnson et al. | 2009 | 75 | 12 | Sweden | 732 | 558 | 174 | 0 | 0 | One-way crossover | Y | NR | ADHD-RS | 12 | n/a |
| Kirby et al. | 2010 | 49 | 9.1 | UK | 456 | 56 | 400 | 0 | 0 | Parallel | Y | Y | SNAP-IV | 16 | n/a |
| Manor et al. | 2012 | 78 | 9.3 | Israel | 120 | 86 | 34 | 0 | 0 | Parallel | Y | Y | CPR-T, CPR-P | 15 | Phosphatidylserine |
| Milte et al. | 2012 | 87 | 8.9 | Australia | 2513 | 1373 | 1140 | 0 | 0 | Three-way crossover | Y | N | CPRS-L | 16 | n/a |
| Perera et al. | 2012 | 94 | 9.3 | Sri Lanka | 955 | 593 | 0 | 0 | 0 | Parallel | Y | N | DSM-IV, SNAP | 24 | Methylphenidate |
| Raz et al. | 2009 | 63 | 10.5 | Israel | 120 | 0 | 0 | 120 | 0 | Parallel | Y | Y | ADHD-RS | 7 | n/a |
| Richardson et al. | 2005 | 117 | 8.5 | UK | 732 | 558 | 174 | 0 | 0 | Parallel/crossover | N | Y | CTRS-L | 12 | n/a |
| Richardson & Puri | 2002 | 29 | 10.3 | UK | 762 | 186 | 480 | 0 | 0 | Parallel | Y | N | CPRS-L | 12 | n/a |
| Richardson et al. | 2012 | 362 | 10.1 | UK | 600 | 0 | 600 | 0 | 0 | Parallel | Y | Y | CPRS-L, CTRS-L | 16 | n/a |
| Sinn et al. | 2007 | 104 | 9.8 | Australia | 732 | 558 | 174 | 0 | 0 | Parallel | Y | NR | CPRS-L | 15 | n/a |
| Stevens et al. | 2003 | 33 | 9.8 | USA | 560 | 80 | 480 | 0 | 0 | Parallel | Y | Y | ASQ and DBD | 16 | Stimulants |
| Vaisman et al. | 2008 | 60 | 9.3 | Israel | 262 | 156 | 95 | 7 | 4 | Parallel | Y | N | CPRS-S | 12 | n/a |
| Voight et al. | 2001 | 54 | 9.3 | USA | 345 | 0 | 345 | 0 | 0 | Parallel | Y | N | CBCL | 16 | n/a |
N = number of completers in sample.
NR = not reported.
Rating scale: ADHD-RS = ADHD rating scale; ASQ = Connors' Parental Abbreviated Symptom Questionnaire; CBCL = Connors' Behavioral Checklist; CPRS-L = Connors' Parents Rating Scale-Long Version; CPRS-S = Connors' Parents Rating Scale-Revised Short Version; DBD = Disruptive Behavior Disorders Rating Scale; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and SNAP = Swanson, Nolan and Pelham version IV.
3.5. Overall effect on ADHD symptoms
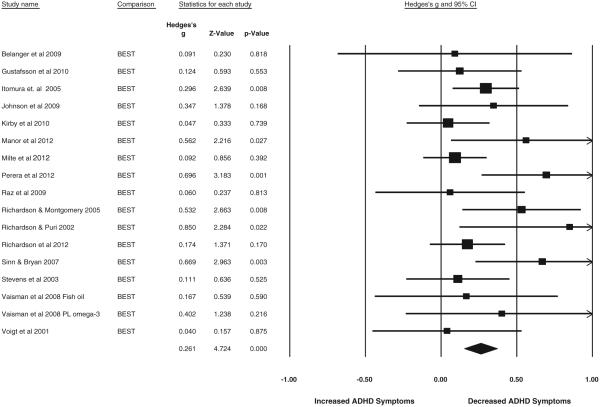
Examining the ADHD composite measure using the best available single measure pooled within study, across parent and teacher, revealed a small but statistically reliable reduction in ADHD symptoms (g = 0.26, 95% CI = 0.15–0.37, z = 4.73, p < .001) and showed minimal heterogeneity (I2 = 25%. Q = 16, p = 0.16). The results are depicted as a forest plot in Fig. 4. This effect replicated well when examined by a reporter. In parent report (n = 1201), g = 0.22, 95% CI = 0.11–0.33, z = 3.99, p < .001; minimal heterogeneity was observed (I2 = 19%, Q = 14.8, p = 0.25). In teacher report (n = 640), g = 0.17, 95% CI = 0.02–0.32, z = 2.18, p < .05; no heterogeneity was observed (I2 = 0%, Q = 2.3, p = 0.80).
Fig. 4.
Best available parent and teacher report of inattention/hyperactivity combined. Study 2 forest plot of symptoms of inattention and hyperactivity post intervention using the best available rating by either parent or teacher. The size of the square indicates the study weight, and the width of the diamond is the 95% confidence interval (CI).
3.6. Symptom specific reports
Our next analysis looked at individual symptom domains across reporters. For the hyperactivity–impulsivity symptom domain, pooled parent/teacher data showed a reliable symptom reduction post supplementation (n = 1254, g = 0.26, 95% CI = 0.13–0.39, z = 3.96, p < .001). Parent report alone showed essentially the same effect (n = 1201, g = 0.23, 95% CI = 0.10–0.36, z = 3.48, p < .01); although heterogeneity was observed (I2 = 41%, Q = 20.4, p = 0.06). The heterogeneity was completely removed with one outlier eliminated (Perara et al., 2012) (I2 = 0%, Q = 6.9, p = 0.81), but this reduced the overall effect (g = 0.17, 95% CI = 0.07–0.26, z = 3.41, p < .01). Teachers reported a nearly identical reduction in hyperactivity (n = 640, g = 0.22, 95% CI = 0.06–0.37, z = 2.79, p < 0.01), with no heterogeneity.
For the inattention symptom domain, pooled parent/teacher report showed a slightly smaller reduction in inattentive symptoms (n = 1171, g = 0.22, 95% CI = 0.10–0.34, z = 3.61, p < .001). Parent report indicated the same reduction in inattention (n = 1118, g = 0.22, 95% CI = 0.09–0.34, z = 3.36, p < .01) with modest heterogeneity (I2 = 26%, Q = 14.8, p = 0.19). However, teacher report of inattention was not significant (n = 639, g = 0.12, 95% CI = −0.03–0.28, z = 1.57, p = 0.78).
3.7. Replication analysis
A replication analysis was performed on the meta-analysis reporting the largest effect size of SMD = 0.31 (Bloch & Qawasmi, 2011). We first analyzed only the studies included in their analysis using a random effects design (they used a fixed effects model), and found a similar result of g = 0.29. We then included two studies that were not published at the time of their analysis but which we judged would have met their inclusion criteria (Manor et al., 2012; Perara et al., 2012). This increased the effect slightly to g = 0.35, 95% CI = 0.20–0.50, z = 4.62, p < .001.
3.8. Moderators
Moderator effects were investigated secondarily only for the best single composite measure (the largest effect) using meta-regression. Overall n—3 dose effects were non-significant (z = −0.687, p = 0.49). However, when investigating long chain fatty acids separately, a significant positive effect was shown for EPA dose (z = 2.280, p < .05), suggesting that increased dosages of EPA improved ADHD symptoms. A scatterplot of the results is shown in Fig. 5. Conversely, although DHA was associated with improved symptoms at all dosages, it showed a slightly negative dosage effect (z=−2.164, p < .05), although this dosage effect was non-significant when one outlier (Milte et al. (2012)), was removed (p = .11). EPA dosage slope remained significant (p = .01) after removing Milte et al. (2012). A negative correlation of −.25 between DHA and EPA dosage was noted, suggesting that the dosage relationship of DHA and EPA may explain the apparent negative dosage effect for DHA. That is, EPA showed more benefit at higher dosage.
Fig. 5.
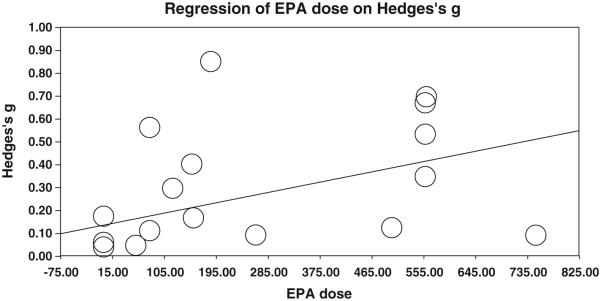
Meta-regression of EPA dosage effects. Study 2 meta-regression of EPA dosage effects of inattention and hyperactivity symptom improvement using best available rating by either parent or teacher. EPA dose is in mg.
Dose duration, reporter, nation, and child age all showed no significant effect (all p > .59) and no obvious outliers. A sensitivity (one-study-removed) analysis revealed no significant effect (point estimate range g = 0.20 to 0.28, all still significant at p < .001). Nation was also investigated and grouped by “western” and “non-western” countries in an attempt to look at possible cross-cultural differences; this result was also non-significant (z = −0.69, p = 0.49).
Investigation of publication bias again indicated the presence of bias. Duval and Tweedie’s (2000) Trim and Fill analysis required imputation of 5 studies, reducing the effect (g = 0.16, 95% CI 0.03–0.28, Q = 29.9). Orwin’s fail-safe N indicated that 17 studies with a zero effect would have to exist unpublished to reduce the effect size of Study 2 to a trivial g = 0.10.
3.9. Summary of Study 2
The beneficial effects of n—3 supplementation replicated across parent and teacher report at an effect size just shy of 1/4 of a standard deviation, with a confidence interval from 0.15 to 0.37, but were slightly more reliable for hyperactivity than for inattention and slightly inflated by possible publication bias.
4. Discussion
Ongoing public and scholarly interest in the relevance of Omega—3 fatty acid supplementation for ADHD mandates further study. Here, we followed up recent meta-analyses of supplementation trials with (a) a first meta-analysis of blood levels and (b) a refined and updated meta-analysis of intervention trials with a larger pool of studies and participants than previously examined. Study 1 found that children with ADHD, in fact, do exhibit lower blood levels of Omega—3 fatty acids. This is important and requires discussion. Study 2 largely confirmed, using alternative methods and additional studies, that intervention does have a small but reliable benefit on ADHD symptoms, supporting two prior meta-analyses and disagreeing with one prior meta-analysis. The estimates of effect size were also largely congruent. Our best estimate of the intervention effect size is g = .26, 95% CI = 0.15–0.37. Sonuga-Barke et al. (2013), reported an effect of SMD = 0.21 (best studies, ‘probably blind’ raters SMD = 0.16), Gillies et al. (2012), SMD = 0.17 (parent) and Bloch and Qawasmi (2011), SMD = .31 (teacher if available, otherwise parent); results are compared in more detail in Table 3. We consider these findings in turn and where this leaves the field.
Table 3.
Details of fatty acid meta-analyses: comparison of recent meta-analytic results.
| Study | Outcome | Studies | N | SMD | 95% CI | p |
|---|---|---|---|---|---|---|
| Bloch & Qawasmi (2011) | Parent & teacher, total ADHD symptoms | 10 | 699 | 0.31 | 0.16-047 | <.001 |
| Gillies et al. (2012) a | Parent & teacher, inatt and hyp | 5 | 413 | 0.17 | −0.03-0.38 | ns |
| Sonuga-Barke et al. (2013) | Proximal rater, total ADHD symptoms | 11 | 827 | 0.21 | 0.05-0.36 | 0.007 |
| Present study: Hawkey & Nigg | Parent & teacher inatt, hyp, composite | 16 | 1408 | 0.26 | 0.15-0.37 | <.001 |
Gillies et al. (2012) was converted to a positive SMD and CI.
Study 1 demonstrated that participants with ADHD have reduced blood levels of EPA and DHA. This is the first time to our knowledge these reports have been pooled in a meta-analysis and it is an important conclusion. Data were insufficient for us to examine several obvious potential confounders of the blood level finding, such as SES. That said that two possible explanations warrant follow up: either because these individuals have less intake through diet, or because these are long chain fatty acids, which are partially obtained through enzymatic elongation, there is a disruption in the conversion process from ALA to EPA/DHA in the ADHD population. Although 8 of 10 studies in this analysis included a measure of dietary intake, unfortunately, most failed to report actual mean intake levels or even to report statistics about their dietary intake data. Seven of these eight studies claimed no statistically reliable differences in dietary intake of fatty acids across groups (the exception was Stevens et al., 1995), although these within-study comparisons were generally low-powered. Three of these studies reported sufficient data that effect sizes could be examined. We pooled these three (all of which had been non-significant individually) for EPA/DHA and saw a reduced dietary intake for those in the ADHD group (n = 81, g = 0.48, p < 0.05). Because of the obvious potential for study selection bias, we do not consider this to be a reliable conclusion. However, it does suggest that the first hypothesis to pursue is that children with ADHD often have inadequate dietary intake. Recommended dietary intake levels of long-chain Omega—3 fatty acids vary widely across nations and organizations; current recommendations range from 140 mg to 1000 mg per day with average of around 500 mg per day and a safe upper limit of 1500 mg per day (Molendi-Coste, Legry, & Leclercq, 2011; Hibbeln, Nieminen, Blasbalg, Riggs, & Lands, 2006).
Future observational studies of children with ADHD are encouraged to obtain and report actual dietary intake levels of Omega—3 fatty acids (as opposed to group differences), as it remains unclear, and critical to know, whether effects of supplementation on ADHD symptoms are more pronounced in children with reduced baseline blood levels. Should this be the case, further investigation of an inefficient or disrupted metabolic pathway could be indicated, and investigation of possible genetic underpinnings would be of keen interest. In all, it is important to discover whether there is a target subgroup of children that would preferentially benefit from supplementation.
In our second meta-analysis, parent and teacher reports replicated a significant reduction in ADHD composite symptoms. This supports and strengthens the conclusion reported by Bloch and Qawasmi (2011) and Sonuga-Barke et al. (2013), which did not distinguish by reporter, and contradicts the conclusion from Gillies et al. (2012). These differences in conclusions probably have to do with study inclusion criteria. As indicated in Table 3, Gillies et al. (2012) only pooled 5 studies; this could be the reason for their failure to find a significant effect despite having a generally similar effect size to other studies. Our estimate of effect size (g = 0.26), benefitting from more studies, is somewhat smaller than that reported by Bloch and Qawasmi (2011). However, a replication of their methods including 2 new studies showed an effect size of g = 0.35, the largest to date. This may indicate that clearly defined ADHD groups are necessary in the study of LC-PUFA on symptoms of attention—based on our Study 1, this could be because such groups tend to have lower blood levels of Omega—3 fatty acids to start with.
With regard to symptom dimensions, we agreed nonetheless with Gillies et al. (2012), that there were no differential effects on inattention/disorganization versus hyperactivity/impulsivity. Parent report revealed a reduction in ADHD symptoms across both domains. Parents may be more sensitive reporters of symptoms, but they may also over-report improvement due to the difficulty of maintaining full blinding of parents (e.g., children reporting fishy after taste). Teacher report was significant only for hyperactivity–impulsivity, but not for inattention. The sample size of teacher report was about half the size of parent report, which may contribute to the reduced effect sizes.
Interestingly, the parent and teacher ADHD index effect was essentially identical in effect size (g = 0.23). This finding may indicate that the index proves to be a more reliable measure across reporters. Larger studies of teacher report may clarify this situation. At this point the most parsimonious conclusion is that effects are similar in both parent and teacher reports across symptom domains. However, more study may reveal differential parent versus teacher sensitivity to aspects of inattention or hyperactivity. Future studies could be improved by expanding the outcome measures to include neuropsychological tasks and/or neuroimaging to validate findings from ratings.
Our analysis showed that larger doses of EPA showed a greater reduction of ADHD symptoms, but showed no significant effects for overall n—3 dosage, agreeing with Bloch and Qawasmi (2011). Our analysis showed that larger doses of DHA did not further improve ADHD symptoms, disagreeing with Bloch and Qawasmi (2011), although studies with increased doses of DHA had decreased doses of EPA which leads us to conclude that dose effects should be investigated further in future research.
Study quality is always of concern in meta-analyses and there are several limitations to consider in this literature. Due to the pungent nature of fish oil, failures of blinding at least in regard to participant awareness must be considered as a possible confound in the intervention studies although replication across parent and teachers helps alleviate this concern. These results also count on the proper dosage ingested daily and rely on accurate report by children and parents, opening potential for bias in recall in relation to perceived symptom improvement. Gillies et al. (2012), conducted extensive analysis of study quality and noted several possible sources of bias. Those concerns remain. However, Sonuga-Barke et al. (2013), reported an analysis restricted to best quality studies and probably blind reporters and still saw a statistically reliable, albeit somewhat smaller effect, at SMD = .16. That finding plus our analysis of publication bias suggests that it is unlikely that study bias fully accounts for the observed effect. In addition, the age range varied between our Study 1 (mean age 16.3 years) and our Study 2 (mean age of 9.7 years).
In conclusion, the overall effect of supplementation, while apparently real, is nonetheless quite small. A recent review of pharmacological treatment effect size shows effect sizes ranging from 0.6 to 1.8 (Banaschewski et al., 2006) with a best estimate of about 1.0. Compared to this effect, the effect of Omega—3 fatty acid supplementation is 1/4 as large. However, it may contribute to treatment options given its low incidence of side effects. Moreover, consumer fears of unknown longterm side effects (or known immediate side effects) of stimulant and non-stimulant ADHD medication may make dietary supplementation an alternative for those unwilling or unable to use these drugs. Finally, this small effect may mask larger effects for some children. As a result, the critical direction for this literature in our view concerns determining whether some children, perhaps those with low baseline levels, benefit more noticeably from supplementation.
Thus, despite finding a statistically reliable effect, the small effect size leads us to agree with Gillies et al. (2012) that there is not enough evidence to recommend Omega—3 fatty acids as an alternative to existing empirically supported pharmacological and behavioral treatments for most children. However, the presence of a reliable though small effect and the mild side effect profile leads us also to suggest, like Sonuga-Barke et al. (2013) and Bloch and Qawasmi (2011), that there is enough evidence to justify the use of Omega—3 fatty acids for ADHD as a supplement to other empirically supported therapies. However, this recommendation is made rather cautiously in view of the remaining uncertainty as to for whom the intervention is effective and about quality of placebo control in the reviewed studies, most of which did not include formal testing of effectiveness of the blind. Effects may depend on baseline blood levels or on genotype (Stevenson et al., 2010) and these factors should be considered in future research. In conclusion, long-chain Omega—3 fatty acids show promise as a supplementary treatment for at least some children with ADHD and further investment and work on this possibility are warranted.
HIGHLIGHTS.
Meta-analysis found lower blood levels of n-3 fatty acids in ADHD versus controls.
Study verified efficacy with small effect of n-3 supplementation for improving ADHD symptoms.
Evidence may justify n-3 as a potential supplementary treatment for ADHD.
Acknowledgments
The OHSU internal institutional funds provided financial support for this study.
Funding/support
The OHSU internal institutional funds.
Abbreviations
- EFA
essential fatty acid
- PUFAs
polyunsaturated fatty acids
- LC-PUFAs
long-chain polyunsaturated fatty acids
- n—3
Omega—3 fatty acids
- ALA
Alpha-linolenic acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- n—6
omega—6 fatty acids
Footnotes
Financial disclosure
There are no financial interests to disclose for either author.
References
- Aman MG, Mitchell EA, Turbott SH. The effects of essential fatty acid supplementation by efamol in hyperactive children. Journal of Abnormal Child Psychology. 1987;15(1):75–90. doi: 10.1007/BF00916467. [DOI] [PubMed] [Google Scholar]
- Antalis CJ, Stevens LJ, Campbell M, Pazdro R, Ericson K, Burgess JR. Omega—3 fatty acid status in attention-deficit/hyperactivity disorder. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2006;75:299–308. doi: 10.1016/j.plefa.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Kleykamp D, Votolato NA, Taylor WA, Kontras SB, Tobin K. Gamma-linolenic acid for attention-deficit hyperactivity disorder: Placebo-controlled comparison to D-amphetamine. Biological Psychiatry. 1989;25:222–228. doi: 10.1016/0006-3223(89)90167-4. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Lofthouse N, Hurt E. Artificial food colors and attention-deficit/hyperactivity symptoms: Conclusions to dye for. Neurotherapeutics. 2012;9(3):599–609. doi: 10.1007/s13311-012-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T, Coghill D, Santosh P, Zuddas A, Asherson P, Buitelaar J, et al. Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. European Child and Adolescent Psychiatry. 2006;15(8):476–495. doi: 10.1007/s00787-006-0549-0. [DOI] [PubMed] [Google Scholar]
- Belanger SA, Vanasse M, Spahis S, Sylvestre M, Lippe S, l'Heureux F, et al. Omega—3 fatty acid treatment of children with attention-deficit hyperactivity disorder: A randomized, double-blind, placebo-controlled study. Paediatrics and Child Health. 2009;14(2):89–98. doi: 10.1093/pch/14.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort MB, Rifas-Shiman SL, Kleinman KP, Guthrie LB, Bellinger DC, Taveras EM, et al. Infant feeding and childhood cognition at ages 3 and 7 years: Effects of breastfeeding duration and exclusivity. JAMA Pediatrics. 2013;29 doi: 10.1001/jamapediatrics.2013.455. http://dx.doi.org/10.1001/jamapediatrics.2013.455 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Qawasmi A. Omega—3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: Systematic review and meta-analysis. JAACAP. 2011;50(10):991–1000. doi: 10.1016/j.jaac.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis version 2. Biostat; Englewood, NJ: 2005. [Google Scholar]
- Brue AW, Oakland TD, Evans RA. The use of a dietary supplement combination and an essential fatty acid as an alternative and complementary treatment for children with attention-deficit/hyperactivity disorder. The Scientific Review of Alternative Medicine. 2001;5(4):187–194. [Google Scholar]
- Chen J, Hsu S, Hsu C, Hwang LH, Yang SC. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. Journal of Nutrition and Biochemistry. 2004;15:467–472. doi: 10.1016/j.jnutbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Colter AL, Cutler C, Meckling KA. Fatty acid status and behavioural symptoms of attention deficit hyperactivity disorder in adolescents: A case–control study. Nutrition Journal. 2008;7:8. doi: 10.1186/1475-2891-7-8. http://dx.doi.org/10.1186/1475-2891-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. American Journal of Clinical Nutrition. 2011;94(6):1914S–1919S. doi: 10.3945/ajcn.110.000893. Suppl. [DOI] [PubMed] [Google Scholar]
- Drane DL, Logemann JA. A critical evaluation of the evidence on the association between type of infant feeding and cognitive development. Paediatric and Perinatal Epidemiology. 2000;14(4):349–356. doi: 10.1046/j.1365-3016.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Germano M, Meleleo D, Montorfano G, Adorni L, Negroni M, Berra B, et al. Plasma, red blood cells phospholipids and clinical evaluation after long chain omega—3 supplementation in children with attention deficit hyperactivity disorder (ADHD) Nutritional Neuroscience. 2007;10(1/2):1–9. doi: 10.1080/10284150601153801. [DOI] [PubMed] [Google Scholar]
- Gillies D, Sinn JKH, Lad SS, Leach MJ, Ross MJ. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents (review) 7. The Cochrane Library; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow RV, Matsudaira T, Taylor E, Rubia K, Crawford M, Ghebremeskel K, et al. Total red blood cell concentrations of omega-3 fatty acids are associated with emotion-elicited neural activity in adolescent boys with attention-deficit hyperactivity disorder. Prostaglandins Leukotrienes and Essential Fatty Acids. 2009;80(2–3):151–156. doi: 10.1016/j.plefa.2008.12.007. http://dx.doi.org/10.1016/j.plefa.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Grayson DS, Kroenke CD, Neuringer M, Fair DA. Dietary omega-3 fatty acids modulate large-scale systems organization in the rhesus macaque brain. Journal of Neuroscience. 2014;34(6) doi: 10.1523/JNEUROSCI.3038-13.2014. http://dx.doi.org/10.1523/JNEUROSCI.3038-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln JR, Nieminen LRG, Blasbalg TL, Riggs JA, Lands WEM. Healthy intakes of n—3 and n—6 fatty acids: Estimations considering worldwide diversity. American Journal of Clinical Nutrition (American Society for Nutrition) 2006;83(6):1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. Supplement. PMID 16841858. [DOI] [PubMed] [Google Scholar]
- Hirayama S, Hamazaki T, Terasawa K. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder—A placebo-controlled doubleblind study. European Journal of Clinical Nutrition. 2004;58:467–473. doi: 10.1038/sj.ejcn.1601830. [DOI] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods. 2006;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- Itomura M, Hamazaki K, Sawazaki S, Kobayashi M, Terasawa K, Watanabe S, et al. The effect of fish oil on physical aggression in schoolchildren—A randomized, double-blind, placebo-controlled trial. Journal of Nutritional Biochemistry. 2005;16:163–171. doi: 10.1016/j.jnutbio.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Progress in Lipid Research. 2013;53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Johnson M, Ostulund S, Fransson G, Kadesjo B, Gillberg C. Omega—3/omega—6 fatty acids for attention deficit hyperactivity disorder. Journal of Attention Disorders. 2009;12(5):394–401. doi: 10.1177/1087054708316261. [DOI] [PubMed] [Google Scholar]
- Joshi K, Lad S, Kale M, Patwardhan B, Mahadik SP, Patni B, et al. Supplementation with flax oil and vitamin C improves the outcome of attention deficit hyperactivity disorder (ADHD) Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2006;74:17–21. doi: 10.1016/j.plefa.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Kirby A, Woodward A, Jackson S, Wang Y, Crawford MA. Childrens' learning and behaviour and the association with cheek cell polyunsaturated fatty acid levels. Research in Developmental Disabilities. 2010;31(3):731–742. doi: 10.1016/j.ridd.2010.01.015. http://dx.doi.org/10. 1016/j.ridd.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Kohlboeck G, Glaser C, Tiesler C, Demmelmair H, Standl M, Romanos M, et al. Effect of fatty acid status in cord blood serum on children’s behavioral difficulties at 10 y of age: Results from the LISAplus Study. American Journal of Clinical Nutrition. 2011;94(6):1592–1599. doi: 10.3945/ajcn.111.015800. [DOI] [PubMed] [Google Scholar]
- Laasonen M, Hokkanen L, Leppämäki S, Tani P, Erkkilä AT. Project DyAdd: Fatty acids in adult dyslexia, ADHD, and their comorbid combination. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2009;81(1):89–96. doi: 10.1016/j.plefa.2009.04.005. http://dx.doi.org/10.1016/j.plefa.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Manor I, Magen A, Keidar D, Rosen S, Tasker H, Cohen T, et al. The effect of phosphatidylserine containing Omega3 fatty-acids on attention-deficit hyperactivity disorder symptoms in children: A double-blind placebo-controlled trial, followed by an open-label extension. European Psychiatry. 2012;27(5):335–342. doi: 10.1016/j.eurpsy.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Millichap JG, Yee MM. The diet factor in attention-deficit/hyperactivity disorder. Pediatrics. 2012;129(2):330–337. doi: 10.1542/peds.2011-2199. http://dx.doi.org/10.1542/peds.2011-2199. [DOI] [PubMed] [Google Scholar]
- Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PR. Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: A randomized controlled trial. Nutrition. 2012;28(6):670–677. doi: 10.1016/j.nut.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Milte CM, Sinn N, Howe PR. Polyunsaturated fatty acid status in attention deficit hyperactivity disorder, depression, and Alzheimer’s disease: Towards and omega—3 index for mental health? Nutrition Reviews. 2009;67(10):573–590. doi: 10.1111/j.1753-4887.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- Molendi-Coste O, Legry V, Leclercq IA. Why and how meet n—3 PUFA dietary recommendations? Gastroenterology Research and Practice. 2011;364040 doi: 10.1155/2011/364040. http://dx.doi. org/10.1155/2011/364040 (Article ID 364040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Lewis K, Edinger T, Falk M. Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(1):86–97. doi: 10.1016/j.jaac.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Nikolas M, Burt SA. Measured gene-by-environment interaction in relation to attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(9):863–873. doi: 10.1016/j.jaac.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perara H, Jeewandara KC, Seneviratne S, Guruge C. Combined w3 and w6 supplementation in children with attention-deficit hyperactivity disorder (ADHD) refractory to methylphenidate treatment: A double-blind, placebo-controlled study. Journal of Child Neurology. 2012;27(6):747–753. doi: 10.1177/0883073811435243. [DOI] [PubMed] [Google Scholar]
- Raz R, Carasso RL, Yehuda S. The influence of short-chain essential fatty acids on children with attention-deficit/hyperactivity disorder: A double-blind placebo-controlled study. Journal of Child and Adolescent Psychopharmacology. 2009;19(2):167–177. doi: 10.1089/cap.2008.070. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Burton JR, Sewell RP, Spreckelsen TF, Montgomery P. Docosahexaenoic acid for reading, cognition and behavior in children aged 7–9 years: A randomized, controlled trial (the DOLAB study) PLoS One. 2012;7(9):e43909. doi: 10.1371/journal.pone.0043909. http://dx.doi.org/10.1371/journal.pone.0043909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AJ, Puri BK. A randomized double-blind, placebo controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2002;26:233–239. doi: 10.1016/s0278-5846(01)00254-8. [DOI] [PubMed] [Google Scholar]
- Schuchardt JP, Huss M, Stauss-Grabo M, Hahn A. Significance of long chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. European Journal of Pediatrics. 2009;169(2):149–164. doi: 10.1007/s00431-009-1035-8. [DOI] [PubMed] [Google Scholar]
- Sinn N, Bryan J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. Journal of Developmental and Behavioral Pediatrics. 2007;28:82–91. doi: 10.1097/01.DBP.0000267558.88457.a5. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: Systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. American Journal of Psychiatry. 2013;170:275–289. doi: 10.1176/appi.ajp.2012.12070991. [DOI] [PubMed] [Google Scholar]
- Sorgi PJ, Hallowell EM, Hutchins HL, Sears B. Effects of an open-label pilot study with high-dose EPA/DHA concentrates on plasma phospholipids and behavior in children with attention deficit hyperactivity disorder. Nutrition Journal. 2007;6(16) doi: 10.1186/1475-2891-6-16. http://dx.doi.org/10.1186/1475-2891-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LJ, Zentall SS, Abate ML, Kuczek T, Burgess JR. Omega—3 fatty acids in boys with behavior, learning, and health problems. Physiology and Behavior. 1996;59(4–5):915–920. doi: 10.1016/0031-9384(95)02207-4. [DOI] [PubMed] [Google Scholar]
- Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. American Journal of Clinical Nutrition. 1995;62:761–768. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- Stevens L, Zhang W, Peck L, Kuczek T, Grevstad N, Mahon A, et al. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids. 2003;38(10):1007–1021. doi: 10.1007/s11745-006-1155-0. [DOI] [PubMed] [Google Scholar]
- Stevenson J, Buitelaar J, Cortese S, Ferrin M, Konofal E, Lecendreux M, et al. Research review: The role of diet in the treatment of attention-deficit/hyperactivity disorder—An appraisal of the evidence on efficacy and recommendations on the design of future studies. Journal of Child Psychology and Psychiatry. 2014 doi: 10.1111/jcpp.12215. http://dx.doi.org/10. 1111/jcpp.12215 [Epub ahead of print] [DOI] [PubMed]
- Stevenson J, Sonuga-Barke E, McCann D, Grimshaw K, Parker KM, Rose-Zerilli MJ, et al. The role of histamine degradation gene polymorphisms in moderating the effects of food additives on children’s ADHD symptoms. The American Journal of Psychiatry. 2010;167(9):1108–1115. doi: 10.1176/appi.ajp.2010.09101529. http://dx.doi.org/10.1176/appi.ajp.2010.09101529. [DOI] [PubMed] [Google Scholar]
- Vaisman N, Kaysar N, Zaruk-Adash Y, Pelled D, Brichon G, Zwingelstein G, et al. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: Effect of dietary n—3 fatty acids containing phospholipids. American Journal of Clinical Nutrition. 2008;87:1170–1180. doi: 10.1093/ajcn/87.5.1170. [DOI] [PubMed] [Google Scholar]
- Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. The Journal of Pediatrics. 2001;139:189–196. doi: 10.1067/mpd.2001.116050. [DOI] [PubMed] [Google Scholar]
- Young GS, Maharaj NJ, Conquer JA. Blood phospholipid fatty acid analysis of adults with and without attention deficit/hyperactivity disorder. Lipids. 2004;39(2):117–123. doi: 10.1007/s11745-004-1209-3. [DOI] [PubMed] [Google Scholar]