Abstract
OBJECTIVE
Refractory chronic low back pain (CLBP) often leads to treatment with long-term opioids. Our goal was to describe the pharmaco-toxicological profile of opioid-treated CLBP patients and identify potential areas for care optimization.
DESIGN
Cross-sectional analysis.
SETTING
Outpatient primary care.
PARTICIPANTS
CLBP patients prescribed >30 mg/day of morphine-equivalent dose (MED) for ≥3 months.
INTERVENTION
N/A
OUTCOME MEASURES
Self-reported clinical, medication (verified) and substance use, and urine drug testing (UDT) data were collected.
RESULTS
Participants (N=35) were 51.8±9.7 years old, 80% female with CLBP for 14.2±10.1 years, treated with opioids for 7.9±5.7 years, with severe disability (Oswestry Disability Index score: 66.7±11.4), and average pain score of 5.6±1.5 (0–10 rating scale). Participants reported using tobacco (N=14), alcohol (N=9) and illicit drugs or unprescribed medications (N=10). On average, participants took 13.4±6.8 daily medications, including 4.7±1.8 pain-modulating and 4.7±2.0 sedating medications. Among prescribed opioids, 57.1% were long-acting and 91.4% were short-acting, with a total of 144.5±127.8 mg/day of MED. Sixteen participants were prescribed benzodiazepines and/or zolpidem/zaleplon. Fifteen participants had UDT positive for illicit drugs or unprescribed medications; in addition, 8 tested positive for alcohol and 19 for cotinine. Compared to those with negative UDTs, those with positive UDTs (N=15) received lower daily “total” and “extended release” opioid doses, and were more likely to test positive for cotinine (p<0.05).
CONCLUSIONS
Study findings corroborate existing evidence for high medication burden and high likelihood of substance misuse among opioid-treated CLBP patients. Further research is needed to help understand causality and ways to optimize care and clinical outcomes.
INTRODUCTION
Chronic low back pain (CLBP) is common, costly, often disabling and refractory in spite of the best possible care.1,2 Approximately 80% of U.S. adults experience low back pain during their lifetime, with 15–20% developing protracted pain and 2–8% developing chronic back pain; 5% of working-age adults are disabled due to CLBP and back pain is the second leading cause of lost work time.1,2 Americans spend at least $50 billion per year on low back pain, with CLBP making up at least 90% of the costs.3 Patients with CLBP are often prescribed opioid therapy to alleviate pain and improve function. Although this can be beneficial in a subset of patients, long-term opioid therapy for non-cancer pain is controversial. It is often marginally effective, and can result in harm such as opioid-induced hyperalgesia, sedation, respiratory depression, overdose, and death. Patients treated with long-term opioid therapy are at increased risk for aberrant drug-use behaviors and development of substance abuse.3–5
Prescription drug abuse, especially opioid, has been identified as a public health epidemic in the U.S.6 With CLBP being the leading non-cancerous condition for which long-term opioids are prescribed,4,7 it dramatically contributes to the circulating opioid supply available for abuse and diversion; prescribed opioids constitute the main supply for 70% of abusers.8 Recent years have seen a dramatic rise in the number of filled opioid prescriptions (174 million in 2000, and 257 million in 2009) and the quantity prescribed per person (74mg in 1997, and 369mg in 2007).9,10 From 2004 to 2008, emergency department visits linked to prescription opioid abuse more than doubled11 and prescription opioids have become the major contributor of drug-related deaths.12 Correlating with these adverse consequences, the proportion of patients who entered addiction treatment in 2009 and endorsed opioid abuse increased more than four-fold.8 Therefore, development of new, effective, safe and non-addictive therapies, and optimizing existing care for CLBP is a national priority.2
There is a relative paucity of research and unified clinical guidelines on how to best optimize therapy of individuals treated with long-term opioids for non-cancer chronic pain, especially in terms of identifying and monitoring for misuse and overuse.5 Combined with the fact that the majority of opioid prescriptions are issued by primary care providers and close to 50% of providers report high level of discomfort and burden associated with the management of this clinical population,13 evaluating avenues for care “improvement” is essential. This may include optimizing pharmacotherapy and treatment adherence, possibly leading to decreased opioid prescribing and/or decreased patient’s reliance on and need for opioid pain medications. Reduced opioid use may, in turn, reduce the risk of developing opioid-related adverse consequences (which are dose-dependent5,14,15) by CLBP-affected individuals and lessen the nationwide problem of opioid abuse and diversion.
To address existing knowledge gaps, we initiated a 26-week randomized controlled trial (RCT) to a) test methods feasibility, b) gather preliminary data on efficacy of a novel mindfulness-based behavioral intervention, developed specifically for this population, and c) characterize opioid-treated CLBP patients to identify potential areas for care optimization. The current analysis included baseline data of the RCT participants (N=35) and focused on the latter aim.
METHODS
Trial Design
Current cross-sectional analyses were performed on the baseline data from participants (N=35) enrolled in an ongoing, open-label, two-parallel-arm, 26-week long randomized controlled trial (RCT). The primary goal of the RCT was to test methods feasibility and gather preliminary data on efficacy of “Mindfulness for Chronic Pain” intervention in improving quality of life and pain coping among CLBP patients, treated with long-term opioids. The study was approved by the University’s Health Sciences Institutional Review Board and informed consent was obtained prior to enrollment into the study.
Participants
Participants were adult patients treated by a clinician for CLBP with long-term opioids. Individuals were identified in several ways. An initial search of the University’s Family Medicine electronic medical record (EMR) database identified active patients meeting pre-specified criteria of age, daily opioid dose and therapy duration, low back pain diagnosis, and geographical proximity. In addition, a study brochure was sent (postal service, email) to local clinicians to facilitate referral of potential participants. Interested potential participants could also call directly to inquire about the study. Eligibility criteria were based on self-report. Inclusion criteria included: fluency in English; at least 21 years of age; presence of CLBP defined as daily pain, for at least 3 months, in the lumbosacral region or radiating to the leg(s) (sciatica); and prescribed, for at least 3 prior months, 30 mg/day or more of Morphine Equivalent Dose (MED) of opioids. Exclusion criteria included: prior formal training in or current, regular practice of mindfulness meditation; inability to participate in study activities; existing diagnoses of delusional, bipolar or borderline personality disorders; and pregnancy. Potential participants were screened by phone, and if eligible and interested were invited to the in-person enrollment meeting.
Study Setting
The enrollment and assessment procedures took place at the University’s Clinical Research Unit.
Outcome Measures
Participants filled out questionnaires on quality of life, medication and other substance use, psychological health, pain and stress coping, and provided a urine sample for toxicology testing.
Demographic and Self-reported Clinical Characteristics
Demographic data was collected. The validated Oswestry Disability Index (ODI) evaluated the percent of CLBP-specific disability “today,” with scores ranging: 0 (none) through 100 (complete disability).16 Questions on pain severity ratings “in the last week” (average, least and worst pain) were derived from the Brief Pain Inventory and followed an 11-point numerical rating scale (0=no pain, 10= “pain as bad as you can imagine”).17–19 Additional questions inquired about the duration of low back pain and opioid therapy.
Self-reported Medication and Other Substance Use
Data on daily use of prescription-based and over-the-counter medications, vitamins and herbal products for “the past 28 days” were collected based on self-reports, and verified against the medication bottle information (medication name, strength per unit, daily dose, when last taken). Daily use and dose of opioids was further quantified by collecting data on daily use (past 28 days) of prescription-based opioid medications using the Timeline Followback (TLFB) method, a validated tool for daily substance use evaluation.20,21 The TLFB was also used to collect daily data on alcohol (number of standard drinks/day) and illicit drug use (drug class, yes/no to use) in the past 28 days. Tobacco use was quantified as an average number of cigarettes per day. To allow for opioid dose comparison, all opioid doses were converted to a morphine-equivalent dose (MED), by multiplying daily dose of a given opioid by published conversion factors.22–25 Similarly, to allow for benzodiazepine dose comparison, all benzodiazepine doses were converted to a lorazepam-equivalent dose using the Benzodiazepine Converter.26
Urine Drug Test (UDT)
Urine specimens were shipped to the Alere™ Toxicology laboratory for comprehensive toxicology testing which included testing for “parent” substances and selected metabolites. Screening for selected substances was conducted using Enzyme Immunoassay (EIA). Confirmatory-level testing was conducted using Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) technology, which is highly specific and sensitive. UDT results were classified as abnormal when there was: evidence of an illicit drug or an additional non-prescribed medication (“positive” or “inconsistent” result), lack of evidence of a prescribed opioid or benzodiazepine, or an adulterated sample.
Sample Size
The sample size for the current feasibility pilot was a result of balance between available financial resources and striving to achieve optimal group size (15–20 participants) for the study intervention sessions.
Statistical Methods
The data was double-entered to and managed using the RedCap™ electronic secure database hosted at our institution.27 It was analyzed using the IBM SPSS Statistics28 program. Baseline characteristics were described using descriptive statistics; mean (standard deviation, SD) or number (percentage) were used to describe the sample, unless indicated otherwise. Cross-sectional correlational analyses or between group comparisons were conducted using nonparametric tests due to the fact that majority of variables showed non-normal distribution. Two-tailed p value < 0.05 constituted a level of statistical significance.
RESULTS
Screening of the University’s Family Medicine electronic medical record (EMR) database identified 264 potential participants; a number of interested individuals were also acquired by clinician or friend (other patient) referral. Of the 87 individuals screened, 39 were ineligible, 13 were eligible but declined participation, and 35 were eligible and enrolled.
Demographics
All enrolled participants (N=35) completed the baseline assessments, providing data for this cross-sectional analysis. Participants’ socio-economic characteristics are summarized in Table 1. They were on average 51.8 ± 9.7 years old, 80% female, 80% White, and 40% single. Majority (77%) reported at least “some college” education. About one-third were unemployed, and one-third were working part- or full-time in paid jobs. Approximately three fourths were receiving Social Security Disability Insurance (SSDI) or Supplemental Security Income (SSI), and two thirds reported individual income of $15,000 or less annually; one third of the participants reported a household income in the same range.
Table 1.
Socio-demographic characteristics of the sample (N=35).
| Total (N=35) | Male (N=7) | Female (N=28) | |
|---|---|---|---|
|
| |||
| Age, mean (SD) | 51.8 (9.7) | 54.4 (3.6) | 51.2 (10.7) |
|
| |||
| Ethnicity: Hispanic or Latino, n (%) | 1 (2.9) | 0 (0.0) | 1 (3.6) |
|
| |||
| Race | |||
| White/Caucasian, n (%) | 28 (80.0) | 3 (42.9) | 25 (89.3) |
| Black/African American, n (%) | 6 (17.1) | 4 (57.1) | 2 (7.1) |
| Other, n (%) | 1 (2.9) | 0 (0.0) | 1 (3.6) |
|
| |||
| Relationship Status1 | |||
| Single, n (%) | 14 (40.0) | 4 (57.1) | 10 (35.7) |
| In a relationship, not married, n (%) | 10 (28.6) | 1 (14.3) | 9 (32.1) |
| Married, n (%) | 11 (31.4) | 2 (28.6) | 9 (32.1) |
|
| |||
| Education | |||
| High School/GED, n (%) | 8 (22.9) | 3 (42.9) | 5 (17.9) |
| Some College, n (%) | 13 (37.1) | 1 (14.3) | 12 (42.9) |
| College Graduate,2 n (%) | 11 (31.4) | 3 (42.9) | 8 (28.6) |
| Post College Degree, n (%) | 3 (8.6) | 0 (0.0) | 3 (10.7) |
|
| |||
| Current Employment | |||
| Employed,3 n (%) | 12 (34.3) | 3 (42.9) | 9 (32.1) |
| Unemployed, n (%) | 10 (28.6) | 1 (14.3) | 9 (32.1) |
| Homemaker, n (%) | 5 (14.3) | 2 (28.6) | 3 (10.7) |
| Retired, n (%) | 8 (22.9) | 1 (14.3) | 7 (25.0) |
|
| |||
| Sources of Income4 | |||
| No income / unemployment, n (%) | 2 (5.7) | 0 (0.0) | 2 (7.1) |
| Public Assistance, n (%) | 2 (5.7) | 2 (28.6) | 0 (0.0) |
| Retirement/Pension, n (%) | 4 (11.4) | 1 (14.3) | 3 (10.7) |
| Paid work, n (%) | 12 (34.3) | 3 (42.9) | 9 (32.1) |
| SSI/SSDI,5 n (%) | 27 (77.1) | 5 (71.4) | 22 (78.6) |
| Other, 6 n (%) | 4 (11.4) | 1 (14.3) | 3 (10.7) |
|
| |||
| Gross Individual Income, $, mean (SD) | 18,290.5 (19,345.1) | 26,797.1 (28,815.7) | 16,163.9 (16,226.1) |
| ≤ $15,000, n (%) | 23 (65.7) | 3 (42.9) | 20 (71.4) |
| $15,001–$30,000, n (%) | 7 (20.0) | 3 (42.9) | 4 (14.3) |
| $30,001–$45,000, n (%) | 2 (5.7) | 0 (0.0) | 2 (7.1) |
| > $45,000, n (%) | 3 (8.6) | 1 (14.3) | 2 (7.1) |
|
| |||
| Gross Household Income, $, mean (SD) | 36,089.4 (33,112.7) | 32,154.3 (39,344.3) | 37,073.2 (32,120.1) |
| ≤ $15,000, n (%) | 11 (31.4) | (28.6) | 9 (32.1) |
| $15,001–$30,000, n (%) | 10 (28.6) | 4 (57.1) | 6 (21.4) |
| $30,001–$45,000, n (%) | 3 (8.6) | 0 (0.0) | 3 (10.7) |
| > $45,000, n (%) | 11 (31.4) | 1 (14.3) | 10 (35.7) |
|
| |||
| # people relying on your income, mean (SD) | 1.9 (1.3) | 1.4 (0.5) | 2.0 (1.4) |
|
| |||
| # people living in household, mean (SD) | 2.4 (1.7) | 1.9 (0.7) | 2.6 (1.9) |
60% of individuals have been previously Divorced/Separated, Widowed or both.
Includes both 2- and 4-year institutions.
Includes full- and part- time work.
Some participants had multiple sources of income.
SSDI and/or SSI
Includes income from private disability insurance, child support, alimony, or family members
Self-reported Clinical Characteristics
On average, participants suffered from low back pain for 14.2 ± 10.1 years (median: 10, range: 1–36 years) and were treated with opioid medications for the past 7.9 ± 5.7 years (median: 7, range 0.5–25 years) (Table 2). They reported, on average, 66.7 ± 11.4 % disability level (total ODI score) in relation to their CLBP. The ODI scores of 31.4% of the participants “qualified” them for severe disability level, with the remaining participants’ scores indicating “crippled” (62.9%) or “bed-bound” (5.7%) status; none of the participants “scored” in the range of mild or moderate disability. In spite of pharmacotherapy, participants reported substantial pain severity (average pain: 5.6 ± 1.5 points). Self-reports of substance use revealed that 40% used tobacco, 25.7% reported drinking (on average, on 24.6 ± 32.3 %, median: 11% of days in the “prior 28 days”), and 28.6% endorsed using illicit drugs or medications that were not prescribed to them in the prior 28 days.
Table 2.
Self-reported clinical characteristics of the study participants (N=35).
| Total (N=35) | Male (N=7) | Female (N=28) | |
|---|---|---|---|
|
| |||
| Low back pain duration, years, mean (SD) | 14.2 (10.1) | 13.0 (9.8) | 14.5 (10.3) |
|
| |||
| Opioid therapy duration, years, mean (SD) | 7.9 (5.7) | 5.4 (3.8) | 8.5 (6.0) |
|
| |||
| Oswestry Disability Index (ODI) | |||
| Percent disability (total score), mean (SD) | 66.7 (11.4) | 68.6 (8.7) | 66.2 (12.1) |
| Minimal & Moderate Disability (total score: ≤ 40%), n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe Disability (total score: 41 – 60%), n (%) | 11 (31.4) | 1 (14.3) | 10 (35.7) |
| Crippled (total score: 61 – 80%), n (%) | 22 (62.9) | 6 (85.7) | 16 (57.1) |
| Bed-bound (total score: 81 – 100%), n (%) | 2 (5.7) | 0 (0.0) | 2 (7.1) |
|
| |||
| Brief Pain Inventory: Pain Severity | |||
| Average pain, past week, mean (SD) | 5.6 (1.5) | 6.4 (1.0) | 5.4 (1.5) |
| Worst pain, past week, mean (SD) | 7. 9 (1.5) | 8.4 (0.8) | 7.8 (1.7) |
| Least pain, past week, mean (SD) | 3.9 (1.9) | 5.3 (2.5) | 3.6 (1.5) |
| Pain “right now,” mean (SD) | 5.7 (1.9) | 6.4 (1.4) | 5.5 (2.0) |
|
| |||
| Tobacco use, yes,1 n (%) | 14 (40.0) | 5 (71.4) | 9 (32.1) |
| Among cigarette smokers, # cigarettes/day, mean (SD) | 10.8 (7.5) | 6.6 (3.7) | 12.2 (8.1) |
|
| |||
| Alcohol use in the past 28 days, yes, n (%) | 9 (25.7) | 2 (28.6) | 7 (25.0) |
| Among drinkers: | |||
| # drinking days, | |||
| mean (SD) | 6.9 (9.0) | 3.5 (3.5) | 7.9 (10.1) |
| median | 3.0 | 3.5 | 3.0 |
| # heavy drinking days,2 | |||
| mean (SD) | 0.6 (1.3) | 0.5 (0.7) | 0.6 (1.5) |
| median | 0.0 | 0.5 | 0.0 |
| # drinks per drinking day, | |||
| mean, (SD) | 2.3 (1.5) | 3.6 (2.0) | 2.0 (1.3) |
| median | 2.0 | 3.6 | 2.0 |
| # total drinks, | |||
| mean (SD) | 15.1 (18.6) | 9.0 (5.7) | 16.9 (20.9) |
| median | 6.0 | 9.0 | 6.0 |
|
| |||
| Illicit/unprescribed drug use, yes,3 n (%) | 10 (28.6) | 2 (28.6) | 8 (28.6) |
Twelve participants smoked cigarettes, one smoked cigars, and one chewed tobacco
Heavy drinking day: 5 drinks or more for a man, 4 drinks or more for a woman per day.
Cannabis (N=7); Opium (N=1); Oxycodone (N=2).
Self-reported Medication Use
Participants were using an average of 13.4 ± 6.8 different types of daily medications (range: 3–32), including 4.7 ± 1.8 pain-modulating (median 5, range 2–9) and 4.7 ± 2.0 sedating (median 4, range 1–9) medications (Table 3); seven participants reported the use of 20 or more daily medications.
Table 3.
Medication profile by gender (self-reports, verified against medication bottle information)
| Total (N=35) | Male (N=7) | Female (N=28) | |
|---|---|---|---|
|
| |||
| Total number of medication types, mean (SD) | 13.4 (6.8) | 9.4 (4.9) | 14.4 (7.0) |
|
| |||
| Total number of pain-modulating medications,1 mean (SD) | 4.7 (1.8) | 3.7 (1.8) | 4.8 (1.8) |
|
| |||
| Total number of sedating medications,2 mean (SD) | 4.7 (2.0) | 3.4 (2.3) | 5.0 (1.9) |
|
| |||
| Opioid Medications | |||
|
| |||
| Opioids, yes, n (%) | 35 (100.0) | 7 (100.0) | 28 (100.0) |
| Pure μ-agonist, n (%) | 35 (100.0) | 7 (100.0) | 28 (100.0) |
| Long-acting μ-agonist,3 n (%) | 20 (57.1) | 2 (28.6) | 18 (64.3) |
| Short-acting μ-agonist, n (%) | 32 (91.4) | 7 (100.0) | 25 (89.3) |
| Tramadol, n (%) | 1 (2.9) | 1 (14.3) | 0 (0.0) |
|
| |||
| Opioid MED, mg/day, mean (SD) | 144.5 (127.8) | 118.5 (140.4) | 151.0 (126.4) |
| Long-acting opioid MED, mg/day, mean (SD) | 96.3 (122.4) | 55.7 (112.8) | 106 (124.0) |
| Short-acting opioid MED, mg/day, mean (SD) | 48.3 (37.7) | 62.7 (51.9) | 44.7 (33.5) |
|
| |||
| Nonopioid Pain-Modulating Medications1 | |||
|
| |||
| Number of nonopioid pain-modulating medications, mean (SD) | 3.0 (1.6) | 2.3 (1.6) | 3.1 (1.6) |
|
| |||
| Acetaminophen,4 n (%) | 20 (57.1) | 3 (42.9) | 17 (60.7) |
|
| |||
| NSAIDs, n (%) | 16 (45.7) | 4 (57.1) | 12 (42.9) |
|
| |||
| GABA analogs,5 n (%) | 16 (45.7) | 4 (57.1) | 12 (42.9) |
|
| |||
| Skeletal muscle relaxants, n (%) | 16 (45.7) | 1 (14.3) | 15 (53.6) |
| Cyclobenzaprine, n (%) | 6 (17.1) | 1 (14.3) | 5 (17.9) |
| Carisoprodol, n (%) | 2 (5.7) | 0 (0.0) | 2 (7.1) |
| Tizanidine, n (%) | 4 (11.4) | 0 (0.0) | 4 (14.3) |
| Baclofen, n (%) | 3 (8.6) | 0 (0.0) | 3 (10.7) |
| Metaxalone, n (%) | 1 (2.9) | 0 (0.0) | 1 (3.6) |
|
| |||
| SNRIs, n (%) | 9 (25.7) | 2 (28.6) | 7 (25.0) |
|
| |||
| TCAs, n (%) | 6 (17.1) | 1 (14.3) | 5 (17.9) |
|
| |||
| Benzodiazepines,6 n (%) | 11 (31.4) | 2 (28.6) | 9 (32.1) |
| Lorazepam equivalent dose, mg/day, mean (SD) | 5.4 (3.8) | 10 (2.8) | 4.3 (3.2) |
|
| |||
| Other selected medications | |||
|
| |||
| SSRIs,7 n (%) | 5 (14.3) | 0 (0.0) | 5 (17.9) |
|
| |||
| Stimulant medications,8 n (%) | 5 (14.3) | 0 (0.0) | 5 (17.9) |
|
| |||
| Headache medications,9 n (%) | 8 (22.9) | 0 (0.0) | 8 (28.6) |
|
| |||
| Bowel Regimen medications,10 n (%) | 12 (34.3) | 1 (14.3) | 11 (39.3) |
|
| |||
| Diabetic medications,11 n (%) | 5 (14.3) | 2 (28.6) | 3 (10.7) |
|
| |||
| Cardiovascular medications, n (%)12 | 18 (51.4) | 4 (57.1) | 14 (50.0) |
Abbreviations: MED, morphine equivalent dose; NSAIDs, non-steroidal anti-inflammatory drugs; GABA, gamma-aminobutyric acid; SNRIs, serotonin norepinephrine reuptake inhibitors; TCAs, tricyclic antidepressants; SSRIs, selective serotonin reuptake inhibitors.
Pain-modulating medications used by participants: opioids, acetaminophen, NSAIDs, anticonvulsants, GABA analogs, glucosamine-chondroitin, skeletal muscle relaxants, SNRIs, TCAs, benzodiazepines, topical lidocaine and other topical pain agents.
Sedating medications used by participants: opioids, anticonvulsants, GABA analogs, skeletal muscle relaxants, tricyclic antidepressants, benzodiazepines, antiemetics, antihistamines, antipsychotics, anxiolytics, non-benzodiazepine sedative hypnotics, mirtazapine, trazodone, and Calms Forte®.
Long-acting opioids used by participants: morphine ER, oxycodone ER, methadone, and fentanyl patches.
Acetaminophen category included: “plain” acetaminophen and acetaminophen combinations (with oxycodone, hydrocodone, aspirin/caffeine or butalbital).
GABA analogs used by participants: gabapentin and pregabalin.
Benzodiazepines used by participants: diazepam (N=2), lorazepam (N=3), and clonazepam (N=8)
SSRIs used by study participants: citalopram, escitalopram, and sertraline
Stimulant medications used by participants: methylphenidate, dextroamphetamine, and caffeine.
Headache medications used by participants: triptans, acetaminophen/aspirin/caffeine, and acetaminophen/butalbital
Bowel regimen medications used by participants: docusate, polyethylene glycol, bisacodyl, senna, and lactulose.
Diabetic medications used by participants: metformin, glargine insulin, lispro insulin
Cardiovascular medications used by participants belong to the following classes: statins, angiotensin receptor blockers, angiotensin converting enzyme inhibitors, alpha agonists, beta blockers, calcium channel blockers, direct acting smooth muscle relaxants, and nitrates
All participants were treated with pure mu-agonist opioids (57.1% were prescribed long-acting, 91.4% were prescribed short-acting preparations), with 17 (48.6%) using a combination of short- and long-acting opioids. Participants were receiving an average daily dose of 144.5 ± 127.8 (median: 106.4, maximum: 510.4) mg of MED, with 14 prescribed less than 100mg/day, 12 prescribed 100–199 mg/day and the remaining 9 participants prescribed 200mg/day or more. Although women appeared to have opioids prescribed in a higher dose-ratio of long-acting to short-acting preparations, no statistically significant gender differences in opioid dose were found. Oxycodone was the most commonly prescribed opioid (N=21), with 12 participants having it prescribed in conjunction with a different opioid; of note, oxycodone was co-prescribed to all participants treated with fentanyl (N=4), methadone (N=1) or tramadol (N=1). Morphine was the second most commonly prescribed opioid (N=11); only 3 participants were prescribed morphine in conjunction with a different opioid. Among those prescribed hydrocodone (N=10), half received it as the only opioid.
Twenty-five participants (71.4%) were prescribed at least one daily nonopioid pain-modulating medication, averaging 3.0 ± 1.6 of such medications per day across all study participants. Approximately half of participants used acetaminophen (“plain” or in a combination with other medications), non-steroidal anti-inflammatory drugs (NSAIDs), gamma-aminobutyric acid analogs or muscle relaxants. Sixteen participants (45.7%) were prescribed benzodiazepines (N=11; average lorazepam-equivalent dose: 5.4 ± 3.8 mg/day) and/or zolpidem/zaleplon (N=6). Only one-third of participants reported using “bowel regimen” medications.
Urine drug testing
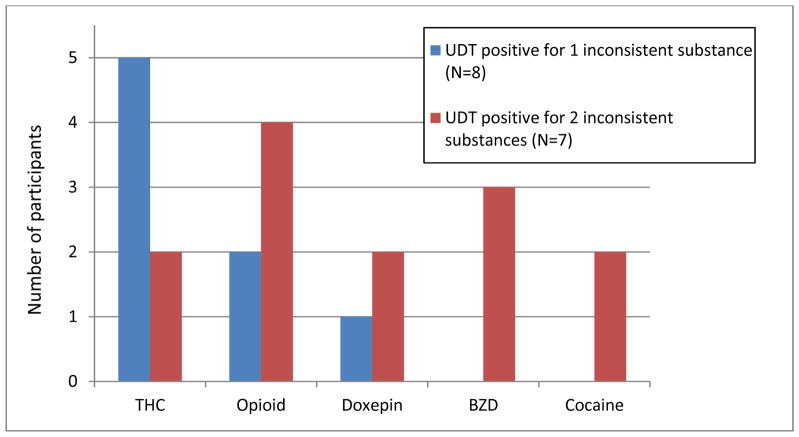
All urine samples were of acceptable quality; none appeared adulterated. The UDT results showed that 15 participants (42.9%) tested positive for illicit or unprescribed prescription-based drugs; these results were labeled as “inconsistent” with the participant’s medication record or “positive”. Marijuana (N=7) and prescription-based opioids (N=6) were the most commonly found “inconsistent” substances. Among these 15 “positive” samples, 8 tested positive for one and 7 tested positive for two “inconsistent” medications or illicit drugs (Figure 1). None of the participants tested positive for phencyclidine, stimulants other than cocaine or synthetic cannabinoids. Nineteen (54.3%) participants tested positive for a nicotine metabolite, cotinine. Eight participants tested positive for alcohol metabolites; among them, three showed presence of other “inconsistent” substances (THC, opioids, benzodiazepines). If counting alcohol as an “inconsistent” substance, a total of 20 participants (57.1%) would have been labeled as having “inconsistent” or “positive” UDT results.
Figure 1.
Illicit drugs or unprescribed medications in urine among 15 participants with “positive” urine drug test (UDT) results.
Abbreviations: THC, marijuana; BZD, benzodiazepines
Comparison of self-reports (past 28-day use) with UDT findings for prescription-based medication, illicit drugs or alcohol revealed discrepancies. Two participants who reported misusing oxycodone were also prescribed oxycodone and, therefore, tested “as expected” (UDT findings were consistent with the prescribed medications). Among those with “positive” (“inconsistent”) UDT results for opioids (N=5), only one reported opioid misuse and had the UDT findings consistent with self-reported opioid (opium) use. Of note, all participants tested “as expected” on UDT for their “regular” prescribed opioids. Among self-reported marijuana users (N=7), six tested positive, and one tested negative for cannabinoids; one additional participant who did not report marijuana use tested positive for it on UDT. Among those who reported drinking (N=9), four tested negative; the UDT was positive for alcohol metabolites in additional 3 participants who did not report drinking.
Spearman’s bivariate correlations
Participants with higher ODI disability scores rated their “current pain” as more severe (r=0.4, p=0.016) and tended to have a higher daily opioid MED (r=0.33, p=0.055). In addition, the daily MED positively correlated with the duration of opioid therapy (r= 0.45, p=0.007). Those with longer duration of low back pain (r=0.35, p=0.042) or opioid therapy (r=0.36, p=0.031) also tended to have higher ratings of “average pain”.
Between group comparisons
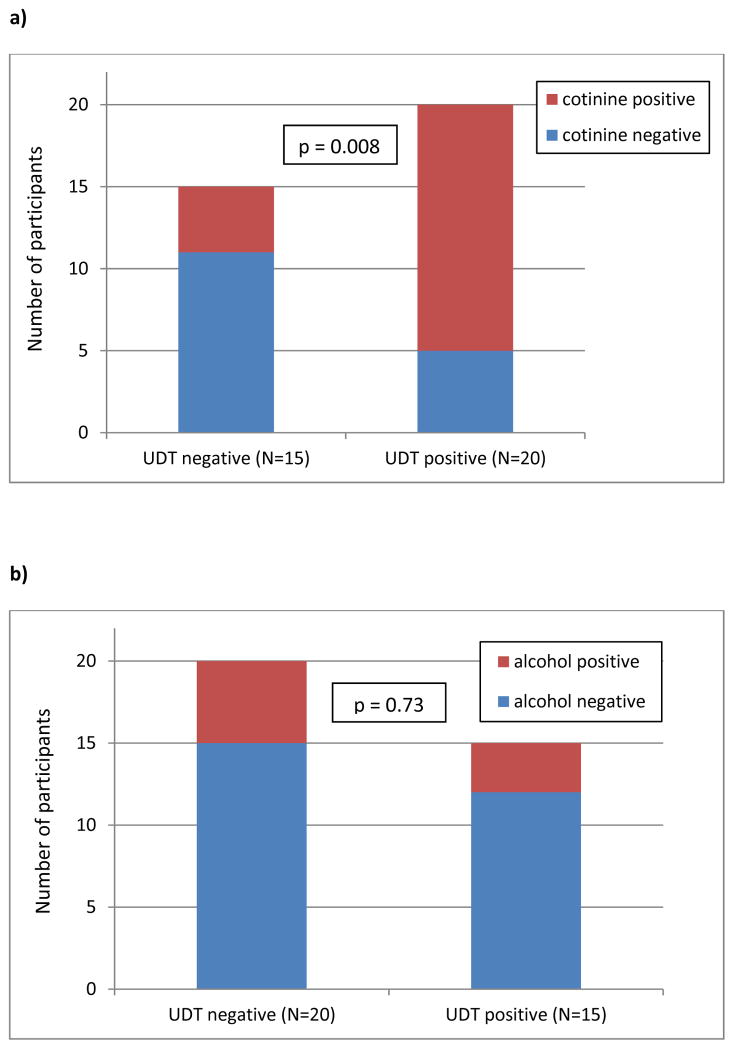
When comparing participants with UDT positive (N=15) and UDT negative (N=20) findings, those with positive UDTs were treated with a lower total daily MED (88.9 ± 61.7 versus 186.2 ± 148.9 mg, p=0.033) and lower daily MED of extended release opioids (36.2 ± 57.4 versus 57.3 ± 79.90 mg, p=0.013); otherwise they did not differ in other clinically-relevant outcomes (Table 4). As shown in Figure 2, those testing positive for cotinine were more likely to have a positive UDT (p=0.008) than those who tested cotinine-negative; presence of alcohol metabolites did not differ between the UDT categories of positive versus negative results.
Table 4.
Chronic low back pain clinically-relevant outcomes by UDT results among study participants (N=35); “positive” UDT refers to the presence of unprescribed medications or illicit drugs.
| UDT positive (N=15) | UDT negative (N=20) | |
|---|---|---|
|
| ||
| Age, years, mean (SD) | 52.9 (10.6) | 51.0 (9.2) |
|
| ||
| LBP duration, years, mean (SD) | 15.0 (11.2) | 13.6 (9.4) |
|
| ||
| Opioid therapy duration, mean (SD) | 7.4 (6.0) | 8.3 (5.6) |
|
| ||
| ODI score, mean (SD) | 67.2 (8.2) | 66.2 (13.5) |
|
| ||
| Pain severity | ||
| average, mean (SD) | 5.7 (1.3) | 5.5 (1.6) |
| worst, mean (SD) | 8.2 (1.1) | 7.7 (1.8) |
| least, mean (SD) | 3.9 (1.7) | 4.0 (2.0) |
|
| ||
| MED (mg/day) | ||
| All opioids, mean (SD) | 88.9 (61.7)1 | 186.2 (148.9) |
| ER opioids, mean (SD) | 36.2 (57.4)2 | 57.3 (79.9) |
| SA opioids, mean (SD) | 52.7 (39.8) | 45.0 (36.6) |
Abbreviations: ODI, Oswestry Disability Index; LBP, low back pain; MED, morphine-equivalent dose; ER, extended release; SA, short-acting; UDT, urine drug test.
p = 0.033, Mann-Whitney U Test for Independent Samples
p = 0.013, Mann-Whitney U Test for Independent Samples
Figure 2.
Cotinine (a) and alcohol (b) in urine across the categories of urine drug test (UDT) results: “negative” (N=20) and “positive” (N=15) for illicit drugs or unprescribed medications. P value refers to statistical significance level for between group comparisons (χ2 test).
DISCUSSION
This study is the first to date to assess meditation intervention in opioid-treated CLBP adults; its goal was to test the methods and inform sample size calculations for future studies. Findings of the study indicated that opioid-treated CLBP patients reported severe-to-crippling disability level and substantial pain in spite of polypharmacy utilizing opioids and other adjuvant pain-modulating medications. Opioid-treated CLBP patients in the study showed a high prevalence of co-prescribing opioids with other (often multiple) medications with sedating properties, including sedative-hypnotics, and a high-prevalence of substance misuse, as documented by self-reports and UDT. Compared to those with negative UDTs, those with “positive” urine samples were treated with lower daily opioid doses, and were more likely to test positive for a nicotine metabolite, cotinine.
This study corroborates findings of others that opioid-treated CLBP patients represent a medically-challenging and complex clinical population, with, on average, unsatisfactory CLBP-related clinical outcomes in spite of the-strongest-possible analgesic therapy.5 Majority (60%) of the participants were treated with a high daily MED of opioids (≥100mg/day), with one-fourth receiving a very-high dose (≥200mg/day). High-dose opioid therapy carries substantial risks, including respiratory depression, unintentional overdose and death, with the likelihood of adverse consequences being dose-dependent.5,14,15 Literature documents that the majority of prescription-opioid related deaths (60%) occur in patients using opioids as prescribed, based on existing prescribing guidelines; the remaining 40% of deaths occurs in individuals abusing opioids obtained through doctor shopping or drug diversion.5 Polypharmacy, as noted among our participants – with seven participants using 20 or more medications, especially when utilizing multiple sedating medications, potentiates the risk of adverse medication effects and drug interactions. Co-prescribing of benzodiazepines and opioids is considered one of the most dangerous “sedating” combinations; this unfortunately appears to be a common practice29 in spite of recommendations against it.30,31 Interestingly, 14.3% of participants were prescribed stimulants which may have been used to “counteract” sedating properties of other medications. As an extreme example, one participant was prescribed nine medications with sedating and two medications with stimulant properties. Close periodic evaluation of the prescribed, over-the-counter, and herbal medications may help “streamline” the patient’s pharmacological profile (reduce the number and/or the doses of medications) and improve safety.32
Results of this study follow the pattern of a current debate and controversy about the net-balance of risks and benefits of long-term opioid therapy for non-cancer pain. Participants were treated with opioids for an average of 7.9 ± 5.7 years, receiving at the study assessment a high average daily dose of opioids. Treatment with high-dose opioids, with concomitant poor clinical outcomes, may suggest lack of long-term efficacy of opioids and/or a presence of opioid-induced hyperalgesia. This is consistent with limited existing evidence showing that long-term opioid therapy for chronic non-cancer pain may worsen outcomes (e.g., disability scores).33 Although opioid-induced hyperalgesia is a known phenomenon,5 it is unclear how to clinically evaluate for its presence or predict which patients may be prone to developing it. Should it have contributed to participants’ situation, an opioid dose reduction could improve clinical outcomes. Prospective research, designed to untangle the influence of these complex forces, is needed in this population.
Many of the participants were not “maximized” on nonopioid pain-modulating medications, especially in regards to non-acetaminophen / non-NSAIDs, leaving a potential for “improvement” (e.g., better clinical outcomes; reduced opioid dose) should these adjuvant medications be added and effective. The use of medications targeting headaches, many of which (e.g., triptans) may have substantial negative effects, was quite common (22.3% of participants), suggesting the presence of other pain comorbidities in this patient population. Of note, only a minority utilized a bowel regimen which is recommended for those on long-term opioids to prevent constipation.30,31
A high-prevalence of positive UDT results for illicit drugs, unprescribed medications or alcohol noted in this study sample corroborates findings of others and calls for close monitoring of therapy progress and adherence among chronic non-cancer pain patients prescribed long-term opioids.10,34,35 Clinical experience and research findings are in agreement that screening for and detecting those with “true” substance use disorders, as opposed to those who, for example, self-medicate to alleviate unrelenting pain (“pseudoaddiction”) are challenging among opioid-treated chronic pain patients. Urine drug testing is recommended and may help stratify patient’s risk level for misuse, but its interpretation can sometimes be challenging and require a nuanced approach.36,37 Although this study did not delineate strong correlates of “positive” UDT results, it found that those treated with a lower daily opioid dose, especially lower daily dose of extended-release opioids, were more likely to have “positive” UDTs. This can be interpreted in a variety of ways. One possibility is that those receiving a lower opioid dose suffered from an under-treated pain which, in turn, steered them toward substance misuse as means of alleviating suffering. The other explanation may be that research participants with positive UDT results tended to test “positive” in clinical settings, and due to that these participants’ regular clinicians (opioid prescribers) had elected to reduce opioid dose.
Alcohol use appears to be rather common among opioid-treated chronic pain patients and can potentiate sedating effects of opioids. A detailed conversation with the patient about avoidance of alcohol, as well as other sedating over-the-counter or prescribed medications and illicit drugs, should be a routine part of “treatment agreement” discussions. Although testing for alcohol metabolites is not recommended as a method for routine screening for drinking and not a part of routine UDTs, the self-reports of alcohol – as well as of cannabis – use appeared to be rather accurate among participants when compared to the UDT results. This was not the case for misused (and detected by UDTs) prescription-based opioids, benzodiazepines or cocaine. In the current climate of political endorsement of legalizing medical marijuana in many states in the U.S., cannabis use may be viewed by some patients or providers as “acceptable” while on long-term opioid therapy. Research evidence does not support this view, with safety concerns additionally potentiated when concomitant use of alcohol and/or other central nervous system depressants takes place.38 In patients who use cannabis and are prescribed opioids, close monitoring for opioid- and other substance related problems is recommended.
Limitations
Cross-sectional nature and small sample size of this study call for caution when interpreting findings and preclude drawing conclusions about causality or directionality of observed relationships. Patients who entered this study may differ from those who were not interested in research or intervention, thus potentially limiting result generalizability. Lack of a detailed assessment for addiction and its spectrum does not allow for determination of prevalence or correlates of addictive disorders; the self-reported and toxicology-based findings can merely suggest presence of substance use disorders.
Directions for future research
The prevalence and impact of long-term opioid therapy for chronic non-cancer pain, combined with overall poor clinical outcomes in this population, limited evidence on effects of long-term opioids and a growing epidemic of prescription-drug abuse, indicate the need for urgent research in this area. In addition to scientific efforts focused on the development of new therapies, there is also a need for basic science-level research on mechanisms underlying efficacy and clinical research on how to best optimize existing care, for example, through simplifying pharmacotherapy39 and tailoring care to the specific needs of an individual patient.40
CONCLUSIONS
Study findings corroborate existing evidence for high medication burden and high likelihood of substance misuse among opioid-treated CLBP patients. Further research is needed to help understand causality and identify ways to optimize care and clinical outcomes in this population. Long-term, effective treatment for CLBP could provide enormous benefits to CLBP patients, their families, and to society through reduced suffering and disability, decreased costs, and the alleviation of prescription opioid abuse.
Acknowledgments
Dr. Zgierska is supported by the K23AA017508 from the National Institutes of Health (NIH) National Institute on Alcohol Abuse and Alcoholism (NIAAA). Dr. Wallace is supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin. The project was also supported by the Clinical and Translational Science Award (CTSA) program through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We are grateful to Drs. M.P. George and Roza George, and Alere™ Toxicology for providing toxicology testing. We are also thankful to Ms. Terry Little for help with the manuscript preparation.
Contributor Information
Aleksandra Zgierska, Email: Aleksandra.Zgierska@fammed.wisc.edu, Assistant Professor, Department of Family Medicine, School of Medicine and Public Health, University of Wisconsin-Madison, 1100 Delaplaine Court, Madison, WI 53715, Office: 608 263 7882; Fax: 608 263 5813.
Margaret L. Wallace, Research Fellow, Department of Family Medicine, School of Medicine and Public Health, University of Wisconsin-Madison.
Cindy A. Burzinski, Assistant Researcher, Department of Family Medicine, School of Medicine and Public Health, University of Wisconsin-Madison.
Jennifer Cox, Research Specialist, Department of Family Medicine, School of Medicine and Public Health, University of Wisconsin-Madison.
Miroslav Backonja, Department of Neurology, School of Medicine and Public Health, University of Wisconsin-Madison; Clinical Research Company Lifetree, Salt Lake City, UT.
References
- 1.Martin BI, Turner JA, Mirza SK, Lee MJ, Comstock BA, Deyo RA. Trends in health care expenditures, utilization, and health status among US adults with spine problems, 1997–2006. Spine (Phila Pa 1976) 2009 Sep 1;34(19):2077–2084. doi: 10.1097/BRS.0b013e3181b1fad1. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. [Accessed March 31, 2014];Healthy People 2020: 2020 Topics and Objectives (Arthritis, Osteoporosis, and Chronic Back Conditions) Available at http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=3.
- 3.Manek NJ, MacGregor AJ. Epidemiology of back disorders: prevalence, risk factors, and prognosis. Curr Opin Rheumatol. 2005 Mar;17(2):134–140. doi: 10.1097/01.bor.0000154215.08986.06. [DOI] [PubMed] [Google Scholar]
- 4.Dillie KS, Fleming MF, Mundt MP, French MT. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med. 2008 Mar-Apr;21(2):108–117. doi: 10.3122/jabfm.2008.02.070144. [DOI] [PubMed] [Google Scholar]
- 5.Manchikanti L, Helm S, 2nd, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012 Jul;15(3 Suppl):ES9–38. [PubMed] [Google Scholar]
- 6.Office of National Drug Control Policy (ONCDP) [Accessed March 31, 2014];Epidemic: Responding to America’s Prescription Drug Abuse Crisis. 2011 Available at http://www.whitehouse.gov/sites/default/files/ondcp/issues-content/prescription-drugs/rx_abuse_plan_0.pdf.
- 7.Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009 Feb;10(2):147–159. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Substance Abuse and Mental Health Services Administration (SAMHSA) Summary of National Findings. US Department of Health and Human Services, SAMHSA, Office of Applied Studies; 2010. [Accessed Jan 30, 2015]. Results from the 2009 National Survey on Drug Use and Health: Volume I. Available at http://oas.samhsa.gov/nsduh/2k9nsduh/2k9resultsp.pdf. [Google Scholar]
- 9.FDA Center for Drug Evaluation and Research Division of Anesthesia and Analgesia Division. Risk evaluation and mitigation strategies (REMS) for extended-release and long-acting opioid analgesics. Paper presented at: Joint Meeting of the Anesthetic and Life Support Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee; Adelphie, MD. July 22 and 23, 2010. [Google Scholar]
- 10.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010 Sep-Oct;13(5):401–435. [PubMed] [Google Scholar]
- 11.Substance Abuse and Mental Health Services Administration (SAMHSA) Office of Applied Studies. [Accessed March 24, 2014];The DAWN Report: Trends in Emergency Department visits involving nonmedical use of narcotic pain relievers. 2010 Available at http://www.oas.samhsa.gov/2k10/DAWN016/OpioidED.htm.
- 12.National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. [Accessed March 24, 2014];Unintentional drug poisoning in the United States. 2010 Available at http://www.cdc.gov/HomeandRecreationalSafety/pdf/poison-issue-brief.pdf.
- 13.National Center on Addiction and Substance Abuse at Columbia University (CASA) Missed Opportunity: National Survey of Primary Care Physicians and Patients on Substance Abuse. New York: CASA; 2000. [Google Scholar]
- 14.Gomes T, Juurlink DN, Dhalla IA, Mailis-Gagnon A, Paterson JM, Mamdani MM. Trends in opioid use and dosing among socio-economically disadvantaged patients. Open Med. 2011;5(1):e13–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes T, Redelmeier DA, Juurlink DN, Dhalla IA, Camacho X, Mamdani MM. Opioid dose and risk of road trauma in Canada: a population-based study. JAMA internal medicine. 2013 Feb 11;173(3):196–201. doi: 10.1001/2013.jamainternmed.733. [DOI] [PubMed] [Google Scholar]
- 16.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980 Aug;66(8):271–273. [PubMed] [Google Scholar]
- 17.Cleland J, Gillani R, Bienen EJ, Sadosky A. Assessing dimensionality and responsiveness of outcomes measures for patients with low back pain. Pain Pract. 2011 Jan-Feb;11(1):57–69. doi: 10.1111/j.1533-2500.2010.00390.x. [DOI] [PubMed] [Google Scholar]
- 18.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004 Sep-Oct;20(5):309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine (Phila Pa 1976) 2000 Dec 15;25(24):3140–3151. doi: 10.1097/00007632-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 20.Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42(1):49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- 21.Sobell LC, Sobell MB. Timeline Followback: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. New Jersey: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 22.McPherson ML. Demystifying Opioid Conversion Calculations: A Guide to Effective Dosing. 1. Bethesda, MD: American Society of Health System Pharmacists; 2009. [Google Scholar]
- 23.Jacox AK, Carr DB, Payne R, et al. Management of Cancer Pain, Clinical Practice Guideline, No 9. Rockville, MD: Agency for Health Care Policy and Research, US Department of Helath and Human Services, Public Health Service; 1994. [Google Scholar]
- 24.Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001 Aug;22(2):672–687. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 25.Grond S, Radbruch L, Meuser T, Loick G, Sabatowski R, Lehmann KA. High-dose tramadol in comparison to low-dose morphine for cancer pain relief. J Pain Symptom Manage. 1999 Sep;18(3):174–179. doi: 10.1016/s0885-3924(99)00060-3. [DOI] [PubMed] [Google Scholar]
- 26.McAuley DF. Benzodiazepine Dose Conversions (Oral) [Accessed March 31, 2014];GlobalRPh: The Clinician’s Ultimate Reference. 2013 Available at http://globalrph.com/benzodiazepine_calc.htm.
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomed informatics. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.IBM Corporation. IBM SPSS Statistics Version 21 [computer program] Armonk, NY: IBM Corporation; 2012. [Google Scholar]
- 29.Deyo RA, Smith DH, Johnson ES, et al. Opioids for back pain patients: primary care prescribing patterns and use of services. J Am Board Fam Med. 2011 Nov-Dec;24(6):717–727. doi: 10.3122/jabfm.2011.06.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part I--evidence assessment. Pain Physician. 2012 Jul;15(3 Suppl):S1–65. [PubMed] [Google Scholar]
- 31.Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2--guidance. Pain Physician. 2012 Jul;15(3 Suppl):S67–116. [PubMed] [Google Scholar]
- 32.Taylor R, Jr, Pergolizzi VJ, Jr, Puenpatom RA, Summers KH. Economic implications of potential drug-drug interactions in chronic pain patients. Expert Rev Pharmacoecon Outcomes Res. 2013 Dec;13(6):725–734. doi: 10.1586/14737167.2013.851006. [DOI] [PubMed] [Google Scholar]
- 33.Sjogren P, Gronbaek M, Peuckmann V, Ekholm O. A population-based cohort study on chronic pain: the role of opioids. Clin J Pain. 2010 Nov-Dec;26(9):763–769. doi: 10.1097/AJP.0b013e3181f15daf. [DOI] [PubMed] [Google Scholar]
- 34.Manchikanti L, Manchukonda R, Pampati V, et al. Does random urine drug testing reduce illicit drug use in chronic pain patients receiving opioids? Pain Physician. 2006 Apr;9(2):123–129. [PubMed] [Google Scholar]
- 35.Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: frequency and predictability of abnormal findings. Clin J Pain. 2007 Feb;23(2):173–179. doi: 10.1097/AJP.0b013e31802b4f95. [DOI] [PubMed] [Google Scholar]
- 36.Gupta A, Patton C, Diskina D, Cheatle M. Retrospective review of physician opioid prescribing practices in patients with aberrant behaviors. Pain Physician. 2011 Jul-Aug;14(4):383–389. [PubMed] [Google Scholar]
- 37.Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011 Mar-Apr;14(2):123–143. [PubMed] [Google Scholar]
- 38.Reisfield GM. Medical cannabis and chronic opioid therapy. Journal Pain Palliative Care Pharmacother. 2010 Dec;24(4):356–361. doi: 10.3109/15360288.2010.519431. [DOI] [PubMed] [Google Scholar]
- 39.Bennett MI, Bagnall AM, Raine G, et al. Educational interventions by pharmacists to patients with chronic pain: systematic review and meta-analysis. Clin J Pain. 2011 Sep;27(7):623–630. doi: 10.1097/AJP.0b013e31821b6be4. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien EM, Staud RM, Hassinger AD, et al. Patient-centered perspective on treatment outcomes in chronic pain. Pain Med. 2010 Jan;11(1):6–15. doi: 10.1111/j.1526-4637.2009.00685.x. [DOI] [PubMed] [Google Scholar]