Abstract
Menopause is associated with changes in bone, muscle and fat mass. The importance of postmenopausal estrogen metabolism in bone health has been established. However, its relationship to body composition in postmenopausal women remains undetermined. The objective of this study is to determine the association between estrogen metabolism and body composition in postmenopausal women.
This is a cross sectional study of 97 postmenopausal Caucasian women, 49–80 y.o., ≥ 1 year from the last normal menstrual period or those who have had oophorectomy. Inactive [2-hydroxyestrone (2OHE1)] and active [16α-hydroxyestrone (16α-OHE1)] urinary metabolites of estrogen were measured by ELISA. The whole and regional body composition was measured by DXA.
We have found that both 2OHE1,, and 2OHE1/16α-OHE1 ratio were negatively correlated with % total fat, and % truncal fat but positively correlated with % total lean mass. Comparing the fat and lean parameters of body composition according to tertiles of 2OHE1 and 2OHE1/16αOHE1 ratio showed that subjects in the lowest tertiles, had the highest % total fat, and % truncal fat and the lowest % total lean mass. Multiple regression analysis also showed 2OHE1 and calcium intake as statistically significant predictors of all body composition parameters.
In conclusion, in postmenopausal women, an increase in the metabolism of estrogen towards the inactive metabolites is associated with lower body fat and higher lean mass than those with predominance of the metabolism towards the active metabolites.
Keywords: body composition, estrogen metabolism, obesity
Introduction
While estradiol (E2) is the predominant form of estrogen in premenopausal women, estrone (E1) is the primary form found in postmenopausal women which originates from the peripheral conversion of the adrenal androgen, androstenedione, by the enzyme aromatase [1]. E1 can be reversibly oxidized to E2 or undergo catabolism to metabolites with varying estrogenic activities [2–5]. There are several catabolic pathways for estrone but the main pathway is the irreversible hydroxylation at c-16 and c-2. The 16α-hydroxylation leads to the formation of active estrogenic metabolites, 16α hydroxyestrone (16αOHE1) and estriol (E3). 16α-OHE1 binds with high affinity to estrogen receptors and acts as estrogen agonist [6, 7]. In contrast the 2-hydroxyl pathway results in formation of 2-hydroxyestrone (2OHE1) and 2-methoxyestrone (2-MeOHE1) that are believed to be virtually devoid of estrogenic activity [8]. The predominance of one pathway over another, as reflected by 2OHE1 /16αOHE1 ratio, is believed to determine the overall estrogenic environment in the body and may be more important than the absolute amounts of individual metabolites produced [8, 9].
Our group previously reported that the oxidative metabolism of estrogen is an important determinant of postmenopausal bone loss [9, 10] and bone mineral density in men [11]. Previous observational studies have also demonstrated an association between estrogen metabolism and BMI [12] suggesting that obesity is associated with significant decreases in hydroxylation at C-2 resulting in reduced production of less active or inactive estrogenic metabolites in both sexes. However, these results were limited to analysis of body mass index (BMI) which is highly dependent on weight and height alone, and no data are available on the relationship between estrogen metabolites and body composition (i.e. actual fat and lean mass composition of the body). Thus, the objective of this study was to determine the association between estrogen metabolism and body composition in postmenopausal women. We hypothesize that there is an association between the ratio of inactive to active estrogen metabolites and body composition (distribution and amount of fat) in postmenopausal women.
Materials and Methods
Study population
This is a cross-sectional study conducted on otherwise healthy postmenopausal women who were at least one year from their last menstrual period or had bilateral oophorectomy. Due to the racial differences in estrogen metabolism [13], only Caucasian subjects were included in the analysis. Participants were recruited through advertisements or direct mailing. This study was conducted in accordance with the guidelines in the Declaration of Helsinki for the ethical treatment of human subjects. The protocol was approved by the Washington University School of Medicine institutional review board. All participants provided an informed consent after a discussion of the protocol. Subjects who were taking any medication that affects estrogen status, such as estrogen, selective estrogen receptor modulators (including raloxifene and tamoxifen) or aromatase inhibitors, were excluded from the study. The subjects currently taking the medication or supplements that can affect estrogen metabolism like phytoestrogens, cimitidine, thyroid hormones, and monooxygenase inhibitors or the drugs affecting CYPP450 enzyme activity such as phenytoin, carbamazepine and phenobarbital were excluded [14]. Since smoking can affect estrogen metabolism [15, 16] current smokers were also excluded, but past smokers who quit for more than six months were included. Subjects consuming more than one serving per day of vegetables containing high levels of phytochemicals known to preferentially enhance 2-hydroxylation of estrogen, such as cabbage, cauliflower, Brussels sprouts, broccoli, and kale , were excluded from participation [17].
Clinical, dietary, and anthropometric
Because of our prior finding of an association between calcium intake and estrogen metabolism [18], calcium intake was included in the current analysis to determine if it has any relationship to body composition. Daily intake of calcium from diet and supplements were calculated from a 7 day dietary record which the participants were asked to fill out for seven days. The record contains a list and serving sizes of common dietary sources of calcium. The participants were asked to record daily intake of these foodstuffs, and the average daily intake of calcium from supplements and diet was determined for 7 days [18].
Alcohol intake was expressed as the average number of alcoholic drink-equivalents consumed over a 1-wk period. A can of beer (336 ml), a glass of wine (112 ml), and 28 ml of a heavy alcoholic beverage were considered one drink-equivalent [9]. Previous smoking was expressed in pack-years and was estimated as the number of 20-cigarette packs smoked per day multiplied by the number of years of smoking. Physical activity was expressed as a numerical score and was defined as: sedentary (sitting or lying most of the day, score 1), moderately active (being on feet more than half a day, score 2), and very active (engaging in regular physical exercise, score 3) [19] . The waist to hip ratio was calculated as the ratio between waist circumference, taken at the umbilical level, and hip circumference, measured 6 in. (15.24 cm) below the anterior superior iliac spine. BMI was calculated as weight in kilograms divided by the square of height in meters square (kg/m2).
Body Composition
Total body mass, lean body mass (mineral-free and fat-free), fat mass, and truncal fat were measured using whole body dual-energy X-ray absorptiometry (Hologic Delphi 4500/w; Hologic, Waltham, MA; Enhanced Whole Body 11.2 software version; Hologic) as previously described [20]. The percentage of whole (% total fat) and regional fat mass, in this particular study truncal fat mass (% truncal fat), were obtained from the estimated readings given by the machine for the different regions of interest. Percent of total lean mass (% total lean) was calculated as total lean mass /total body mass.
Biochemical data
Urinary estrogen metabolites were measured in 24-h urine specimens using ESTRAMET immunoassay kits (Immuna Care Corp, Bethlehem, PA). The ESTRAMET series of test kits are monoclonal antibody-based competitive enzyme immunoassays for estrogen metabolites in microtiter plate format as previously described [21]. In this study we assessed 2 metabolites, 2OHE1 and 16α-OHE1, and calculated the 2OHE1 to 16α-OHE1 ratio. The inter- and intraassay CVs for these enzyme-linked immunoassays were <9% and 13% respectively. Each urinary metabolite value was corrected for 24-h urinary creatinine (mg/24 h) and was expressed in ng/mg creatinine. Serum samples were collected in the nonfasting state.
Statistical analysis
Results are expressed as the means±SD. A P < 0.05 was considered statistically significant. BMI data was not normally distributed. Accordingly, BMI data was log transformed for analysis and back transformed to obtain a geometric mean. % trunk fat was uniformly distributed but this doesn’t require transformation to provide valid analyses; the other outcome variables were normally distributed. The association between clinical variables with various parameters of body composition and each urinary metabolite were evaluated by Pearson or Spearman correlation analysis as appropriate. Multiple regression analyses were performed to determine important independent clinical and biochemical predictors of body composition parameters. These variables (estrogen metabolites, average daily calcium intake, age, menopausal age, and past history of smoking) were selected because of their potential to influence body composition. Comparisons of BMI, waist circumference and body composition parameters across the different tertiles of urinary metabolites were performed by analysis of variance (ANOVA) adjusted for age and menopausal age; if a parameter differed significantly among the tertiles, post-hoc testing was performed. Data were managed using Excel 2000 (Microsoft Corp., Redmond, WA) and were analyzed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Our population consisted of 97 Caucasian postmenopausal women, between 49–80 years of age, who were at least one year from their last menstrual period or had bilateral oophorectomy. The age distribution of our subjects consisted of one patient below age 50 y.o. (i.e. the 49 y.o. participant), 30 subjects were between the ages of 50 to 59, 37 were between 60 to 69 and 29 were 70 y.o. and above. Most of the participants were part of a previously published study [18, 22] and participant characteristics of the studied population are reported in Table 1.
Table 1.
Participant characteristics of the study population (n=97)
| Age (yr) | 63.73±7.52 |
| BMI (kg/m2) | 28.91±6.23 |
| Waist (inch) | 36.26±6.51 |
| Menopausal age | 13.79±11.15 |
| Alcohol intake (oz-Eq/week) | 1.02±1.99 |
| History of Past smoking (pack-years) | 6.11±13.65 |
| Physical activity score | 2.27±0.60 |
| Calcium intake (mg/d) | 1187.32±717.61 |
Values are means ± SD, BMI: body mass index
Correlation analysis showed a significant negative correlation between 2OHE1 with % total fat and % truncal fat, and a significant positive correlation between 2OHE1 and % total lean mass (Table 2). The 2OHE1/16αOHE1 ratio also showed a significant negative correlation with % total fat and a significant positive correlation with % total lean mass. Furthermore, both 2OHE1 and 2OHE1/16αOHE1 negatively correlated with BMI. Average daily calcium intake also showed negative correlations with % total fat and % truncal fat, a positive correlation with % total lean mass and negative correlation with BMI (Table 2).
Table 2.
Correlation analyses demonstrating association between urine estrogen metabolites with body composition parameters and participant characteristics.
| % Total Fat | % Truncal Fat | %Total Lean | BMI | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| 2OHE1 | −0.27 | <0.01 | −0.32 | <0.01 | 0.26 | 0.01 | −0.36 | <0.01 |
| 16αOHE1 | −0.14 | 0.19 | −0.19 | 0.06 | 0.14 | 0.19 | −0.12 | 0.25 |
| 2OHE1 /16αOHE1 | −0.22 | 0.03 | −0.10 | 0.31 | 0.21 | 0.04 | −0.29 | <0.01 |
| Age | −0.09 | 0.38 | 0.16 | 0.12 | 0.11 | 0.31 | −0.24 | 0.02 |
| FEI | 0.24 | 0.07 | 0.35 | <0.01 | −0.23 | 0.08 | 0.50 | <0.01 |
| Menopausal age | 0.15 | 0.14 | −0.15 | 0.14 | 0.16 | 0.12 | −0.16 | 0.13 |
| Average daily calcium intake | −0.27 | 0.01 | −0.34 | <0.01 | 0.27 | 0.01 | −0.35 | <0.01 |
| History of past smoking (pack-years) | 0.02 | 0.83 | 0.01 | 0.95 | 0.03 | 0.75 | −0.00 | 0.97 |
r = Pearson correlation except for Spearman correlations with BMI
2OHE1:2-hydroxyestrone, 16αOHE1: 16 alpha-hydroxyestrone, FEI: free estradiol index.
Significant correlations are typed in bold font with corresponding p values.
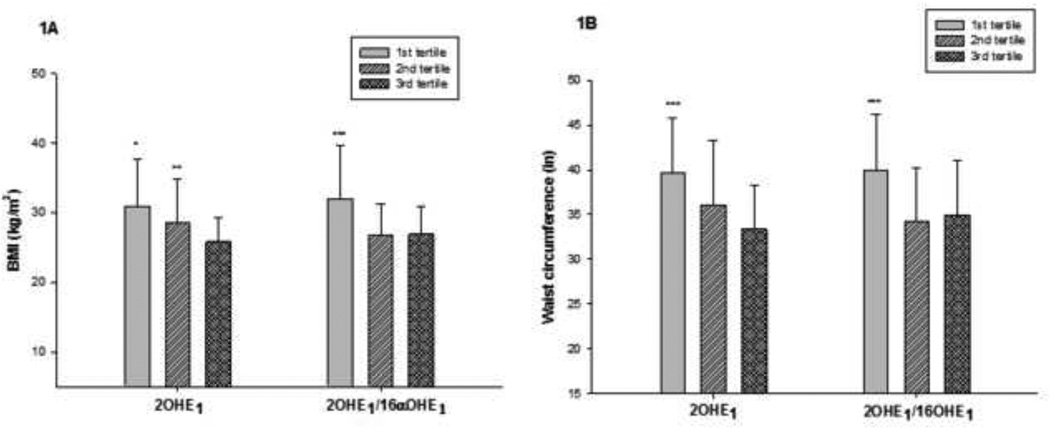
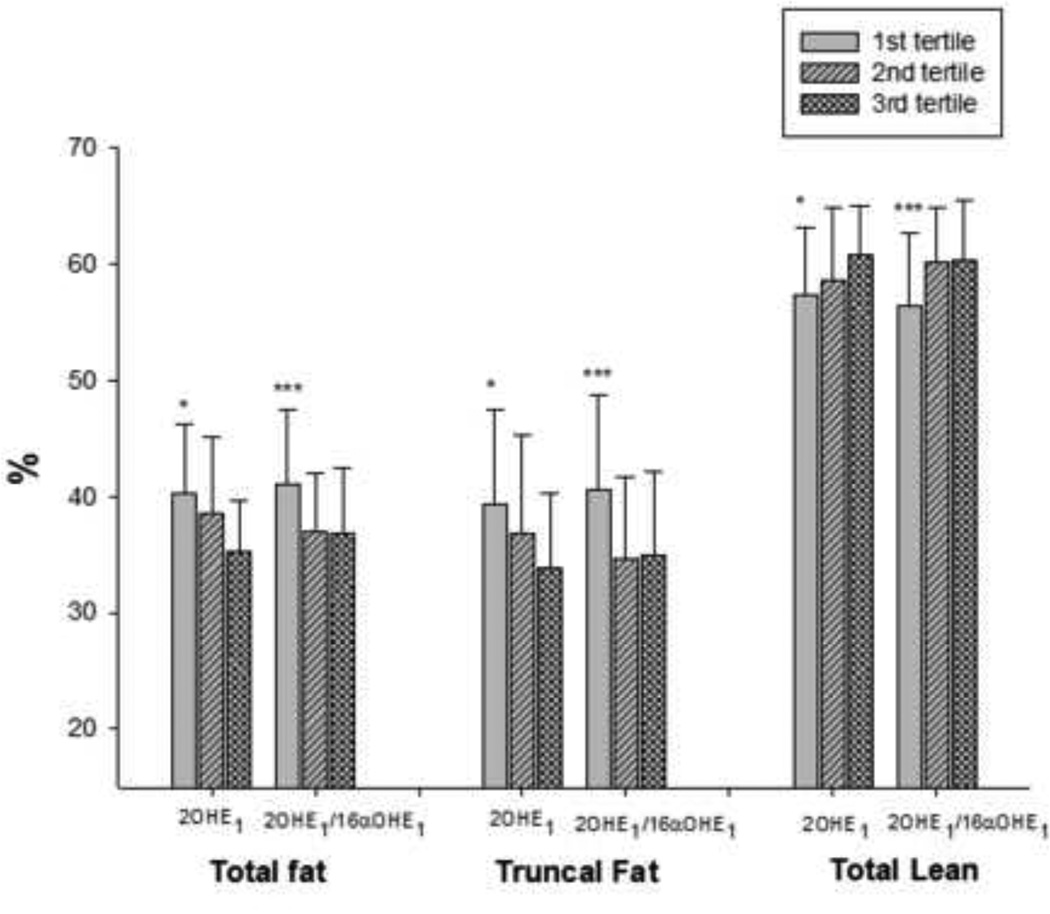
We divided our subjects according to tertiles of 2OHE1 and 2OHE1/16α-OHE1. Metabolite values (means ± SD) for the different tertiles were as follows, 1) for 2OHE1: tertile 1= 1.42±1.02, tertile 2= 1.95±1.27 and tertile 3= 2.60±1.41 ng/g creatinine; and 2) for 2OHE1/16α-OHE1 ratio: tertile 1= 0.89±0.24, tertile 2= 1.69±0.29, and tertile 3= 3.28±1.19. In all instances, comparisons (i.e. p values) presented in the text are results of ANOVA testing, while results from post-hoc analyses are presented in the figures. A comparison of the BMI across the tertiles showed that women in the lowest tertiles of 2OHE1 and 2OHE1/16α-OHE1 (ratio<1.3) had the highest BMI (both p <0.01) compared to the upper 2 tertiles (Figure 1A). Similarly, comparing waist circumference among the different tertiles showed that women in the lowest tertiles of 2OHE1 and 2OHE1/16α-OHE1 have significantly higher waist circumference (both p <0.01) compared to those in the upper 2 tertiles (Figure 1B). Analysis of the body composition parameters according to tertiles of 2OHE1/16αOHE1 ratio showed that women in the lowest tertile had significantly higher body fat (i.e. % total fat and % truncal fat, p=0.01 and p<0.01, respectively) and lower lean body mass (% total lean, p=0.02) relative to the upper 2 tertiles (Figure 2). Moreover, it appears that the changes in body composition occur right at a cut-off value in 2OHE1/16αOHE1 ratio of 1.3, with mean values of body composition parameters among women with ratios at or above 1.3 (upper 2 tertiles) appearing to be almost equal. Analysis according to tertiles of 2OHE1 also showed decreasing body fat (% total and % truncal fat, p=0.01 and p=0.02, respectively) and increasing lean mass with increasing tertiles (p=0.03). There were no significant differences with respect to other variables such as age, menopausal age, smoking history, alcohol intake, physical activity score, and calcium intake across the tertiles of both 2OHE1 and 2OHEI/16α-OHE1 (data not shown).
Figure 1.
Anthropometric parameters, i.e. BMI in kg/m2 (1A) and waist in inches (1B), divided according to tertiles of 2OHE1 and 2OHE1/16α-OHE1. Each group represents the averages (means ± SD) of the BMI and waist circumference in the different tertiles of 2OHE1 and 2OHE1/16α-OHE1 (from left to right). *p<0.05:1st vs. 3rd tertile, **p<0.05:2nd vs 3rd tertile, ***p<0.05 1st vs. 2nd and 3rd tertile by post-hoc analysis
Figure 2.
Parameters of body composition divided according to tertiles of 2OHE1 and 2OHE1/16α-OHE1. Each group represents the averages (mean ± SD) of the % total fat, % truncal fat and % total lean in the different tertiles of 2OHE1 and 2OHE1/16α-OHE1 (from left to right). P values by analysis of variance adjusted for age and menopausal age. *p<0.05:1st vs. 3rd tertile, ***p<0.05 1st vs. 2nd and 3rd tertile by post-hoc analysis
Multiple regression analyses were performed by stepwise selection of variables (estrogen metabolites, average daily calcium intake, age, menopausal age, and past history of smoking) believed to be associated with body composition parameters. Average calcium intake which we reported to be associated with urinary estrogen metabolite levels was also added into the model [18]. Urinary 2OHE1 and average daily calcium intake were found to be significant independent predictors of % total body fat, % truncal fat and % total lean mass (Table 3). 16α-OHE1 (% total fat p=0.70, % truncal fat p=0.49, % total lean mass p=0.70), 2OHE1/16α-OHE1 ratio (% total fat p=0.44, % truncal fat p=0.34, % total lean mass p=0.48), age (% total fat p=0.47, % truncal fat p=0.86, % total lean mass p=0.56), menopausal age (% total fat p=0.12, % truncal fat p=0.38 % total lean mass p=0.14), and past history of smoking (% total fat p=0.81, % truncal fat p=0.76, % total lean mass p=0.75) were not found to be independent predictors with any of the parameters of body composition.
Table 3.
Multivariable analyses identifying predictors of body composition parameters.1
| Body composition parameter and clinical predictor |
R2 | Regression Coefficient |
95% Confidence Intervals |
P values |
|---|---|---|---|---|
| % total fat | 14.76 | <0.0 | ||
| 2OHE1 | −0.26 | −0.48 to −0.05 | 0.01 | |
| Calcium intake | −0.002 | −0.004 to −0. 5× 10−3 | 0.01 | |
| % truncal fat | 18.30 | <0.01 | ||
| 2OHE1 | −0.43 | −0.72 to −0.15 | <0.01 | |
| Calcium intake | −0.003 | −0.006 to −0.9 × 10−3 | <0.01 | |
| % total lean | 14.47 | <0.01 | ||
| 2OHE1 | 0.27 | 0.07 to 0.48 | 0.01 | |
| Calcium intake | 0.002 | 0.48 × 10−3 to 0.004 | 0.02 | |
Significance of predictor variables tested by multiple regression analysis. Candidate predictor variables entered into the model were as follows: urinary estrogen metabolites, average daily calcium intake, age, menopausal age, and past history of smoking.
In order to examine whether the estrogen metabolism parameters are related to age and/or menopausal age, we defined menopausal age as a binary variable with cutscore 10 and age as a trinary variable with cutscores 60 and 70. In the present data (a subset of the parent study) [22], 2-way ANOVA analyses found no statistically significant relationships with these variables to estrogen metabolism.
Our sample size is a subset of convenience from a parent study [22]. The post-hoc power calculations indicated that our sample size of 32, 32, 33 subjects per group in the tertiles of 2OHE1: 16aOHE1 had 80% power for comparing the pattern of 3 means of % total fat as reported in Figure 2 in a one-way ANOVA. This calculation was based on a common standard deviation of 6.0% and α=0.05. Other comparisons had similar power.
Discussion
Our results indicate that women whose estrogen metabolism favors the formation of inactive metabolites, as shown by a high 2OHE1/16αOHE1 ratio and high levels of 2OHE1, have lower BMI and smaller waist circumference. Furthermore, analysis of parameters of body composition indicated that the higher the levels of inactive estrogen metabolites the lower the body fat and the higher the lean body mass proportions suggesting an association between estrogen metabolism and body composition in postmenopausal women.
In addition to the alteration in the risk for hormone-related cancers, the risk for bone loss and response to hormone therapy, [9, 23], our data from the current study suggest that an increase in 2-hydroxylation of estrogen in postmenopausal women is associated with a leaner body habitus and lower body fat. Although this finding does not establish a cause and effect relationship, it is consistent with results from animal studies showing that obese ZSF1 rats given 2-hydroxyestradiol for approximately 6 months had a 25% lower body weight than controls [24, 25]. More importantly the reduced body weight, was associated with significantly improved glucose control (both plasma glucose levels and glycosylated hemoglobin) [24] in these animals. Data from in-vitro experiments showed that 2OHE1 mediates the activation of the AMP-protein kinase phosphorylation by estradiol [26]. Activation of this pathway leads to an increase in fat oxidation, inhibition of fat synthesis and an increase in glucose uptake [27–29] and may perhaps account for the positive effects of 2OHE1 on glucose and lipid metabolism in-vivo.
In a study using tracer estradiol, Schneider and colleagues found that obese individuals had reduced C-2 hydroxylation without any change in 16-hydroxylation compared to age-matched normal weight controls [30]. Although the authors concluded that obesity modulates estrogen metabolism by suppressing 2-hydroxylation, an increase in 16α-hydroxylation of E1 may also explain the relatively lower 2OHE1/16αOHE1 ratio with increase in body weight. Nevertheless, the significant inverse association between 2OHE1 with body weight and body fat in comparison to the absence of any significant correlation with 16αOHE1 in our study seemed to be in agreement with the notion of an alteration in the C-2 rather than C-16 pathway. However, as patterns of estrogen metabolism are determined by the activities of the different CYP450 enzymes, [5, 31, 32], it is also possible that certain individuals may be predisposed to a higher body weight because of reduced 2-hydroxylation instead of weight regulating C-2 activity.
Recent studies suggest important positive effects of 2-hydroxyl products on certain metabolic risk factors. For instance, a recent study demonstrated lower blood pressure in individuals with higher 2/16 ratio and vice-versa [33]. Studies in animal models of obesity and the metabolic syndrome (i.e. ZSF1 rats) indicated that treatment with 2-hydroxyestradiol lowered arterial blood pressure; reduced the development of obesity, nephropathy, and the severity of diabetes; and reduced plasma cholesterol and improved endothelial function [24]. In addition, 2-methoxyestradiol (methoxylated product of 2-hydxoyestradiol), was also found to attenuate proteinuria, increase renal blood flow and glomerular filtration, and had potent antiproliferative and anti-inflammatory effects in diseased kidneys of aged animals [25].
Our results also demonstrate that in addition to 2OHE1, daily calcium intake is also a significant predictor of body composition. We observed that the higher the calcium intake in our subjects, the lower the body fat and the higher the lean mass. These results are not surprising and consistent with findings from other studies showing the women with high calcium intake tend to have lower BMI [34–37]. However, the reported effect of calcium on body weight appears to be mostly limited to those who obtained their calcium from dietary sources [36]. We have previously reported that calcium may modulate estrogen metabolism. [18]. Nevertheless, whether the effect of calcium on body weight and body composition, is in part mediated by its effect on estrogen metabolism remains unknown and needs further investigation.
There are several agents or lifestyle habits that may modulate estrogen metabolism. Diets high in protein increase 2-hydroxylation relative to diets high in carbohydrate [38]. Consumption of vegetables rich in phytochemicals (i.e broccoli, cauliflower, Brussels sprouts), and exercise increased 2-hydroxylation [8, 9]. On the other hand, high soy intake is associated with enhanced 2-hydroxylation with a reduction in 16α-hydroxylation [39]. It is possible that the health benefits of diet rich in vegetables along with regular exercise may be partly mediated by increased 2-hydroxylation of estrogen in addition to lower caloric intake and greater energy expenditure. In fact, a recent study reported that exercise and caloric restriction resulted in increase 2-hydroxylation most especially in those in the lowest tertile of baseline 2OHE1/16α-OHE1 40].
It must be noted that although statistical significance was only attained for 2OHE1, the correlations for both metabolites (2OHE1 and 16αOHE1) with body fat and body weight were both negative. Potential explanations for this finding include the lack of power or, that by itself, 16αOHE1 has no association with body composition. On the other hand, given the background information on the opposing activity between 2OHE1 and 16αOHE1, it would be interesting to know if this negative correlation between 16αOHE1 with body weight and body fat persists in a larger sample size.
We realize the limitation of our study; being cross-sectional, we have no information on the lifelong habits of our patients. Although we carefully excluded women taking medications and those with dietary and lifestyle habits identified to affect estrogen hydroxylation, there may be other food items and medications that we haven’t excluded but potentially may modulate estrogen metabolism.
In summary, our results indicate that patients with predominant 2-hydroxylation of estrogen have lower BMI and body fat relative to those with predominant 16α-hydroxylation. These observations may suggest that perhaps increasing estrogen metabolism to the 2-hydroxyl pathway may have some beneficial effects on body weight. Data from animal studies indicate that administration of 2-hydroxyestradiol reduces body weight and improves metabolic profile [25] it’s potential usefulness in human subjects remains undetermined and deserves investigation. To our knowledge, our study is the first to report the association between estrogen metabolism and body composition; thus, additional studies are needed to confirm our findings.
Acknowledgements
This work has been supported by NIH grants R03 AR049401 (RAV), K12 HD01459 (Building Interdisciplinary Research Careers in Women's Health) and the General Clinical Research Center at Washington University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors’ contributions were as follows: Principal investigator, study design and supervision: RAV; preparation and analysis of the data, data interpretation, and writing of the manuscript: RAV, NN, SV and CQ; recruitment of the participants and specimen collection: NN, and JY; data entry: NN, JY and SN; specimen analysis: JY, SN and TG; Approving final version of manuscript: RAV, NN, SV, SN, JY, TG and CQ.
None of the authors had a personal or financial conflict of interest in the research reported.
Reference List
- 1.Nawata H, Tanaka S, Tanaka S, et al. Aromatase in bone cell: association with osteoporosis in postmenopausal women. J Steroid Biochem Mol Biol. 1995 Jun;53(1–6):165–174. doi: 10.1016/0960-0760(95)00031-t. [DOI] [PubMed] [Google Scholar]
- 2.Martucci CP, Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993 Feb;57(2–3):237–257. doi: 10.1016/0163-7258(93)90057-k. [DOI] [PubMed] [Google Scholar]
- 3.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamazaki H, Shaw PM, Guengerich FP, Shimada T. Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol. 1998 Jun;11(6):659–665. doi: 10.1021/tx970217f. [DOI] [PubMed] [Google Scholar]
- 5.Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001 Sep;50(9):1001–1003. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- 6.Westerlind KC, Gibson KJ, Malone P, Evans GL, Turner RT. Differential effects of estrogen metabolites on bone and reproductive tissues of ovariectomized rats. J Bone Miner Res. 1998 Jun;13(6):1023–1031. doi: 10.1359/jbmr.1998.13.6.1023. [DOI] [PubMed] [Google Scholar]
- 7.Lippert TH, Seeger H, Mueck AO. The impact of endogenous estradiol metabolites on carcinogenesis. Steroids. 2000 Jul;65(7):357–369. doi: 10.1016/s0039-128x(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 8.Martucci C, Fishman J. Direction of estradiol metabolism as a control of its hormonal action--uterotrophic activity of estradiol metabolites. Endocrinology. 1977 Dec;101(6):1709–1715. doi: 10.1210/endo-101-6-1709. [DOI] [PubMed] [Google Scholar]
- 9.Leelawattana R, Ziambaras K, Roodman-Weiss J, et al. The oxidative metabolism of estradiol conditions postmenopausal bone density and bone loss. J Bone Miner Res. 2000 Dec;15(12):2513–2520. doi: 10.1359/jbmr.2000.15.12.2513. [DOI] [PubMed] [Google Scholar]
- 10.Napoli N, Armamento-Villareal R. Estrogen hydroxylation in osteoporosis. Adv Clin Chem. 2007;43:211–227. [PubMed] [Google Scholar]
- 11.Napoli N, Faccio R, Shrestha V, Bucchieri S, Rini GB, rmamento-Villareal R. Estrogen metabolism modulates bone density in men. Calcif Tissue Int. 2007 Apr;80(4):227–232. doi: 10.1007/s00223-007-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews CE, Fowke JH, Dai Q, et al. Physical activity, body size, and estrogen metabolism in women. Cancer Causes Control. 2004 Jun;15(5):473–481. doi: 10.1023/B:CACO.0000036445.04238.87. [DOI] [PubMed] [Google Scholar]
- 13.Coker AL, Crane MM, Sticca RP, Sepkovic DW. Re: Ethnic differences in estrogen metabolism in healthy women. J Natl Cancer Inst. 1997 Jan 1;89(1):89–90. doi: 10.1093/jnci/89.1.89. [DOI] [PubMed] [Google Scholar]
- 14.Grover S, Gourie-Devi M, Baghel R, et al. Genetic profile of patients with epilepsy on first-line antiepileptic drugs and potential directions for personalized treatment. Pharmacogenomics. 2010 Jul;11(7):927–941. doi: 10.2217/pgs.10.62. [DOI] [PubMed] [Google Scholar]
- 15.Michnovicz JJ, Hershcopf RJ, Haley NJ, Bradlow HL, Fishman J. Cigarette smoking alters hepatic estrogen metabolism in men: implications for atherosclerosis. Metabolism. 1989 Jun;38(6):537–541. doi: 10.1016/0026-0495(89)90213-8. [DOI] [PubMed] [Google Scholar]
- 16.Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med. 1986 Nov 20;315(21):1305–1309. doi: 10.1056/NEJM198611203152101. [DOI] [PubMed] [Google Scholar]
- 17.Michnovicz JJ, Bradlow HL. Dietary and pharmacological control of estradiol metabolism in humans. Ann N Y Acad Sci. 1990;595:291–299. doi: 10.1111/j.1749-6632.1990.tb34303.x. [DOI] [PubMed] [Google Scholar]
- 18.Napoli N, Thompson J, Civitelli R, rmamento-Villareal RC. Effects of dietary calcium compared with calcium supplements on estrogen metabolism and bone mineral density. Am J Clin Nutr. 2007 May;85(5):1428–1433. doi: 10.1093/ajcn/85.5.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armamento-Villareal R, Civitelli R. Estrogen action on the bone mass of postmenopausal women is dependent on body mass and initial bone density. J Clin Endocrinol Metab. 1995 Mar;80(3):776–782. doi: 10.1210/jcem.80.3.7883830. [DOI] [PubMed] [Google Scholar]
- 20.Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009 Dec;17(12):2162–2168. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klug TL, Bradlow HL, Sepkovic DW. Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16 alpha-hydroxyestrone in urine. Steroids. 1994 Nov;59(11):648–655. doi: 10.1016/0039-128x(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 22.Napoli N, Villareal DT, Mumm S, et al. Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res. 2005 Feb;20(2):232–239. doi: 10.1359/JBMR.041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.rmamento-Villareal RC, Napoli N, Klug T, Civitelli R. The oxidative metabolism of estrogen modulates response to ERT/HRT in postmenopausal women. Bone. 2004 Sep;35(3):682–688. doi: 10.1016/j.bone.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Tofovic SP, Dubey RK, Jackson EK. 2-Hydroxyestradiol attenuates the development of obesity, the metabolic syndrome, and vascular and renal dysfunction in obese ZSF1 rats. J Pharmacol Exp Ther. 2001 Dec;299(3):973–977. [PubMed] [Google Scholar]
- 25.Zhang X, Jia Y, Jackson EK, Tofovic SP. 2-Methoxyestradiol and 2-ethoxyestradiol retard the progression of renal disease in aged, obese, diabetic ZSF1 rats. J Cardiovasc Pharmacol. 2007 Jan;49(1):56–63. doi: 10.1097/FJC.0b013e31802cb88e. [DOI] [PubMed] [Google Scholar]
- 26.D'Eon TM, Rogers NH, Stancheva ZS, Greenberg AS. Estradiol and the estradiol metabolite, 2-hydroxyestradiol, activate AMP-activated protein kinase in C2C12 myotubes. Obesity (Silver Spring) 2008 Jun;16(6):1284–1288. doi: 10.1038/oby.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005 Jan;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008 Dec;14(12):539–549. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Park H, Kaushik VK, Constant S, et al. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002 Sep 6;277(36):32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- 30.Schneider J, Bradlow HL, Strain G, Levin J, Anderson K, Fishman J. Effects of obesity on estradiol metabolism: decreased formation of nonuterotropic metabolites. J Clin Endocrinol Metab. 1983 May;56(5):973–978. doi: 10.1210/jcem-56-5-973. [DOI] [PubMed] [Google Scholar]
- 31.Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF. Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res. 2000 Jul 1;60(13):3440–3444. [PubMed] [Google Scholar]
- 32.Shimada T, Watanabe J, Kawajiri K, et al. Catalytic properties of polymorphic human cytochrome P450 1B1 variants. Carcinogenesis. 1999 Aug;20(8):1607–1613. doi: 10.1093/carcin/20.8.1607. [DOI] [PubMed] [Google Scholar]
- 33.Masi CM, Hawkley LC, Berry JD, Cacioppo JT. Estrogen metabolites and systolic blood pressure in a population-based sample of postmenopausal women. J Clin Endocrinol Metab. 2006 Mar;91(3):1015–1020. doi: 10.1210/jc.2005-2339. [DOI] [PubMed] [Google Scholar]
- 34.Fleming KH, Heimbach JT. Consumption of calcium in the U.S.: food sources and intake levels. J Nutr. 1994 Aug;124(8 Suppl):1426S–130S. doi: 10.1093/jn/124.suppl_8.1426S. [DOI] [PubMed] [Google Scholar]
- 35.Major GC, Chaput JP, Ledoux M, et al. Recent developments in calcium-related obesity research. Obes Rev. 2008 Sep;9(5):428–445. doi: 10.1111/j.1467-789X.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- 36.Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J Am Coll Nutr. 2002 Apr;21(2):146S–151S. doi: 10.1080/07315724.2002.10719212. [DOI] [PubMed] [Google Scholar]
- 37.Lind L, Lithell H, Hvarfner A, Pollare T, Ljunghall S. On the relationships between mineral metabolism, obesity and fat distribution. Eur J Clin Invest. 1993 May;23(5):307–310. doi: 10.1111/j.1365-2362.1993.tb00779.x. [DOI] [PubMed] [Google Scholar]
- 38.Anderson KE, Kappas A, Conney AH, Bradlow HL, Fishman J. The influence of dietary protein and carbohydrate on the principal oxidative biotransformations of estradiol in normal subjects. J Clin Endocrinol Metab. 1984 Jul;59(1):103–107. doi: 10.1210/jcem-59-1-103. [DOI] [PubMed] [Google Scholar]
- 39.Fuhrman BJ, Pfeiffer R, Xu X, et al. Soy intake is associated with increased 2-hydroxylation and decreased 16alpha-hydroxylation of estrogens in Asian-American women. Cancer Epidemiol Biomarkers Prev. 2009 Oct;18(10):2751–2760. doi: 10.1158/1055-9965.EPI-09-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerlind KC, Williams NI. Effect of energy deficiency on estrogen metabolism in premenopausal women. Med Sci Sports Exerc. 2007 Jul;39(7):1090–1097. doi: 10.1097/mss.0b013e3180485727. [DOI] [PubMed] [Google Scholar]