Abstract
Aim
Determination of piperaquine (PQ) in pediatric plasma requires a method with a small sample volume.
Results
We report a sensitive LC–MS/MS method for quantitation of PQ with only 25 µI human plasma. Using a deuterated internal standard (PQ-d6), an analytical PFP column, APCI+ as the ion source and MRM (535/288 for PQ and 541/294 for the IS) for detection, the method has a linear calibration range of 1.5–250 ng/ml with a runtime of 3.0 min per sample. The method was applied to plasma samples from children.
Conclusion
The developed LC–MS/MS method is suitable for pediatric studies with small volume plasma samples collected via capillary tubes. One limitation was the performance of PFP columns varied among different brands.
Piperaquine (PQ) is a component of dihy-droartemisinin-piperaquine (DP), one of the standard artemisinin-based combination regimens for the treatment of uncomplicated falciparum malaria [1]. DP is also under study for chemoprevention against malaria with monthly dosing for at-risk populations [2,3]. DP has performed well in treatment [4,5] and chemoprevention [2] trials, likely in part due to the long elimination half-life of PQ [6,7]. PQ (Figure 1), chemically named 1,3-bis-[4-(7-chloroquinolyl-4)-piperazinyl-1]-propane, is a weak base with four pKa values of 8.6, 8.6, 6.5 and 6.5 [8]. It is highly lipophilic (Log p = 6.2) at neutral and alkaline pH. The free base form of PQ is poorly soluble in water, methanol (MeOH) and acetonitrile (MeCN), but very hydrophilic at low pH and easily soluble in acidified solvents [7,9]. Adsorption of PQ to glass occurs if the solution is stored in a glass bottle. These properties present considerable challenges for analytical method development.
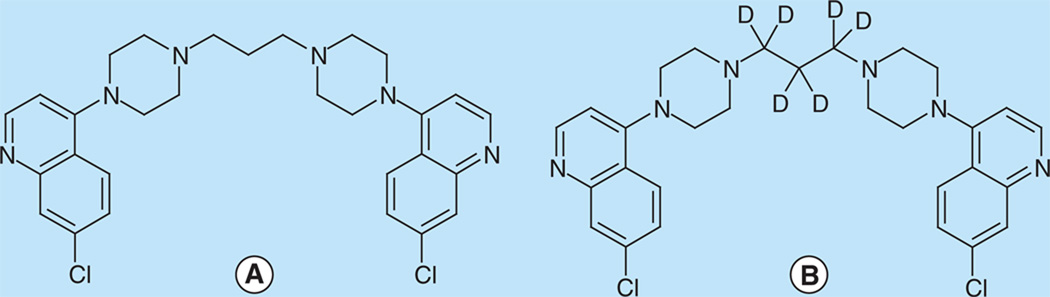
Figure 1. (A) piperaquine (PQ) & (B) piperaquine-d6 (PQ-d6).
Measurement of drugs in pediatric patients requires sensitive quantification methods with a small sample volume. Plasma levels are routinely used to characterize pharmacokinetics and are not impacted by intersubject variability in hematocrit as whole blood assays are. A number of methods have been published for quantification of PQ in plasma, including HPLC–UV [9–11] and LC–MS/MS [8,12,13]. These methods required 50–1000 µl plasma sample volume. The three reported LC–MS/MS methods utilized electrospray ionization in positive mode (ESI+) as the ion source, which is often compromised by matrix effect, even when a deuterated internal standard (IS) is used [8]. Alternatively, atmospheric pressure chemical ionization (APCI) is less sensitive to matrix effect [14]. Here we report an LC–MS/MS method using APCI in positive mode (APCI+) as the ion source and PQ-d6 (Figure 1) as the IS, permitting accurate quantification of PQ for the concentration range expected clinically, in only 25 µl plasma sample volume. This method allows for PQ quantitation in capillary plasma samples collected from children in field-based clinical trials.
Experimental
Chemicals & reagents
Piperaquine tetraphosphate tetrahydrate (MW 999.55, purity 99%) was purchased from AK Scientific, Inc. (CA, USA). Piperaquine-d6 (PQ-d6, MW 541.55, isotopic purity ≥99%) was purchased from AlSAchim, SAS (IllKirch, France). Trichloroacetic acid (TCA) and ammonium formate (NH4FA) (certified ACS reagents), trifluoroacetic acid (TFA) and formic acid (FA) (Optima™ LC/MS grade), acetonitrile (MeCN), methanol (MeOH) and other common solvents (HPLC grade) were purchased from Fisher Scientific, Inc. (NJ, USA). Blank human plasma (K3EDTA added as anticoagulant) was obtained from Biological Specialty Corporation (PA, USA).
LC-MS/MS conditions
The LC–MS/MS system comprises an AB Sciex API5000 Tandem Mass Spectrometer, Shimadzu Prominence 20ADXR UFLC pumps and an SIL-20ACXR autosampler managed with Analyst® 1.5.1 (AB Sciex, CA, USA). The gases for the MS system were supplied by an LC–MS gas generator (Source 5000™, Parker Balston, Inc., MA, USA). The LC columns tested include Synergi polar RP (2.0 × 50 mm, 4 µm), PolymerX RP-1 (4.0 × 50 mm, 5 µm), and pen-tafluorophenyl (PFP) (2.0 × 50 mm, 2.6 µm) columns from Phenomenex, Inc., CA, USA, and Zorbax C8 (2.1 × 50 mm, 5 µm), C18 (2.1 × 30 mm, 1.8 µm) and Pursuit PFP (2.0 × 50 mm, 3 µm) columns from Agilent Technologies, Inc., CA, USA. The LC–MS/MS system was operated in a 25°C room controlled with an air conditioner. The MS conditions for PQ and the IS were optimized by separate infusion of 50 ng/ml PQ or IS into the MS at a flow rate of 10 µl/min while adjusting MS parameters to achieve maximal signal. Ionization utilized APCL+, and detection utilized multiple reaction monitoring mode. Data were processed with Analyst 1.5.1.
Preparation of PQ standards & QC samples
Two sets of PQ stock solutions at 1 mg/ml (base form, converted by multiplying the conversion factor 0.5304) were prepared in 0.5% FA in MeCN-water (1:1, v/v) with separately weighed PQ (in salt form). One solution was used for standard samples and the other for QC samples. Calibration standard samples comprised 1.5, 5, 10, 25, 50, 100 and 250 ng/ml, and QC samples comprised 3, 20 and 200 ng/ml. The IS solution was prepared by dissolving PQ-d6 in 5% TCA in MeOH-water (1:1, v/v) to yield a final concentration of 0.25 ng/ml. The stock solutions, standards, QC samples and the IS solution were aliquoted and stored at −70°C between uses.
Sample preparation
Plasma samples were thawed and a 25 µl aliquot of each sample was pipetted into a micro Eppendorf centrifuge tube. To each tube was added 100 µl MeOH-water (1:1, v/v) containing 5% TCA and 0.25 ng/ml PQ-d6. The mixture was vortexed for 10 s and centrifuged at 25,000 × g for 3 min. The supernatant (75 µl) was transferred to a plastic autosampler vial (250 µl capacity) and 10 µl were injected into the LC–MS/MS system.
Method validation
The assay was validated according to the NIH-sponsored Clinical Pharmacology Quality Assurance (CPQA) program guidelines [15], which were developed based on FDA guidelines [16]. Intraday precision and accuracy were determined by analysis of six replicates of each QC sample at low (3 ng/ml), medium (20 ng/ml) and high (200 ng/ml) concentrations, with a set of standards in one batch. The same procedure was repeated on two additional days with new samples to determine interday precision and accuracy (n = 3 days). Carryover was tested by injecting solvent or double blank (plasma extract) after the upper limit of quantification (ULOQ).
Evaluation of matrix effect followed the approach published by Matuszewski, et al. [17]. Three sets of validation samples at low, medium and high concentration were prepared. Set 1 samples were prepared by spiking both PQ (at 0.6, 4, and 40 ng/ml, respectively) and IS (0.2 ng/ml) in 5% TCA in MeOH-water (1:1, v/v), corresponding to the final concentrations of PQ and IS after protein precipitation (fivefold dilution). Set 2 samples were prepared by extracting six lots of blank plasma, then spiking PQ and IS into each extracted matrix at the same concentration as set 1. Set 3 samples were prepared by spiking PQ at 3, 20 and 200 ng/ml in six lots of plasma and extracting the samples as described in the sample preparation section. The data from set 1 and set 2 were used to define overall system and detector performance, absolute and relative matrix effects, results from set 3 defined recovery and overall process efficiency.
The stability of PQ in plasma was evaluated at room temperature (21–24°C), −70°C and after three freeze-thaw cycles by comparing with freshly spiked and processed QC samples. The processed samples in the autosampler vials were also tested for 4-day stability by comparing with the values determined immediately after processing. Each condition was tested with low and high QC samples in three or four replicates. The stability of stock solution was tested for 18 months at −70°C and 6 days at room temperature (21–24 °C) in MeCN-water (1:1, v/v) with 0.5% FA by comparing with the peak area from a freshly prepared stock solution. The stability of IS was tested at room temperature (21–24°C) overnight (17 h) in MeOH-water (1:1, v/v) with 5% TCA.
Sample dilution was evaluated with an extra-high QC sample at 1000 ng/ml undergoing four, eight, and 12-fold dilution with blank plasma. The diluted samples were analyzed in triplicate. The percent deviation of the measured concentration from nominal value was expected to be within ±15% and the precision within ≤15%. Potential concomitant drug intereference was tested by spiking lumefantrine, lopinavir, efavirenz, zidovudine, lamivudine, saquinavir at 5000 ng/ml, ritonavir, nelfinavir, indinavir, amprenavir, artemether and dihydroartemisinin at 500 ng/ml into medium QC samples individually, compared with a medium QC sample spiked with solvent only.
Clinical sample analysis
The method was applied to plasma samples collected previously from young children enrolled in a clinical trial based in Uganda, comparing different chemopreventative regimens, including DP [3]. The study was approved by the institutional review board at University of California, San Francisco, USA. DP (Duo-Cotexin, Holley-Cotec, Beijing, China) was prescribed once daily for three consecutive days each month according to weight-based guidelines to the nearest one-quarter tablet (targeting a total dose of 6.4 and 51.2 mg/kg of DHA and PQ, respectively). Venous blood was collected by phlebotomy into EDTA tubes and centrifuged at 2000 × g for 10 min, and plasma was transferred to cryovials and stored at −80°C. Samples were shipped in dry ice to analytical lab at University of California, San Francisco, CA, USA. Plasma samples were collected each time a study participant was diagnosed with malaria, ranged from 1 to 30 days post dose reported. A total of 184 plasma samples were analyzed.
Results & discussion
LC–MS/MS optimization
PQ is a weak base having multiple pKa values [8]. It is a challenge to achieve a symmetric peak of PQ while maintaining good retention on the column (k≥2) as previously reported [12,18], tailing peaks were initially observed in the commonly used silica-based C8 and C18 columns at pH 4–10. We also tested polystyrene divinylbenzene-based PolymerX RP-1 (50 × 4.0 mm, 5 µm, Phenomenex, Inc.), which again gave a broad and tailing PQ peak. Good retention and peak shape were achieved with a Pursuit PFP column (50 × 2.1 mm, 3 µm, Agilent Technologies, Inc.) using TFA as a modifier in the mobile phase. Of note, a broad and tailing peak for PQ was observed when using a Kinetex PFP column (50 × 2.1 mm, 2.6 µm, Phenomenex, Inc.). Therefore, we chose the Pursuit PFP column for further analytical development.
Using water containing 10 mM NH4FA and 0.14% TFA as mobile phase A and MeCN as mobile phase B, a symmetric sharp peak was obtained. Initially, significant carryover was observed: residual PQ peak height was greater than 2000 cps. Higher NH4FA concentration led to a longer retention time, and higher TFA concentration reduced peak tailing. After adding 0.1% TFA in MeCN as mobile phase B and increasing initial B% from 10 to 20%, carryover was reduced but still present at a significant level, most likely from autosampler. This was confirmed by analyzing a ULOQ sample with a reprogrammed LC gradient: two identical additional gradient elution segments were added which followed the first injection analysis so that the column was eluted twice without injection. No residual PQ peak was observed in the following two periods of elution. To resolve this, different needle wash solvents including 0.1–1% FA or TCA or TFA or NH4OH in MeOH-water or MeCN-water, and 2% DMSO in isopropanol-water (1:1, v/v) were tested. Ultimately, the carryover peak was reduced to 600 cps at 0.04–0.08% of ULOQ (250 ng/ml), using MeCN-water (80:20, v/v) containing 0.3% TFA and 20 mM NH4FA as the needle wash solvent. Notably, the carryover was injection volume dependent and no residual PQ peak was observed when injecting air from an empty vial, suggesting carryover might be associated with the injection solvent 5% TCA in MeOH-water (1:1, v/v). The final optimized LC conditions are as follows. Chromatographic separation was achieved on a Pursuit PFP column (2.1 × 50 mm, 3 µm) equipped with a precolumn filter (MAC-MOD, Inc., PA, USA). The mobile phase solvent A was 20 mM NH4FA, 0.14% TFA, pH 2.96; solvent B was 0.1% TFA in MeCN; needle wash solvent was MeCN-water (80:20, v/v) containing 0.3% TFA and 20 mM NH4FA. PQ was eluted at a flow rate of 0.5 ml/min in a gradient program comprising 20% solvent B (0–0.1 min), 20–80% B (0.1–1.5 min), 80% B (1.5–2.0 min), 80–20% B (2.0–2.01 min) and 20% B (2.01–3.0 min). The divert valve was set to direct LC eluent to the MS source at 0.7 min and to the waste line at 2.4 min. Under these conditions, the retention times for PQ and the IS (PQ-d6) were 1.09 and 1.08 min, respectively. Dead volume may be estimated with the fomula: V0 = 0.5 × d2 × L, where d and L are column diameter and length, respectively. The estimated dead volume for the PFP column (50 × 2.1 mm) was 0.11 ml (0.5 × 0.212 × 5 = 0.11) and the dead time was 0.22 min (0.11/0.5). Thus the k value for PQ was estimated to be (1.09–0.22)/0.22 = 4.0.
Two different ionization methods were tested. APCI+ was threefold more sensitive than ESI+ in the select mobile phase and was less sensitive to matrix effect. Therefore, APCI+ was selected as the ion source. Multiple reaction monitoring mode with ion pair m/z 535/288 for PQ and m/z 541/294 for the IS (PQ-d6) was selected for quantification. Ion pair m/z 535/260 for PQ was used for confirmation. The optimized compound-dependent MS parameters were as follows. Declustering potential, entrance potential and the dwell time were 150 v, 10 v and 80 ms, respectively for all ion pairs. For both PQ m/z 535/288 and the IS m/z 541/294, collision energy and collision cell exit potential were 47 and 22 v, respectively; for PQ ion pair m/z 535/260, collision energy and collision cell exit potential were 53 and 24 v, respectively. The optimized instrument-dependent parameters were as follows. The turbo heater was set at 400°C; curtain gas, 30 psi; nebulizer gas (gas 1), 55 psi; collision-activated dissociation gas: 11 psi; nebulizer current, 4.0 v.
Sample preparation
Protein precipitation was selected for its simplicity. Various protein precipitation agents were tested. Bad peak shape was observed when adding 100 µl 10% ZnSO4–MeOH (1:1, v/v) into 25 µl samples. Aqueous 20% TCA gave low recovery (14%). High recovery was obtained with pure MeCN (plasma/solvent at 1:4 ratio, v/v), but the peak was fronting if the sample was not diluted with water. Pure MeOH gave a decent peak with 73% recovery, but an interfering peak appeared afterward (0.2–0.3 min apart); a similar interfering peak was observed when using 10% TCA in MeCN– water (1:1, v/v, and 1:3, v/v) and 10% TFA in MeOH– water (1:1, v/v). This interfering peak was reduced significantly after using 10% TCA in MeOH–water (1:1, v/v). If the supernatant from protein precipitation was passed through a Captiva™ NDlipid plate to remove phospholipids, the recovery of PQ was less than 10%. Finally, the plasma sample (25 µl) was precipitated with 100 µl MeOH–water (1:1, v/v) containing 5% TCA and 0.25 ng/ml PQ-d6 as the IS. The processed sample (supernatant) was transferred into a plastic sample vial (250 µl capacity) and 10 µl were injected for LC–MS/MS analysis. Notably, low IS concentration was used in this assay to avoid carryover, at the select concentration, the IS S/N was 58.
Method validation
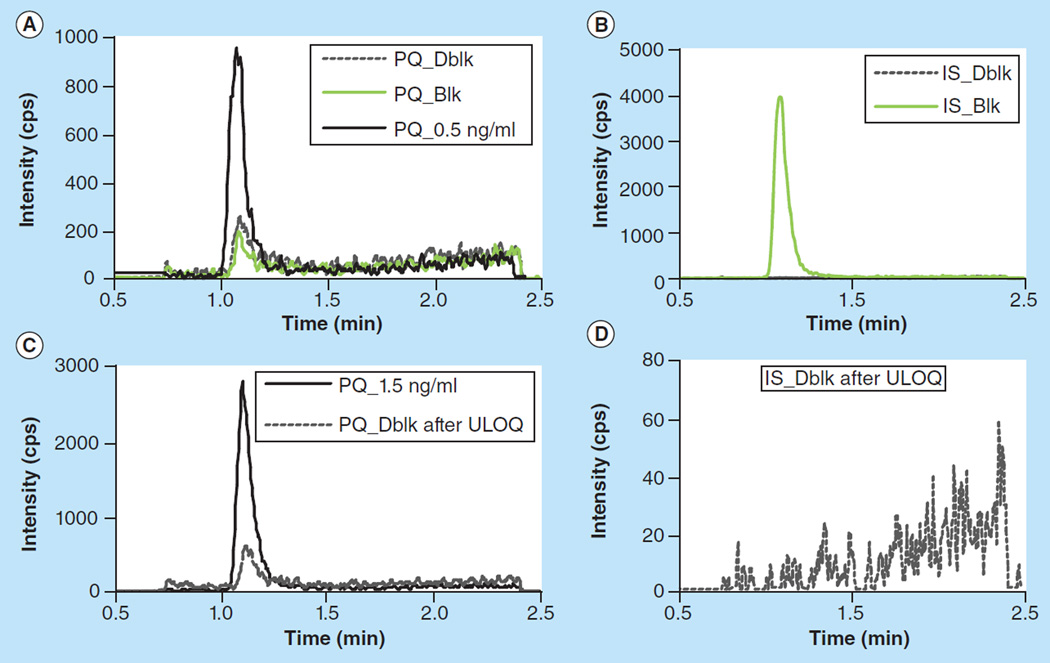
Based on published studies [8,19,20], the clinical PQ concentration range is expected to be 0–250 ng/ml. We aimed to develop an assay with the target calibration range of 0.5–250 ng/ml. With the optimized LC–MS/MS conditions and sample preparation, the signal intensity for a 0.5 ng/ml plasma sample was 2200 cps (peak height) and the S/N was 21. However, using the optimized conditions, carryover following the upper limit of quantification (ULOQ) remained at a significant level (peak height was 700 cps) and a residual PQ peak (300–400 cps) was constantly observed for multiple blank injections after the first blank injection. To meet the criteria that blank signal should be less than 20% LLOQ signal, the LLOQ for this assay was set at 1.5 ng/ml, and the calibration range was 1.5–250 ng/ml. Based on previous studies, 1.68 ng/ml was detected from a patient 63 days after standard dose [8], and PQ trough concentrations less than 31 ng/ml were associated with treatment failure [19], thus patients who take PQ regularly and monthly are expected to have trough concentrations exceeding 1.5 ng/ml, and the LLOQ of 1.5 ng/ml is more than adequate to detect concentrations clinically relevant. The calibration curve was fitted with least square linear regression weighted by 1/x. The correlation coefficient (r) was typically greater than 0.9990 (Supplementary Table S1). Representative MRM ion chromatograms of blank plasma extract and of blank plasma extract with IS and PQ at LLOQ levels are shown in Figure 2.
Figure 2. Chromatograms of blank plasma and plasma spiked with PQ at 0.5 ng/mL and 1.5 ng/mL.
Elevated residual PQ peak was observed in Dblk after ULOQ, indicating carryover in the assay. No carryover was found for the IS.
Blk: blank plasma processed with IS; Dblk: blank plasma processed without IS; IS: Internal standard; PQ: Piperaquine; ULOQ: Upper limit of quantification.
The intraday precision (n = 6) over 3 days ranged from 2.35 to 6.18 at the three concentrations (3, 20 and 200 ng/ml), and interday precisions ranged from 3.3 to 5.6, all of them within 15%. The intra- and interday accuracy ranged from 2.08 to 12.0 and 4.4 to 8.2, respectively. At the LLOQ 1.5 ng/ml level, the precision and accuracy met the criteria of less than 20% (Table 1).
Table 1.
Intra- and inter-day precision and accuracy based on nominal values (ng/ml).
| Intraday |
Interday |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1.50 | 3.00 | 20.0 | 200 | 1.50 | 3.00 | 20.0 | 200 | |
| Mean (ng/ml) | 1.51–1.76 | 3.09–3.34 | 21–22 | 204–214 | 1.63 | 3.25 | 21.6 | 208.7 |
| SD | 0.02–0.05 | 0.08–0.21 | 0.53–0.63 | 5.04–6.79 | 0.11 | 0.18 | 0.8 | 6.9 |
| RSD (%) | 1.48–2.88 | 2.65–6.18 | 2.54–2.80 | 2.35–3.27 | 6.8 | 5.6 | 3.7 | 3.3 |
| Percentage deviation | 0.56–17.0 | 3.00–11.2 | 5.42–12.0 | 2.08–7.08 | 8.6 | 8.2 | 8.1 | 4.4 |
| n | 6 | 6 | 6 | 6 | 18 | 18 | 18 | 18 |
Matrix effect was evaluated based on data from set 1, 2 and 3 (see Supplementary Table S2). Absolute matrix effect was evaluated with mean peak area values from sets 1 and 2. A value of 100% indicated no matrix effect. If the value was greater than 100%, ion enhancement was observed, and if less than 100%, ion suppression was observed. At low, medium and high concentrations, the matrix effect for PQ was 112, 124 and 119%, respectively. However, the IS exhibited the same trend of matrix effect (115, 128 and 122%, respectively). The difference of matrix effect between PQ and the IS was less than 5% and the normalized matrix effect was close to 100% (Table 2). These results indicated that matrix effect was well compensated by the deuterated IS.
Table 2.
Matrix effect, recovery and process efficiency (n = 6).
| Conc (ng/ml) |
PQ peak area, × 104 |
IS peak area, × 104 |
Matrix effect |
Recovery |
PE |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Set1 | Set 2 | Set 3 | Set1 | Set 2 | Set 3 | PQ | IS | IS-normalized | PQ | IS | PQ | IS | |
| Low (3.0) |
4.77 ± 0.25 |
5.34 ± 0.09 |
4.22 ± 0.11 |
1.53 ± 0.11 |
1.76 ± 0.08 |
1.34 ± 0.03 |
112 | 115 | 97.2 | 79.0 | 76.1 | 88.5 | 87.6 |
| Medium (20) |
30.0 ± 1.6 |
37.2 ± 0.9 |
28.4 ± 1.0 |
1.44 ± 0.08 |
1.84± 0.05 |
1.36 ± 0.02 |
124 | 128 | 97.0 | 76.3 | 73.9 | 94.7 | 94.4 |
| High (200) |
327 ± 16 |
389 ± 14 |
305 ± 5 |
1.59 ± 0.09 |
1.94 ± 0.04 |
1.46 ± 0.04 |
119 | 122 | 97.5 | 78.4 | 75.3 | 93.3 | 91.8 |
Conc: Concentration; IS: Internal standard: PE: Process efficiency; PQ: Piperaquine.
Relative matrix effect was evaluated by comparing the CV% from set 1 and 2 (Table 3). The differences between CV% of peak areas from set 1 and 2 were −3.6, −2.8 and −1.3 at low, medium and high concentrations, respectively; the corresponding values for IS were −3.0, −3.0 and −3.4, respectively. When comparing CV% from the peak area ratios, these values were even smaller (−0.4, 0.9 and 3.1, respectively), all within 5%, suggesting that IS compensated for the variation and there was no significant relative matrix effect.
Table 3.
Precision of peak areas and peak area ratio in set 1 and 2 (n = 6).
| Conc (ng/ml) |
PQ peak area CV (%) |
IS peak area CV (%) |
Peak area ratio CV (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Set 1 | Set 2 | Set 2–Set 1 | Set 1 | Set 2 | Set 2–Set 1 | Set 1 | Set 2 | Set 2–Set 1 | |
| Low (3.0) | 5.2 | 1.6 | −3.6 | 7.5 | 4.5 | −3.0 | 4.3 | 3.9 | −0.4 |
| Medium (20) | 5.3 | 2.5 | −2.8 | 5.9 | 2.9 | −3.0 | 3.2 | 4.1 | 0.9 |
| High (200) | 4.8 | 3.5 | −1.3 | 5.5 | 2.2 | −3.4 | 1.1 | 4.1 | 3.1 |
Set 1 samples prepared in 5% trichloroacetic acid in MeOH-water(1:1, v/v), set 2 samples prepared in six different lots of plasma.
Conc: Concentration; CV: Coefficient of variation; IS: Internal standard; PQ: Piperaquine.
Furthermore, slopes of lines connecting low, medium and high QC samples from each lot of plasma were calculated. The CV% from set 3 was 3.50% (<5%), confirming the absence of significant matrix effect on quantification (Table 4).
Table 4.
Slopes of PQ standard lines plotted through low, medium and high QC from each matrix in set 1–3.
| Matrix number | Slope |
||
|---|---|---|---|
| Set 1 | Set 2 | Set 3 | |
| 1 | 1.019 | 0.983 | 1.022 |
| 2 | 1.012 | 0.957 | 1.078 |
| 3 | 1.027 | 0.985 | 1.035 |
| 4 | 1.037 | 0.994 | 1.067 |
| 5 | 1.013 | 1.020 | 0.993 |
| 6 | 1.039 | 1.084 | 1.088 |
| Mean | 1.024 | 1.004 | 1.047 |
| SD | 0.012 | 0.044 | 0.037 |
| Percentage CV | 1.16 | 4.39 | 3.50 |
The recovery of PQ at 3, 20 and 200 ng/ml was 79.0, 76.3 and 78.4%, respectively (Table 2). The mean recovery for the IS was 75.1%.
PQ was stable in both plasma and MeCN–water solution. No degradation was found for plasma samples at room temperature (21–24°C) for 9 days, at −70°C for 8 months and after three freeze-thaw cycles. The processed samples were also stable in autosampler vials for at least 4 days. PQ stock solution in MeCN-water (1:1, v/v) containing 0.5% FA was stable for at least 18 months at −70°C and 6 days at room temperature. The IS (PQ-d6) working solution in MeOH–water (1:1, v/v) containing 5% TCA was stable for at least 17 h at room temperature. In all tested conditions, less than 15% deviation from the control was found (Supplementary Table S3). Further investigation is ongoing to define long-term stability at −70°C.
Sample dilution was validated with an extra-high QC sample at 1000 ng/ml. The precision of the four-, eight- and 12-fold diluted samples was 1.9, 9.7 and 0.9%, respectively, and the percent deviation from nominal value was −7.6, −1.9 and 0.3%, respectively.
To test interference of potential concomitant drugs, lumefantrine, artemether and dihydroartemisinin, lopinavir, efavirenz, zidovudine, lamivudine, saquinavir, ritonavir, nelfinavir, indinavir and amprenavir were separately spiked into medium QC samples, analyzed in triplicate, and compared with a control medium QC that spiked with equal volume of solvent. No interference was found from these drugs (Supplementary Table S4).
The assay was applied to 184 clinical samples, among which 76 samples were below the LLOQ (1.5 ng/ml), attributed to nonadherence in some children [3]. These were provided only to confirm the utility of the method. Interpretation of these clinical specimen levels will be published separately. PQ trough concentrations less than 31 ng/ml were associated with treatment failure [19]. As all these samples were collected from children who developed malaria, the PQ concentrations are expected to be low. For samples greater than LLOQ, the median PQ concentration (interquartile range) was 6.22 (3.47 to 9.98) ng/ml. All samples were below ULOQ. During the analysis, 29 QC samples at three concentration levels were analyzed along with the samples. The precision (relative SD) of the QC samples was 6.83% at 3.0 ng/ml (n = 9), 4.76% at 20 ng/ml (n = 10) and 3.77% at 200 ng/ml (n = 10). All QCs were within the acceptable accuracy range (±15%), and the mean accuracy was 2.26, 4.45 and 9.50% for low, medium and high QC samples, respectively.
Two considerable challenges for quantification of PQ are peak tailing and carryover. These challenges increase for LC–MS/MS-based methods because mobile phases are limited to volatile solvents and additives. Several LC–MS/MS methods have been published [8,12,13,18]. All these methods used C18 columns. Peak tailing was obvious in two of the methods [8,18]. A third method had good peak shape but a low retention factor (k<1) [12], resulting in poor separation, which may introduce variation due to matrix effect. Here we used a high concentration of TFA in the mobile phase solvents to improve peak shape and a PFP column to achieve good retention of PQ. Noticeably, 0.5% FA was used to acidify PQ in stock solution to facilitate dissolution, while TCA was added in MeOH–water (1:1, v/v) for its protein precipitation and ion pairing effects. To minimize matrix effect from plasma samples and TFA, APCI+ was used in this assay. Although ion enhancement matrix effect was still observed, the deuterated IS (PQ-d6) compensated for the matrix effect effectively.
PQ is very sticky and adsorbs to glass. The signal intensity of PQ dropped 79% after overnight storage in a glass sample vial. Therefore, glass tubes and vials should be avoided during analysis. However, no significant effect of a glass flask on PQ concentration was observed during preparation of plasma standard and QC samples, probably because 97% of PQ was bound to plasma proteins [10]. Carryover was observed in previous assays [12,13,18]. Carryover in those assays might be from both the column (due to a tailing peak) and the autosampler (due to absorption). Since we did not have a tailing peak issue, carryover from the column was minimized in our assay. Hodel et al. reported the carryover was 0.2% of ULOQ (4000 ng/ml), corresponding to 8 ng/ml, fourfold higher than LLOQ (2 ng/ml) and three consecutive blank injections were applied following the higher calibrator [13]. This presented a challenge during unknown sample analysis. Samples should be analyzed from low-to-high concentration based on expectation; otherwise, re-analysis should be performed for samples following a high-level unknown sample. The carryover in our method was 0.04–0.08% of ULOQ (250 ng/ml), corresponding to 0.2 ng/ml PQ, an LLOQ at 0.5 ng/ml could still be used if the study samples were analyzed in a low-to-high concentration order [21]. To be conservative, the LLOQ in our assay was increased to 1.5 ng/ml to ensure the carryover peak was ≤20% LLOQ. This method provides the same or better sensitivity but less sample volume than most previously published methods [8–13].
A limitation of using a PFP column is that the performances of PFP columns from different vendors or lots vary. In the early phase of method development, we used a 2.6 µm core-shell PFP column from Phenomenex, Inc., but the peak was too broad and tailing. Therefore, we chose the PFP column from Agilent Technologies, Inc. Noticeably, a second PFP column from Agilent Technologies, Inc. used during method validation gave broader peak shape than the first column, but comparable good peak shape was obtained after multiple days of use. We suggest equilibration of new column with mobile phase overnight.
Conclusion & future perspective
Simple finger or heel pricking followed by blood collection with capillary tubes is a convenient sample collection method for pediatric patients. It will likely become more popular if coupled with a sensitive analytical method requiring less than 50 µl sample volume or dried blood spot. We previously developed a method to measure lumefantrine in small volume capillary plasma and applied it to field studies [22,23]. The method reported here was applied to venous plasma samples collected previously to simply confirm feasibility in plasma. Since sample volumes necessary for the assay are only 25 µl, the method is fully applicable to measuring PQ in capillary plasma as well. However, caution should be taken when comparing PQ concentrations measured from different sample sources. It has been previously reported that PQ concentrations in capillary blood are about 1.7-fold higher than in venous blood in part due to the extensive distribution of PQ [24]. It is therefore likely that PQ concentrations in capillary plasma (peripheral source from finger-prick) are also different from concentrations in venous plasma. In addition, stability of PQ in blood samples collected with capillary tubes may need investigation in the future.
Supplementary Material
Executive summary.
A PFP column is better than C18 column to retain piperaquine in acidic mobile phase, with a retention factor of k = 4.0.
Only 25 µl human plasma sample was used in the assay, allowing for PQ quantitation in capillary plasma samples collected from children in field-based clinical trials.
Matrix effect was minimized by using PQ-d6 as the IS and APCI+ as the ion source.
A simple, fast and sensitive method for determination of piperaquine.
Acknowledgments
This work was supported by grants to the University of California, San Francisco (UCSF); Center for AIDS Research from the NIH (AI027763), R01 (HD068174) and a grant through the Resource Allocation Program (RAP) of the UCSF Clinical and Translational Science Institute.
Key terms
- Carryover
The residual peak of an analyte from a previous injection, measured by injecting a blank sample or solvent following the highest calibrator (ULOQ) injection, and expressed as percentage of peak area in the blank sample compared with the ULOQ
- PFP column
The stationary phase is pentafluorophenyl, giving extra retention for halogenated compounds and can also be used for selective analysis of nonhalogenated polar compounds
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.future-science.com/doi/full/10.4155/BIO.14.254
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.World Health Organization. Guidelines for the Treatment of Malaria. (2nd Edition) 2010 Mar; http://whqlibdoc.who.int. [PubMed]
- 2.Nankabirwa J, Cundill B, Clarke S, et al. Efficacy, safety, and tolerabilky of three regimens for prevention of malaria: a randomized, placebo-controlled trial in Ugandan schoolchildren. PLoS ONE. 2010;5(10):e13438. doi: 10.1371/journal.pone.0013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bigira V, Kapisi J, Clark TD, et al. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med. 2014;11(8):e1001689. doi: 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poespoprodjo JR, Fobia W, Kenangalem E, et al. Dihydroartemisinin-piperaquine treatment of multidrug resistant falciparum and vivax malaria in pregnancy. PLoS ONE. 2014;9(1):e84976. doi: 10.1371/journal.pone.0084976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zani B, Gathu M, Donegan S, Olliaro PL, Sinclair D. Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane database Sys. Rev. 2014;1:CD010927. doi: 10.1002/14651858.CD010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creek D, Bigira V, Arinakwe E, et al. Increased risk of early vomiting among infants and young children treated with dihydroartemisinin-piperaquine compared with artemether-lumefantrine for uncomplicated malaria. Am. J. Trop. Med. Hyg. 2010;83(4):873–875. doi: 10.4269/ajtmh.2010.10-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. Piperaquine: A resurgent antimalarial drug. Drugs. 2005;65(1):75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- 8. Lindegardh N, Annerberg A, White NJ, Day NP. Development and validation of a liquid chromatographic-tandem mass spectrometric method for determination of piperaquine in plasma stable isotope labeled internal standard does not always compensate for matrix effects. J. Chromatog. B Analyt. Technol. Biomed. Life Sci. 2008;862(1–2):227–236. doi: 10.1016/j.jchromb.2007.12.011.. •• A sensitive LC–MS/MS method (LLOQ = 1.5 ng/ml, 50 ul plasma) but complex SPE method.
- 9. Lindegardh N, Ashton M, Bergqvist Y. Automated solid-phase extraction method for the determination of piperaquine in plasma by peak compression liquid chromatography. J. Chromatog. Sci. 2003;41(1):44–49. doi: 10.1093/chromsci/41.1.44.. • Trichloroacetic acid was used to improve peak shape and retention time.
- 10. Hung TY, Davis TM, Ilett KF. Measurement of piperaquine in plasma by liquid chromatography with ultraviolet absorbance detection. J. Chromatog. B Analyt. Technol. Biomed. Life Set. 2003;791(1–2):93–101. doi: 10.1016/s1570-0232(03)00209-5.. • Reported high protein binding of piperaquine (PQ) in plasma.
- 11.Lindegardh N, White NJ, Day NP. High throughput assay for the determination of piperaquine in plasma. J. Pharm. Biomed. Anal. 2005;39(3–4):601–605. doi: 10.1016/j.jpba.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 12. Singhal P, Gaur A, Gautam A, Varshney B, Paliwal J, Batra V. Sensitive and rapid liquid chromatography/tandem mass spectrometric assay for the quantification of piperaquine in human plasma. J. Chromatog. B Analyt. Technol. Biomed. Life Sci. 2007;859(1):24–29. doi: 10.1016/j.jchromb.2007.09.021.. •• A sensitive LC–MS/MS method with a small retention factor, vulnerable to matrix effect.
- 13. Hodel EM, Zanolari B, Mercier T, et al. A single LC-tandem mass spectrometry method for the simultaneous determination of 14 antimalarial drugs and their metabolites in human plasma. J. Chromatog. B Analyt. Technol. Biomed. Life Sci. 2009;877(10):867–886. doi: 10.1016/j.jchromb.2009.02.006.. • Carry-over of PQ was reported.
- 14.Remane D, Wissenbach DK, Meyer MR, Maurer HH. Systematic investigation of ion suppression and enhancement effects of fourteen stable-isotope-labeled internal standards by their native analogues using atmospheric-pressure chemical ionization and electrospray ionization and the relevance for multi-analyte liquid chromatographic/mass spectrometric procedures. Rapid Commun. Mass Spectrom. 2010;24(7):859–867. doi: 10.1002/rcm.4459. [DOI] [PubMed] [Google Scholar]
- 15.Clinical Pharmacology Quality Assurance (CPQA) Program: CPQA guidelines for chromatographic method development and validation based on (and including) FDA guidelines dated May 2001. Version 4.0. (2012) www.fstrf.org.
- 16.Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. Washington, DC, USA: US Department of Health and Human Services; 2001. www.fda.gov/downloads. [Google Scholar]
- 17. Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HVLC–MS/MS. Anal. Chem. 2003;75(13):3019–3030. doi: 10.1021/ac020361s.. • Comprehensive method evaluating matrix effect.
- 18.Lee TM, Huang L, Johnson MK, et al. In vitro metabolism of piperaquine is primarily mediated by CYP3A4. Xenohiotica. 2012;42(11):1088–1095. doi: 10.3109/00498254.2012.693972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lwin KM, Phyo AP, Taming J, et al. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin-piperaquine chemoprevention in adults at high risk of malaria. Antimicrob. Agents Chemother. 2012;56(3):1571–1577. doi: 10.1128/AAC.05877-11.. • Define minimium PQ concentration for prevention of malaria.
- 20.Creek DJ, Bigira V, Mccormack S, et al. Pharmacokinetic predictors for recurrent malaria after dihydroartemisinin-piperaquine treatment of uncomplicated malaria in Ugandan infants. J. Infect. Dis. 2013;207(11):1646–1654. doi: 10.1093/infdis/jit078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. European Medicine Agency. Guideline on Bioanalytical Method Validation. London, UK: Committee for Human Medicinal Products; 2011. . • Provided guidance for carryover.
- 22.Huang L, Li X, Marzan F, Lizak PS, Aweeka FT. Determination of lumefantrine in small-volume human plasma by LC-MS/MS: using a deuterated lumefantrine to overcome matrix effect and ionization saturation. Bioanalysis. 2012;4(2):157–166. doi: 10.4155/bio.11.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achan J, Kakuru A, Ikilezi G, et al. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N. Engl. J. Med. 2012;367(22):2110–2118. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashley EA, Stepniewska K, Lindegardh N, et al. Comparison of plasma, venous and capillary blood levels of piperaquine in patients with uncomplicated falciparum malaria. Eur. J. Clin. Pharmacol. 2010;66(7):705–712. doi: 10.1007/s00228-010-0804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.