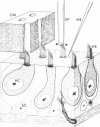
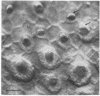
Abstract
Hair cells, the primary receptors of the auditory, vestibular, and lateral-line sensory systems, produce electrical signals in response to mechanical stimulation of their apical hair bundles. We employed an in vitro preparation and intracellular recording to investigate the transduction mechanism of hair cells in the sacculus from the inner ear of the bullfrog (Rana catesbeiana). When stimulated directly by mechanical deflection of their hair bundles, these cells gave graded responses up to 15 mV in amplitude; the peak sensitivity was about 20 mV/micron deflection. The depolarizing component of the receptor potential corresponding to stimuli directed towards the kinocilium. Depolarizing responses were associated with a membrane resistance decrease, and hyperpolarizing responses with a resistance increase. Action potentials, possibly calcium spikes, were occasionally evoked in hair cells by mechanical or electrical stimulation.
Full text
PDF
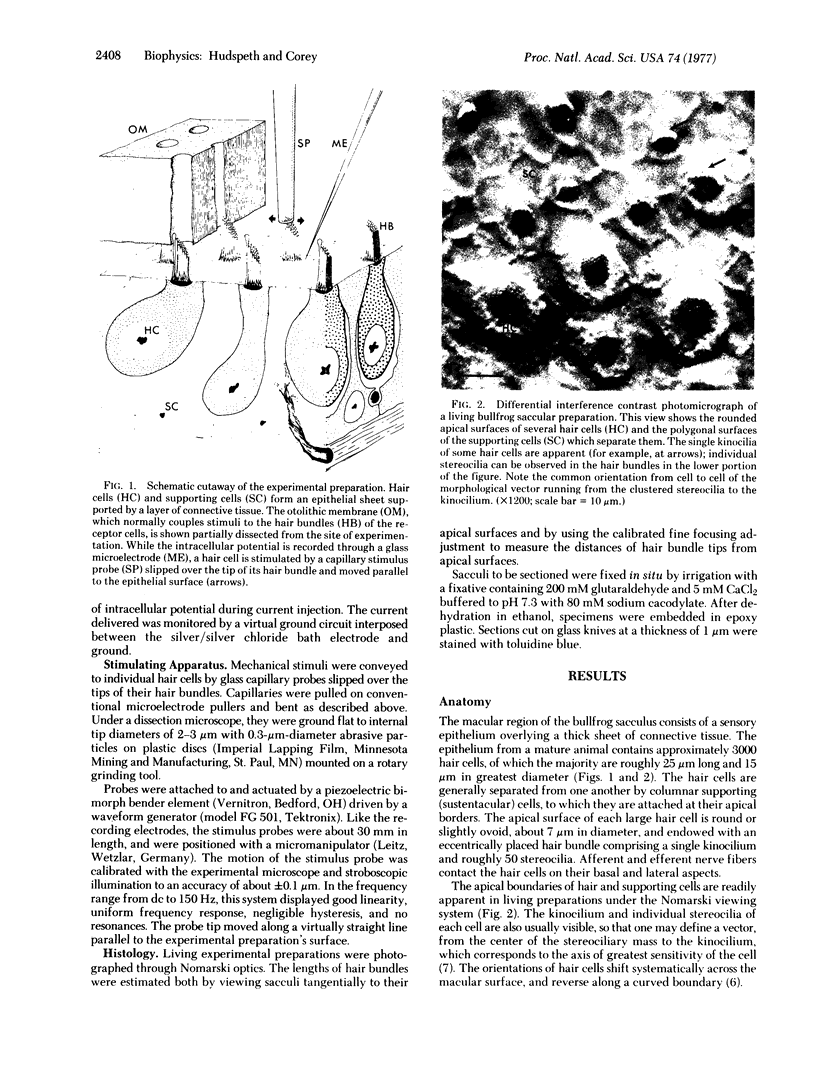
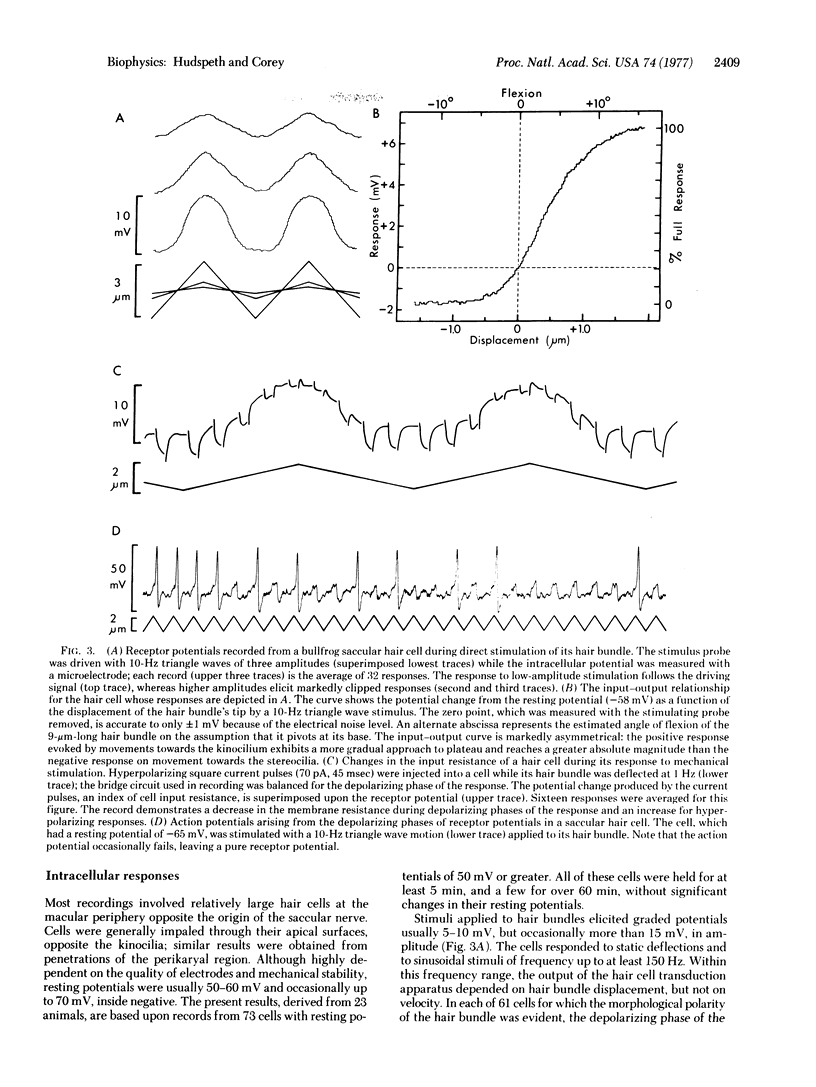


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L. Responses of hair cells to statocyst rotation. J Gen Physiol. 1975 Oct;66(4):507–530. doi: 10.1085/jgp.66.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. A model for transducer action in the cochlea. Cold Spring Harb Symp Quant Biol. 1965;30:181–190. doi: 10.1101/sqb.1965.030.01.020. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Fuortes M. G. Responses of hair cells in the statocyst of Hermissenda. J Physiol. 1975 Sep;251(1):107–129. doi: 10.1113/jphysiol.1975.sp011083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Granda A. M., Maxwell J. M. Voltage signal of photoreceptors at visual threshold. Nature. 1977 Jan 13;265(5590):181–183. doi: 10.1038/265181a0. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Ishii Y., Matsuura S. An analysis of microphonic potentials of the sacculus of goldfish. Jpn J Physiol. 1972 Dec;22(6):603–616. doi: 10.2170/jjphysiol.22.603. [DOI] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Harris G. G., Frishkopf L. S., Flock A. Receptor potentials from hair cells of the lateral line. Science. 1970 Jan 2;167(3914):76–79. doi: 10.1126/science.167.3914.76. [DOI] [PubMed] [Google Scholar]
- Hillman D. E., Lewis E. R. Morphological basis for a mechanical linkage in otolithic receptor transduction in the frog. Science. 1971 Oct 22;174(4007):416–419. doi: 10.1126/science.174.4007.416. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oman C. M., Young L. R. The physiological range of pressure difference and cupula deflections in the human semicircular canal. Theoretical considerations. Acta Otolaryngol. 1972 Nov;74(5):324–331. doi: 10.3109/00016487209128458. [DOI] [PubMed] [Google Scholar]
- Rhode W. S., Robles L. Evidence from Mössbauer experiments for nonlinear vibration in the cochlea. J Acoust Soc Am. 1974 Mar;55(3):588–596. doi: 10.1121/1.1914569. [DOI] [PubMed] [Google Scholar]
- TERZUOLO C. A., WASHIZU Y. Relation between stimulus strength, generator potential and impulse frequency in stretch receptor of Crustacea. J Neurophysiol. 1962 Jan;25:56–66. doi: 10.1152/jn.1962.25.1.56. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- WERSAELL J., FLOCK A. SUPPRESSION AND RESTORATION OF THE MICROPHONIC OUTPUT FROM THE LATERAL LINE ORGAN AFTER LOCAL APPLICATION OF STREPTOMYCIN. Life Sci. 1964 Oct;3:1151–1155. doi: 10.1016/0024-3205(64)90132-8. [DOI] [PubMed] [Google Scholar]
- Weiss T. F., Mulroy M. J., Altmann D. W. Intracellular responses to acoustic clicks in the inner ear of the alligator lizard. J Acoust Soc Am. 1974 Mar;55(3):606–619. doi: 10.1121/1.1914571. [DOI] [PubMed] [Google Scholar]