Abstract
Background
Both the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR) are expressed in adipose tissue and assumed to mediate cortisol actions on adipose tissue. The relative significance of the two receptors in mediating glucocorticoid regulation of adipogenesis and adipokine expression in human adipocytes has not been addressed.
Methods
We investigated the differential roles of the GR and MR in mediating glucocorticoid actions on adipogenesis and adipokine production using RNA interference in primary cultures of human preadipocytes and adipocytes.
RESULTS
Both types of receptors are expressed, but levels of GR were several hundred fold higher than MR in both human preadipocytes and adipocytes. As expected, cortisol added during adipogenesis increased the differentiation of human preadipocytes. Silencing of GR, but not MR, blocked these proadipogenic actions of cortisol. In differentiated human adipocytes, addition of cortisol increased leptin and adiponectin, while suppressing IL-6, mRNA levels and protein secretion. Knockdown of GR by 65% decreased leptin and adiponectin while increasing IL-6 production. In addition, GR silencing blocked the effects of cortisol on adipokine expression. In contrast, although MR knockdown increased leptin, it did not affect adiponectin and IL-6 expression.
Conclusion
Our data demonstrate that although both GR and MR have roles in regulating leptin expression, GR plays more important roles in mediating the actions of cortisol to regulate adipogenesis and adipokine production in human adipocytes.
Keywords: cortisol, glucocorticoid receptor, mineralocorticoid receptor, adipogenesis, adipokine
INTRODUCTION
Glucocorticoids (GCs) affect almost every aspect of adipose tissue biology. They are required for the full differentiation of adipose precursors and for the maintenance of key genes in glucose and lipid metabolism in cultured adipocytes and adipose tissue (1-5). As expected from their well known anti-inflammatory actions, GCs decrease the expression of inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα) that are mainly expressed in non-adipocyte fraction in human adipose tissue (6;7). In contrast, GCs increase the expression of adipokines including leptin and adiponectin as well as acute phase reactant proteins that are mainly expressed in adipocytes (2). Although the powerful actions of GCs on adipose tissue biology are well documented, the molecular events and mechanisms through which GCs regulate adipose tissue development and function are not fully elucidated.
The action of GCs on target cells is thought to be mediated by the type 2 glucocorticoid receptor (GR, NR3C1), a member of nuclear receptor superfamily that is expressed in almost every tissue, including adipose tissue. The type 1 glucocorticoid receptor, the mineralocorticoid receptor (MR, NR3C2), is also expressed in human adipose tissue and has been suggested to mediate GC actions (8). MR has been shown to be expressed in 3T3-L1 preadipocytes at 30-50 times lower levels than GR and its expression levels increase with differentiation (9). In addition, it has been shown that MR plays a more important role than GR in the regulation of adipogenesis in 3T3-L1 preadipocytes and adipogenic precursors isolated from brown adipose tissue (9;10). The relative expression levels of MR and GR in human preadipocytes and adipocytes and whether the well-known proadipogenic effects of GCs in human preadipocytes (11-13) is mediated through GR or MR has not been addressed. In addition, although a previous study suggests that MR also mediates cortisol regulation of adipokine production in 3T3-L1 adipocytes (8), it is not clear whether GC activation of MR pathway significantly contributes to the cortisol regulation of human adipocyte function.
In the current study, we measured the expression levels of GR and MR in primary cultures of human preadipocytes and adipocytes and then used an RNAi-mediated knockdown approach to compare the relative contribution of GR and MR to cortisol actions on adipocyte differentiation and adipokine production. Our data demonstrate GR rather than MR plays more important roles in GC stimulation of adipogenesis. In addition, we demonstrated that GC regulation of leptin, adiponectin and IL-6 expression in human adipocytes is also mediated through GR. Overall, our data suggest that GR plays a more important role than MR in human adipose biology.
METHODS
Materials
All chemicals, dexamethasone and hydrocortisone (cortisol) were purchased from Sigma (St. Louis, MO), except Rosiglitazone (Enzo, Farmingdale, NY) and recombinant human insulin (Lilly, Indianapolis, IN). Collagenase type I was purchased from Worthington Biochemical (Lakewood, NJ). Cell culture media and fetal bovine serum (FBS) were obtained from Life Technologies (Carlsbard, CA). GR, MR and control siRNA were purchased from Qiagen and transfection reagents were purchased from Qiagen (HiPerFect, Germantown, MD) and Life Technologies (Lipofectamine and PLUS reagents, Carlsbard, CA).
Isolation and culture of adipose stromal vascular cells (SVC)
Abdominal sc adipose tissues were obtained from 6 subjects (mean age 45.6±4.1 years, current BMI 33.5±3.9 kg/m2, 4 female, 2 male) during elective surgery. All subjects were free of diabetes, endocrine, or inflammatory diseases by medical record, and weight stable for at least 1 month prior to surgery. All subjects provided signed informed consent and the protocol was approved by Institutional Review Board of Boston University Medical Center.
Adipose stromal cells, often called preadipocytes, were obtained with collagenase digestion as previously described (13;14). Cells from total 6 individual subjects, subcultured 5 to 6 times, were used without pooling. Experiments were repeated at least 4 times using cells derived from different subjects, as indicated in figure legends.
Knockdown of GR and MR in preadipocytes and differentiation in the absence or presence of cortisol
In the morning of transfection, preadipocytes were trypsinized and replated at 15,000 cells/cm2. In the late afternoon, cells were transfected with control, GR or MR siRNA (10 nM) using HiPerfect (Qiagen). siRNA was diluted in serum-free α-MEM, mixed with HiPerfect reagents and incubated for 15 min at room temperature. After refeeding cells with the growth media, the siRNA-HiPefect mix was added to each well for overnight transfection. Cells were refed on the following day and allowed to grow. 4~5 days after transfection, knockdown efficiency was confirmed at the mRNA and protein levels. At the post-confluent stage, cells were induced to differentiate with or without cortisol (200 nM) for 7 days in DMEM/F12 supplemented with 500 μM IBMX, 100 nM insulin, 2 nM T3, 10 μg/ml transferrin, 1 μM rosiglitazone, 33 μM biotin and 17 μM pantothenic acid and then maintained in a maintenance media (DMEM/F12 with 10 nM insulin) with or without cortisol (200 nM) till harvest on day 14.
Knockdown of GR and MR in differentiated human adipocytes and cortisol treatment
Cells were plated and differentiated as previously described (13). On day 9 of differentiation, adipocytes were transfected with siRNA using Lipofectamine and PLUS reagents (Life Technologies, Carlsbard, CA). siRNA (10 nM) was diluted in DMEM/F12, mixed with PLUS reagent and incubated at room temperature for 15 min. Lipofectamine reagents were diluted in DMEM/F12, mixed with the siRNA-PLUS mix, and incubated for additional 15 min. The siRNA-PLUS-Lipofectamine mixture was then added to cells. After overnight transfection, cells were refed with the maintenance media (DMEM/F12 with 10 nM insulin and 10 nM dexamethasone) and maintained for an additional 4 to 5 days with refeeding. GR or MR silenced adipocytes were deprived of dexamethasone overnight and then treated with cortisol (200 nM) for 24 hours in the presence of 10 nM insulin. After cortisol treatment, cells were harvested for RNA and protein analysis. Culture media were collected and saved at -80°C for leptin, adiponectin and IL-6 measurement.
Oil Red O staining
Cells were fixed in 4% paraformaldehyde for 15 min and stained with Oil Red O solution for 1 h at room temperature as previously described (13). Representative images were acquired with a Nikon TE 200 microscope (Tokyo, Japan) equipped with an Olympus DP72 camera.
Measurement of leptin, adiponectin and IL-6
Concentrations of leptin, adiponectin and IL-6 in culture media were measured using commercial ELISA Kits (R & D, Minneapolis, MN) following the manufacturer’s protocol. Intra-assay and inter-assay coefficient of variation values were 3±1.5% and 8±3.9% for IL6, 6±2.3% and 8±3.8% for leptin, and 3±0.5% and 8±3.2% for adiponectin.
RNA extraction and gene expression
Total RNA was extracted using Qiazol (Qiagen, Germantown, MD) and quantity and quality were assessed spectrophotometrically. 0.5 to 1 μg total RNA was reverse transcribed using Transcriptor First Strand Synthesis Kits (Roche, Indianapolis, IN). qPCR was performed on Light Cycler 480 II (Roche, Indianapolis, IN) with Taqman probes (Life Technologies, Carlsbard, CA). Cyclophilin A (PPIA) was used as a reference gene.
Immunoblotting
Cells were washed 3 times with ice-cold PBS, scraped into cell lysis buffer (Cell Signaling, Beverly, MA) supplemented with 5% SDS and protease inhibitors and processed as previously described (13). 5 to 10 μg total protein was resolved in 10% NuPAGE gels (Life Technologies, Carlsbard, CA), transferred to PVDF membranes and probed for GR (gift from Dr. Garabedian at NYU), MR (Santa Cruz Biotech, Santa Cruz, CA), perilipin (gift from Dr. A. Greenberg at Tufts University), ATGL (gift from Dr. Gong at University of Maryland), adiponectin (BD Bioscience, San Jose, CA), FABP 4 (gift from Dr. J. Storch at Rutgers University), and loading controls (total ERK from Cell Signaling and HSP 90 from Santa Cruz Biotech). Chemiluminescence images were captured using an Imager (LAS 4000, Fuji Film, Tokyo, Japan) and band intensities were quantified using Multi Gauge software (Fuji Film, Tokyo, Japan).
Statistical analysis
Data are expressed as means ± standard error mean (SEM) and analyzed using GraphPad Prism. A 2-way ANOVA was used to assess the interaction of siRNA treatment, GR or MR, and cortisol treatment. When the main effect or interaction was significant, Student T tests were used to test the cortisol effect within control, GR and MR siRNA conditions. Differences between means were considered statistically different when p values were less than 0.05.
Results
GR and MR were expressed in human preadipocytes and adipocytes
Both GR and MR were expressed in human preadipocytes and adipocytes. GR mRNA levels were ~500-fold and ~250-fold greater than MR in human preadipocytes and adipocytes, respectively (data not shown). MR protein levels did not change, while GR protein levels decreased during differentiation of human preadipocytes (Fig 1A).
Figure 1. Changes in GR and MR protein levels during differentiation of human preadipocytes.
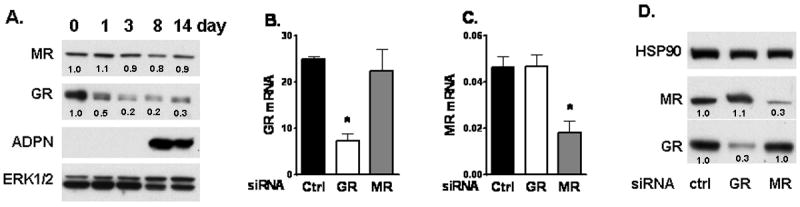
(A) Protein levels of GR and MR on days 0, 1, 3, 8 and 14 of differentiation were measured with immunoblotting. Adiponectin (ADPN) was used a marker of differentiation. Representative blots of three independent experiments using cells from different subjects are shown. Band intensities were quantified and expression levels relative to the loading control, total ERK, are . Validation of knockdown of (B) GR and (C) MR mRNA levels. Data are from cells derived from 5 independent subjects, *, p<0.05 compared to control siRNA. (D) Validation of GR and MR knockdown at the protein level. Band intensities were quantified and expression levels relative to HSP90 (loading control) are .
Knockdown of GR, but not MR, blocked the proadipogenic actions of cortisol on human preadipocyte differentiation
To test whether GR or MR mediated the GC induction of adipogenesis, GR and MR levels were reduced using RNAi. siRNA-mediated gene silencing decreased GR levels by ~70% both at the mRNA and protein levels in primary human preadipocytes (Fig 1B-D). Similarly, MR siRNA decreased MR expression levels by ~60%. The effects of GR and MR siRNA were specific and did not affect each others expression. Knockdown of GR or MR did not significantly affect the proliferation of human preadipocytes (data not shown).
We next tested whether depletion of MR or GR affected the cortisol induction of differentiation. After confirming knockdown of GR or MR in preadipocytes, cells were differentiated in an adipogenic cocktail with or without a GC, cortisol (200 nM). Although siRNA was transfected in preadipocytes, gene silencing effects were maintained throughout differentiation process (data not shown). In the absence of cortisol, human preadipocytes did not differentiate as shown by lack of lipid droplet accumulation and the low expression of adipogenic makers (Fig 2). Cortisol, similar to dexamethasone (13), significantly increased the differentiation degree of human preadipocytes and overall more than 70% differentiation was observed. When GR levels were reduced by 70%, the proadipogenic actions of cortisol were completely blocked. Knockdown of MR however, did not affect differentiation. Combined, these data demonstrate that GC stimulation of adipogenesis in human preadipocytes is mediated through GR rather than MR.
Figure 2. Knockdown of GR in human preadipocytes blocked the proadipogenic actions of cortisol.
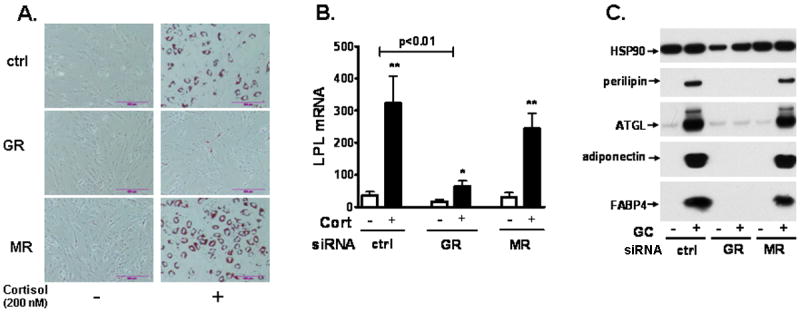
MR or GR silenced human preadipocytes were differentiated with or without cortisol (200 nM) and differentiation degree was determined after 14d of differentiation. (A) Oil Red-O staining. (B) LPL mRNA levels. GR siRNA and cortisol interaction, p<0.01 with 2 way-ANOVA. Cortisol effect within siRNA condition: *, p<0.05, **, p<0.01, n=5. (C) Protein levels of adipogenic markers (perilpin, ATGL, adiponectin, and FABP4).
Knockdown of GR and MR in newly-differentiated human adipocytes
To test whether GC regulation of adipokine expression is mediated through GR or MR, we silenced GR and MR in adipocytes. Differentiated adipocytes were transfected with MR or GR siRNA on day 9 and cultured in the maintenance media. Gene silencing effects were effective after 4 days of transfection and maintained for at least additional 5 days (data not shown). GR or MR silenced adipocytes were deprived of dexamethasone overnight (the maintenance media contains 10 nM dexamethasone and 10 nM insulin), and then treated with cortisol (200 nM) for 24h in the presence of 10 nM insulin. siRNA mediated-knockdown in primary human adipocytes was effective, reducing GR mRNA expression by 65±7% and MR mRNA expression by 60±9% (n=5, p<0.01, Fig 3A and B). Similar levels of knockdown were achieved at the protein expression (Fig 3C).
Figure 3. Knockdown of GR blocked the cortisol-induction of GILZ mRNA in human adipocytes.
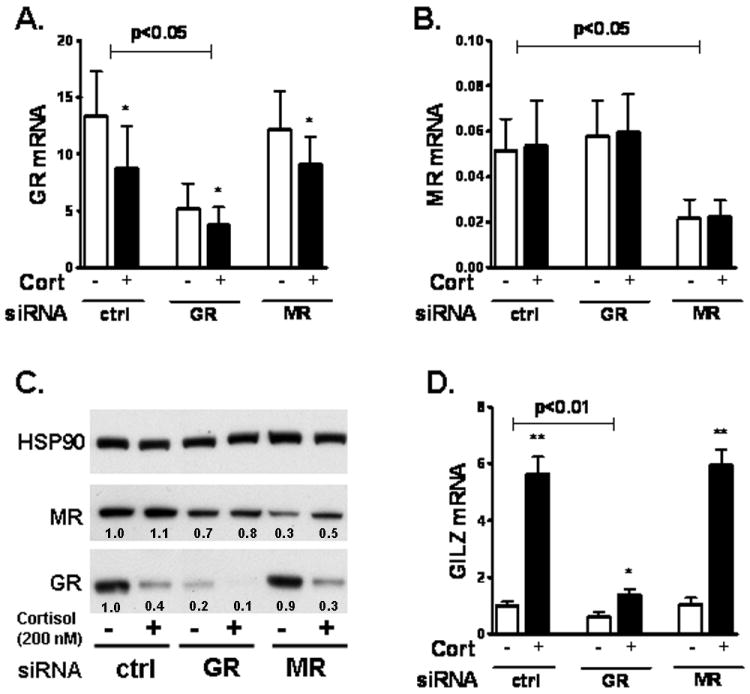
Differentiated adipocytes (d9) were transfected with siRNA and treated for 24h with cortisol (200 nM) on day 14. Knockdown of GR and MR were confirmed on d15. (A) GR mRNA levels. GR siRNA decreased GR mRNA levels (p<0.05 with 2-way ANOVA); effect of cortisol vs. control treatment, *, p<0.05. (B) MR mRNA levels. MR siRNA reduced MR mRNA levels (p<0.05 with 2-way ANOVA). (C) Immunoblotting of GR and MR protein. Band intensities were quantified and expression levels relative to the control gene, HSP90, are . (D) GILZ mRNA expression levels. Interaction of GR siRNA and cortisol with 2 way-ANOVA, p<0.01, n=4. Effect of cortisol vs. control, *, p<0.05, ** p<0.01 by paired T test.
Cortisol significantly decreased GR expression, both at the mRNA (40±9%, p<0.05, n=4) and protein levels (50±10%, p<0.05, n=4), in human adipocytes (Fig 3 A-C). MR expression levels however, were not affected by cortisol. As expected, cortisol significantly induced GILZ mRNA expression, a known primary target of GR (15), by 5.7±0.3 fold in the control group (Fig 3D). Knockdown of GR decreased the basal mRNA expression levels of GILZ by 45±9%. In addition, GR silencing reduced the cortisol-induction of GILZ mRNA (5.7±0.3 fold in control vs. 1.6±0.4 fold in GR siRNA, p=0.01, n=4). MR knockdown did not affect basal or the cortisol-stimulation of GILZ expression.
GR knockdown suppressed while MR knockdown increased leptin expression in human adipocytes
We next tested if the effects of cortisol on two major adipokines, leptin and adiponectin, as well as a proinflammatory cytokine, IL-6, were mediated through GR or MR. Cortisol (200 nM, 24h) increased leptin mRNA by 10±2.4-fold (p<0.01) and secretion by 2.2-fold (p<0.05) in human adipocytes (Fig 4A and B). Although GR knockdown tended to suppress basal leptin mRNA levels by 39±11% (p=0.08), it did not affect basal leptin secretion into the culture media. GR knockdown however, blocked the cortisol-induction of leptin mRNA expression and secretion (p<0.05). In contrast, MR knockdown increased leptin mRNA levels in both basal (by 42±12.8%, p=0.04) and cortisol induced (43±6.7%, p=0.01) conditions.
Figure 4. GR mediated cortisol-regulation of adipokine production in differentiated human adipocytes.
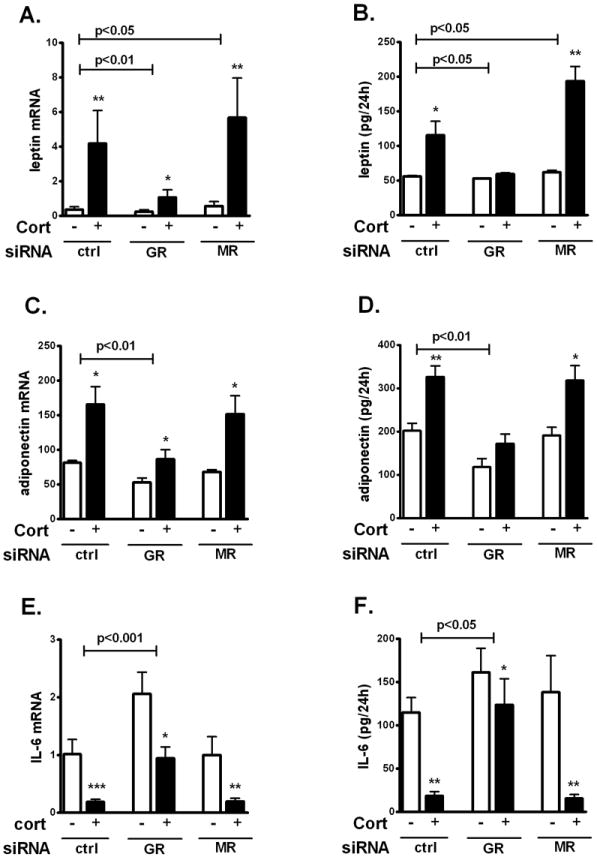
GR or MR silenced human adipocytes were starved for GCs overnight and treated with 200 nM cortisol for 24h. (A) Leptin mRNA. (B) Leptin secretion. (C) Adiponectin mRNA. (D) Adiponection secretion. (E) IL-6 mRNA. (F) IL-6 secretion. Interaction GR or MR siRNA and cortisol by 2 way-ANOVA, p<0.05, p<0.01, p<0.001 for the n=4-5. Effects of cortisol treatment vs. control: *, p<0.05, **, p<0.01 by paired T test.
GR knockdown decreased adiponectin expression levels, no effects of MR silencing
Cortisol, added in the presence of insulin, increased adiponectin mRNA levels by 2.1±0.4 fold (p<0.05) and secretion by 1.7±0.1 fold (p<0.01) in cultured human adipocytes (Fig 4C and D). GR knockdown decreased adiponectin mRNA levels and protein secretion in both basal (by 34±9.4% at the mRNA levels and by 43±5% in secretion) and cortisol-stimulated (by 48±6.0% at the mRNA levels and by 47±6% in protein secretion) conditions. GR silencing also blocked the stimulatory effects of cortisol on adiponectin expression (p<0.01). MR knockdown did not have any significant effects on the adiponectin mRNA levels or secretion.
Cortisol suppression of IL-6 was mediated through GR rather then MR
Cortisol significantly decreased IL-6 mRNA expression by 82±18% (p<0.001) and secretion by 84±37% (p<0.01) in cultured human adipocytes, as expected from its well known anti-inflammatory actions (Fig 4E and F). Knockdown of GR significantly increased IL-6 mRNA expression levels (basal; 73±12.9% and cortisol-repressed condition; 410±41.5%) and IL-6 secretion (basal; 40±7% and cortisol-suppressed condition; 680±202%). Although cortisol suppressed IL-6 expression in both control and GR silenced cells, the magnitude of the effects was much lower when GR was reduced (mRNA levels: 84±3% in the control vs. 26±6% in GR knockdown, p<0.01; IL-6 secretion; 85±3.5% in the control vs. 26±6.3% in GR knockdown, p<0.01). Knockdown of MR did not significantly affect IL-6 expression levels.
DISCUSSION
The type 2 glucocorticoid receptor (GR) has been assumed to mediate the pleiotropic effects of GCs on adipogenesis as well as adipose tissue metabolic and endocrine functions. However, several studies demonstrated that the MR is expressed in human adipose tissue and plays important roles in adipogenesis and adipokine production (8-10;16). When selective agonists for GR (dexamethasone) or MR (aldosterone) were used to test the roles of each receptor in the regulation of adipogenesis and adipokine expression, discrepant results were obtained. Using RNAi-mediated knockdown of GR and MR in primary cultures of human preadipocytes and adipocytes, we show that GR plays a more important role than MR in the cortisol regulation of adipogenesis and adipokine production in human adipocytes.
In contrast to our results in human adipocytes, other studies suggest that MR rather than GR plays more important roles in adipogenesis in mouse adipocytes (9;10;16). Differences in experimental design and cell culture model systems, 3T3-L1 (9;16) and brown adipose tissue stromal cells (10), may have contributed to the contradictory results. For example, Caprio et al reported that aldosterone increased, while dexamethasone decreased, adipocyte maturation when added during a late stage of differentiation in 3T3-L1 cells (9). However, this experimental design did not test the requirement of GC for priming 3T3-L1 preadipocytes for adipogenic programming during the initial 2d of differentiation (17), a time when a transient enrichment of the GR near genes involved in cell proliferation, development and differentiation, including the master regulator of adipogenesis, PPARgamma2 gene, is known to occur (18). In human preadipocytes, we and others find that when GCs, either cortisol or dexamethasone, are present throughout differentiation continuously, they promote differentiation (13;14;19) and are required for maintaining the expression of mature adipocyte genes (3). The fact that the siRNA knockdown by Caprio et al in 3T3-L1 cells was transient (9), while in human adipocytes we obtained a sustained knockdown during differentiation, may also have contributed to the apparent discrepancies. In fact, we found that when GR expression was continuously reduced with lentiviral delivery of GR-shRNA in 3T3-L1 preadipocytes, the dexamethasone induction of adipogenesis was completely blocked (unpublished observation, Lee MJ and Fried SK).
Another factor that may contribute to the reported differences in the requirement for GR in adipogenesis is that GCs are required for cell survival during the clonal expansion that precedes terminal differentiation in 3T3-L1 but not in human preadipocytes (20). While the post-confluent mitosis is closely linked to, and may be required for, adipogenesis in 3T3-L1 (21-23), human primary preadipocytes do not require cell division to enter differentiation (24). Thus, the previously demonstrated anti-adipogenic effects of drospirenone, a potent synthetic anti-mineralocorticoid, in 3T3-L1 preadipocytes might have been due to its effects on cell proliferation (16). In human preadipocytes, knockdown of GR or MR did not significantly affect proliferation.
In vivo, the relative abundance and physiologic importance of MR and GR are far from clear. Circulating levels of cortisone are 100-1000 times higher than aldosterone and 11beta-hydroxysteroid dehydrogenase (HSD) 2 is expressed at much lower levels than HSD1 in human adipose tissue (25-27). It is therefore conceivable that locally activated cortisol within human adipose tissue may activate both GR and MR. Interestingly, we previously found that HSD2 expression was higher in omental than abdominal subcutaneous adipose tissue (27), so depot differences in the fine-tuning of cortisol actions are likely. Although MR is expressed at lower levels than GR, both HSD1 and MR expression levels are increased in adipose tissue of obesity, while the expression of HSD2 and GR are not (8;26;27). Thus, the relative contribution of the MR pathway may be increased in adipose tissue of obesity, resulting in adipokine dysregulation, increased adipose inflammation and reactive oxygen species. Accordingly, the blockade of MR with selective agonists has been shown to improve adipokine expression and insulin resistant in obesity rodent models (28;29).
It is important to note that the activation of GR or MR often have opposite effects in the regulation of adipogenesis and adipokine production. Dexamethasone increases adipokine expression (leptin, adiponectin) while decreasing proinflammatory cytokine (IL-6, TNFα, MCP1) (2;6;7;10;30). In contrast, aldosterone increases the expression of proinflammatory cytokines (PAI-1, MCP1) and inhibits adiponectin expression (10;31;32). Herein, we show that in cultured human adipocytes, cortisol, which assumed to bind to both GR and MR, improves adipokine profile in human adipocytes, similar to dexamethasone. Although reducing MR expression in human adipocytes by ~60% increased leptin expression, it did not affect the cortisol regulation of adipokine expression, whereas GR knockdown blocked the cortisol effects. Our data cast doubt on the importance of MR in mediating cortisol effects on human adipocyte biology.
In summary, our findings suggest that in cultured abdominal subcutaneous human adipocytes, cortisol effects on adipogenesis and adipokine production are mediated through GR. Furthermore, MR activation by aldosterone may play independent roles, e.g. in the suppression of leptin expression. Future studies are needed to explore potential depot differences in the prereceptor metabolism of cortisol and the relative abundance of GR and MR in preadipocytes and adipocytes and whether these are altered in obesity and females compared to males. A deepened understanding of relative contribution of GR vs. MR in adipose biology is important for developing treatments for obesity and its related diseases.
Acknowledgments
This work was supported by NIH DK080448 and P30 DK046200 (to S.K.F.). We thank Dr. Monica Miller for assistance with adipose tissue sampling during surgery.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011 doi: 10.1016/j.metabol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Lee MJ, Gong DW, Burkey BF, Fried SK. Pathways regulated by glucocorticoids in omental and subcutaneous human adipose tissues: a microarray study. Am J Physiol Endocrinol Metab. 2011;300(3):E571–E580. doi: 10.1152/ajpendo.00231.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MJ, Fried SK. Glucocorticoids antagonize tumor necrosis factor-alpha-stimulated lipolysis and resistance to the antilipolytic effect of insulin in human adipocytes. Am J Physiol Endocrinol Metab. 2012;303(9):E1126–E1133. doi: 10.1152/ajpendo.00228.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gathercole LL, Morgan SA, Bujalska IJ, Hauton D, Stewart PM, Tomlinson JW. Regulation of lipogenesis by glucocorticoids and insulin in human adipose tissue. PLoS One. 2011;6(10):e26223. doi: 10.1371/journal.pone.0026223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 7.Bruun JM, Lihn AS, Madan AK, Pedersen SB, Schiott KM, Fain JN, et al. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue Implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab. 2004;286(1):E8–13. doi: 10.1152/ajpendo.00269.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hirata A, Maeda N, Nakatsuji H, Hiuge-Shimizu A, Okada T, Funahashi T, et al. Contribution of glucocorticoid-mineralocorticoid receptor pathway on the obesity-related adipocyte dysfunction. Biochem Biophys Res Commun. 2012;419(2):182–187. doi: 10.1016/j.bbrc.2012.01.139. [DOI] [PubMed] [Google Scholar]
- 9.Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007;21(9):2185–2194. doi: 10.1096/fj.06-7970com. [DOI] [PubMed] [Google Scholar]
- 10.Hoppmann J, Perwitz N, Meier B, Fasshauer M, Hadaschik D, Lehnert H, et al. The balance between gluco- and mineralo-corticoid action critically determines inflammatory adipocyte responses. J Endocrinol. 2010;204(2):153–164. doi: 10.1677/JOE-09-0292. [DOI] [PubMed] [Google Scholar]
- 11.Hauner H, Schmid P, Pfeiffer EF. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J Clin Endocrinol Metab. 1987;64(4):832–835. doi: 10.1210/jcem-64-4-832. [DOI] [PubMed] [Google Scholar]
- 12.Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest. 1989;84(5):1663–1670. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MJ, Wu Y, Fried SK. A modified protocol to maximize differentiation of human preadipocytes and improve metabolic phenotypes. Obesity (Silver Spring) 2012;20(12):2334–2340. doi: 10.1038/oby.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauner H, Skurk T, Wabitsch M. Cultures of human adipose precursor cells. Methods Mol Biol. 2001;155:239–247. doi: 10.1385/1-59259-231-7:239. [DOI] [PubMed] [Google Scholar]
- 15.Wang JC, Derynck MK, Nonaka DF, Khodabakhsh DB, Haqq C, Yamamoto KR. Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc Natl Acad Sci U S A. 2004;101(44):15603–15608. doi: 10.1073/pnas.0407008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caprio M, Antelmi A, Chetrite G, Muscat A, Mammi C, Marzolla V, et al. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: potential implications for the treatment of metabolic syndrome. Endocrinology. 2011;152(1):113–125. doi: 10.1210/en.2010-0674. [DOI] [PubMed] [Google Scholar]
- 17.Pantoja C, Huff JT, Yamamoto KR. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol Biol Cell. 2008;19(10):4032–4041. doi: 10.1091/mbc.E08-04-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steger DJ, Grant GR, Schupp M, Tomaru T, Lefterova MI, Schug J, et al. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev. 2010;24(10):1035–1044. doi: 10.1101/gad.1907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G, Floyd ZE, Wu X, Hebert T, Halvorsen YD, Buehrer BM, et al. Adipogenic differentiation of adipose-derived stem cells. Methods Mol Biol. 2011;702:193–200. doi: 10.1007/978-1-61737-960-4_14. [DOI] [PubMed] [Google Scholar]
- 20.Tomlinson JJ, Boudreau A, Wu D, Atlas E, Hache RJ. Modulation of early human preadipocyte differentiation by glucocorticoids. Endocrinology. 2006;147(11):5284–5293. doi: 10.1210/en.2006-0267. [DOI] [PubMed] [Google Scholar]
- 21.Pairault J, Green H. A study of the adipose conversion of suspended 3T3 cells by using glycerophosphate dehydrogenase as differentiation marker. Proc Natl Acad Sci U S A. 1979;76(10):5138–5142. doi: 10.1073/pnas.76.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuri-Harcuch W, Marsch-Moreno M. DNA synthesis and cell division related to adipose differentiation of 3T3 cells. J Cell Physiol. 1983;114(1):39–44. doi: 10.1002/jcp.1041140107. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt W, Poll-Jordan G, Loffler G. Adipose conversion of 3T3-L1 cells in a serum-free culture system depends on epidermal growth factor, insulin-like growth factor I, corticosterone, and cyclic AMP. J Biol Chem. 1990;265(26):15489–15495. [PubMed] [Google Scholar]
- 24.Entenmann G, Hauner H. Relationship between replication and differentiation in cultured human adipocyte precursor cells. Am J Physiol. 1996;270(4 Pt 1):C1011–C1016. doi: 10.1152/ajpcell.1996.270.4.C1011. [DOI] [PubMed] [Google Scholar]
- 25.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Heintze U, Janke J, et al. Regulation of 11beta-HSD genes in human adipose tissue: influence of central obesity and weight loss. Obes Res. 2004;12(1):9–17. doi: 10.1038/oby.2004.3. [DOI] [PubMed] [Google Scholar]
- 26.Veilleux A, Laberge PY, Morency J, Noel S, Luu-The V, Tchernof A. Expression of genes related to glucocorticoid action in human subcutaneous and omental adipose tissue. J Steroid Biochem Mol Biol. 2010;122(1-3):28–34. doi: 10.1016/j.jsbmb.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Lee MJ, Fried SK, Mundt SS, Wang Y, Sullivan S, Stefanni A, et al. Depot-specific regulation of the conversion of cortisone to cortisol in human adipose tissue. Obesity (Silver Spring) 2008;16(6):1178–1185. doi: 10.1038/oby.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117(17):2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, et al. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc Res. 2009;84(1):164–172. doi: 10.1093/cvr/cvp191. [DOI] [PubMed] [Google Scholar]
- 30.Patsouris D, Neels JG, Fan W, Li PP, Nguyen MT, Olefsky JM. Glucocorticoids and thiazolidinediones interfere with adipocyte-mediated macrophage chemotaxis and recruitment. J Biol Chem. 2009;284(45):31223–31235. doi: 10.1074/jbc.M109.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraus D, Jager J, Meier B, Fasshauer M, Klein J. Aldosterone inhibits uncoupling protein-1, induces insulin resistance, and stimulates proinflammatory adipokines in adipocytes. Horm Metab Res. 2005;37(7):455–459. doi: 10.1055/s-2005-870240. [DOI] [PubMed] [Google Scholar]
- 32.Li P, Zhang XN, Pan CM, Sun F, Zhu DL, Song HD, et al. Aldosterone perturbs adiponectin and PAI-1 expression and secretion in 3T3-L1 adipocytes. Horm Metab Res. 2011;43(7):464–469. doi: 10.1055/s-0031-1277226. [DOI] [PubMed] [Google Scholar]


