Abstract
Background/Aims
Cyclosporine (CsA)-induced renal injury causes renal tubular acidosis. The current study was performed to evaluate the influence of CsA-induced renal injury on the ammonia transporter family members, Rh B-glyco-protein (Rhbg) and Rh C-glycoprotein (Rhcg).
Methods
Rats were treated daily for 1 or 4 weeks with vehicle (VH) or CsA. Induction of chronic CsA-induced nephropathy was confirmed by demonstrating impaired renal function and characteristic histopathology. Rhbg and Rhcg expression was evaluated with immunoblot, immunohistochemistry, real-time RT-PCR and electron microscopy.
Results
CsA treatment for 4 weeks developed mild metabolic acidosis and decreased urinary ammonia excretion. Rhcg mRNA expression was unchanged in both the cortex and outer medulla, but Rhcg protein expression in the CsA group was significantly reduced in the cortex and outer medulla. There were no significant differences in Rhbg mRNA and protein expression between the CsA and VH group.
Conclusion
Long-term treatment with CsA in rats results in decreased urinary ammonia excretion accompanied by decreased expression of Rhcg; these changes are likely to mediate the CsA-induced defect in ammonium excretion in the collecting duct.
Keywords: Ammonia transporter, Chronic cyclosporine nephropathy, Kidney
Introduction
Long-term treatment with cyclosporine (CsA) is associated with characteristic histologic lesions (striped inter-stitial fibrosis, arteriolopathy and tubular atrophy). CsA treatment also causes tubular dysfunction characterized by polyuria, magnesium wasting, renal tubular acidosis, and hyperkalemia [1–3] . Of these, chronic metabolic acidosis associated with impaired net acid excretion is one of the predominant features of chronic CsA nephropathy [4], but the molecular mechanisms have yet to be identified.
Ammonia is the principal component of renal net acid excretion [5–7] . Recent evidence leads to the previous model of ammonia transport, which invoked passive NH3 diffusion and acidification-induced specific proteins transport NH3 and [8] . In particular, in the collecting duct, the site of the most renal ammonia excretion, the non-erythroid Rh glycoprotein family members, Rh B glycoprotein (Rhbg) and Rh C glycoprotein (Rhcg), may play critical roles in collecting duct renal ammonia transport.
Heterologous expression studies confirm that both Rhbg and Rhcg transport ammonia and the ammonia analog, methylammonia, and that their affinity for ammonia is similar to physiologically relevant ammonia concentrations present in the kidney [9–12] . Transport studies using cultured mouse collecting duct cells (mIMCD-3) show that carrier-mediated mechanisms, appearing to involve Rhbg and Rhcg, are critical components of both basolateral and apical plasma membrane ammonia transport [13, 14]. Finally, chronic metabolic acidosis, which increases renal ammonia metabolism, increases expression of Rhcg in the outer medulla and inner medulla through multiple mechanisms [15, 16]. Thus, Rhbg and Rhcg are likely to play important roles in renal ammonia metabolism.
The current study examines whether chronic cyclosporine administration decreases ammonia excretion, and whether this is associated with altered expression of Rhbg and/or Rhcg. Our results indicate that CsA treatment decreases Rhcg suggesting that these changes may mediate decreased ammonia excretion which then leads to the development of chronic metabolic acidosis.
Methods
Animals
Male Sprague-Dawley rats (Charles River Co., Korea), weighing 220–240 g, were housed in individual cases (Nalge, Rochester, N.Y.) in a temperature- and light-controlled environment. Prior to starting the treatment, rats were pair-fed on a low-salt diet (0.05% sodium, Teklad Premier, Madison, Wisc.) and daily body weight was monitored. Rats were randomized into 4 groups: the vehicle groups (VH) consisted of rats receiving daily subcutaneous injections of olive oil (Sigma Diagnostics Inc., St. Louis, Mo.) at a dose 1 ml/kg for 1 (VH1, n = 5) or 4 weeks (VH4, n = 5), and the CsA group consisted of rats receiving daily subcutaneous injections of CsA (Novartis Pharma, Basel, Switzerland) at a dose of 15 mg/kg for 1 (CsA1, n = 5) or 4 weeks (CsA4, n = 5). The dose and administration route of CsA were chosen based on a previous report [17]. On days 7 and 28, the animals were placed in metabolic cages (Tecniplast, Buguggiate, Italy) and urine was collected under mineral oil. The urine volume was recorded and an aliquot frozen for later analysis. On the day of the experiment, the animals were euthanized using ketamine. Blood was taken from the aortic vein and placed in a heparinized tube for pH and pCO2 measurement. The remaining blood was placed in a non-heparinized tube for serum collection. After centrifugation, the serum was placed in microcentrifuge tubes, frozen, and stored for later analysis. Kidneys were removed and the cortex and outer medulla were dissected. A portion of each region was placed in RNAzol reagent (Tel-Test Inc., Friendwood, Tex.). The remaining tissues were homogenized and sheared for protein lysate, as described previously [15]. The samples in RNAzol reagent were stored at 4 ° C overnight and then stored at –20 ° C. Protein homogenates were stored at –20 ° C. Tissues were shipped on dry-ice and then stored in a –80 ° C freezer until analyzed. The Animal Care Committee of the Catholic University approved the experimental protocol.
Antibodies
Antisera to rodent Rhbg and Rhcg were generated in our laboratory and have been characterized previously [14, 18]. Antisera were affinity-purified using a commercially available kit (Sulfo-Link, Pierce Biotechnology, Rockford, Ill.) and the immunizing peptides, as described previously [13, 14] .
Immunoblot Analysis
Immunoblot analysis was performed as described previously [19]. For membrane fraction, the kidneys from the cortex, outer medulla, and inner medulla were homogenized in lysis buffer. Homogenates were centrifuged at 4,000 g for 20 min at 4 ° C to remove cell debris. The supernatant was centrifuged at 200,000 g for 1 h (Beckman Instruments, Palo Alto, Calif.) to separate plasma membranes and intracellular vesicles. Afterwards, the protein concentration was determined with a protein microassay using the Bradford method (Bio-Rad). An equal amount of protein was resolved on SDS-PAGE and then electroblotted onto a nitrocellulous membrane (Millipore, Bedford, Mass.). Membranes were then blocked with 5 g/dl nonfat dry milk in Tris-buffered saline (pH 8.0) and incubated overnight at 4 ° C with Rhbg at 1: 2,500 and Rhcg at 1: 5,000 dilutions in blocking buffer. After being washed, membranes were exposed to secondary antibody (goat anti-rabbit IgG conjugated to horseradish peroxidase Amersham Biosciences, Bucks., UK) for 1 h diluted at 1: 1,000. Optical densities were obtained after three determinations for each band.
Electrolyte and Ion Measurement
Serum creatinine was measured with an enzymatic assay (CREA plus, No. 11775642216, Roche Diagnostics, Mannheim, Germany). BUN, sodium and potassium levels were measured by a Cobas autoanalyzer (Hoffmann-La Roche, Nutley, N.J.). The ammonia concentration was measured using a commercially available assay (Sigma Ammonia Assay Kit, Sigma-Aldrich). Urine osmolality was measured with a Fiske 2400 Osmometer (Fiske Associates, Norwood, Mass.). Blood-gas measurements were performed on freshly obtained, heparinized arterial blood using an AVL Opti 1 blood-gas analyzer. The serum bicarbonate concentration was calculated from the pH and the pCO2 measurement. Whole-blood CsA levels were measured by monoclonal radioimmunoassay (Incstar, Stillwater, Minn.).
Tissue Processing for Immunohistochemistry
On the day of fixation, animals were anesthetized with an intraperitoneal injection of ketamine. The kidneys were preserved by in vivo perfusion fixation through the abdominal aorta. The kidneys were first perfused briefly with PBS (pH 7.4) to rinse away all blood and, subsequently, with periodate-lysine-paraformalde-hyde (PLP) solution for 10 min. They were cut into sagittal sections and immersed in PLP solution overnight at 4 ° C. After being rinsed in PBS, tissues were dehydrated in a graded series of ethanol and embedded in polyester wax (polyethylene glycol 400 distearate, Polysciences, Warrington, Pa.). 5-μ m thick sections were cut and mounted on gelatin-coated glass slides. The others were cut using vibratome for pre-embedded immunohistochemistry.
Rhbg and Rhcg RNA Quantifications
Total RNA was extracted using RNeasy MidiKit (Qiagen, Valencia, Calif.) and stored in a –70 ° C freezer until used. Rhbg and Rhcg mRNA were quantified using real-time RT-PCR using techniques reported previously [15]. The forward primer for Rhbg was 5′ -CCT-GCC-GCT-GCT-GTG-TCT-3′, the reverse primer was 5′ -AGC-GGA-CAA-AGA-TCG-CAA-AG-3′, and the fluorescent probe was 6FAM-CTCTTCCAAGGCGCCACCTCCCT-TAMRA. For Rhcg, the forward primer was 5′ -TGT-GGA-TAT-ACTGGC-CTA-GCT-TCA-3′, the reverse primer was 5′ -GAG-GGCTGC-TCG-GTG-TTG-3′, and the fluorescent probe was 6FAMCTC-AGC-CAG-TTC-CTT-CCA-CGG-AGA-CA-TAMRA. GAPDH and 18S mRNA expression was quantified using commercially available primers and probes (Applied Biosystems, Foster City, Calif.). RNA was reverse transcribed using the Super-Script First Strand Synthesis System for RT-PCR (Invitrogen) and random hexamers. Real-time RT-PCR was performed on an ABI Prism GeneAmp 5700 Sequence Detection System (Applied Bio-systems). Amplification was performed using a total fluid volume of 25 μ l and TaqMan RT-PCR Master Mix Reagents (Applied Bio-systems). We used a two-step cycle protocol including an initial 95 ° C denaturation step for 15 s and then 60 ° C for 1 min and 40 cycles of these alternating temperatures. Results were analyzed using GeneAmp 5700 SDS software, version 1.3 (PerkinElmer, Applied Biosystems). Expression was quantified using the ΔΔCT technique with either GAPDH or 18S RNA as an internal standard, and a validation experiment was performed to demonstrate that efficiencies of target and reference are approximately equal before using the ΔΔCT method for quantitation.
Pre-Embedding Immunoperoxidase Method
Vibratome sections (50 μm thick) were used. In brief, before incubation with the primary antibodies, the tissue sections were incubated for 3 h with 1% BSA, 0.05% saponin, and 0.2% gelatin-PBS (solution B). The tissue sections were then incubated overnight at 4 ° C in rabbit polyclonal anti-Rhbg (1: 1,500) and anti-Rhcg (1: 2,500) antibodies in 1% BSA-PBS (solution A). After several washes with 0.1% BSA, 0.05% saponin, and 0.2% gelatin-PBS (solution C), the tissue sections were incubated for 2 h in peroxidase-conjugated donkey anti-rabbit IgG Fab fragment (Jackson ImmunoResearch Laboratories, West Grove, Pa.). The tissues were then rinsed, first in solution C and subsequently in 0.05 M Tris buffer (pH 7.6). For the detection of horseradish peroxidase, sections were incubated in 0.1% 3,3′ -diaminobenzidine and H2O2. The sections were then embedded in poly/Bed 812 resin (Poly-sciences, Warrington, Calif.). For EM, sections were postfixed with 1% glutaraldehyde, 1% osmium tetroxide, and 1% uranyl acetate before being dehydrated and embedded in poly/Bed 812 resin. Ultrathin sections were cut and photographed with a transmission electron microscope (JEOL 1200EX, Tokyo, Japan).
Pre-Embedding Immunogold Method
This method has been described previously [20]. In brief, vibratome sections were incubated with primary antibodies over-night as described above, washed with solution C, and then washed with 0.8% BSA-0.1% gelatin-2 m M NaN3-PBS and 5% normal goat serum (pH 7.4; gold buffer). The tissue sections were then incubated overnight at 4 ° C in NANOGOLD-IgG and Fab′ conjugates (Nanoprobes, Yaphank, N.Y.). After they were washed with PBS, labeled gold was postfixed with 1% glutaraldehyde for 10 min. The tissue sections were enhanced for 7–8 min by HQ silver system (Nanoprobes). The tissue sections were rinsed, postfixed, and embedded for EM as described above.
Statistics
Data are expressed as means ± SEMs. Multiple comparisons among groups were performed by one-way ANOVA using the post-hoc Bonferroni test (SPSS software version 12.0). In each analysis, n refers to the number of animals in each group.
Results
Physiological Analysis
Serum electrolyte measurements are summarized in table 1. One-week treatment with CsA did not induce systemic acidosis, nor were there alterations in urinary ammonia excretion (table 2). On the other hand, 4-week treatment with CsA resulted in mild metabolic acidosis compared with control pair-fed rats. Importantly, serum sodium and potassium concentrations did not change significantly. Physiological parameters in urine are summarized in table 2. One week treatment with CsA did not decrease but 4 weeks treatment with CsA decreased uri-nary ammonia excretion significantly without a significant alteration in urine pH. There was mild polyuria in rats treated for 4 weeks with CsA, as reported previously by our laboratory [19] and others [21] .
Table 1.
Functional parameters in blood
| Parameters | VH1 (n = 5) | CsA1 (n = 5) | VH4 (n = 5) | CsA4 (n = 5) |
|---|---|---|---|---|
| tCO2, mmol/l | 26.7±1.3 | 27.8±0.9 | 26.5±0.3 | 23.0±0.9* |
| pCO2, mm Hg | 42.2±0.6 | 41.6±0.9 | 42.9±1.2 | 41.7±1.9 |
| HCO3, mmol/l | 25.5±1.2 | 26.1±0.9 | 25.4±0.3 | 22.6±0.9* |
| pH | 7.39±0.01 | 7.36±0.01 | 7.37±0.02 | 7.30±0.02* |
| Na, mmol/l | 142±0.4 | 140±0.6 | 142±0.6 | 140±0.8 |
| K, mmol/l | 3.6±0.1 | 3.7±0.1 | 3.9±0.1 | 3.9±0.1 |
| Cl, mmol/l | 105±3 | 104±1 | 106±1 | 104±1 |
| CsA | – | 2,935±130 | – | 2,558±60 |
Values are means ± SE. n = Number of rats. VH = Vehicle-treated group; CsA = cyclosporine A.
p < 0.05 vs. VH.
Table 2.
Functional parameters in urine
| Parameters | VH1 (n = 5) | CsA1 (n = 5) | VH4 (n = 5) | CsA4 (n = 5) |
|---|---|---|---|---|
| Urine volume, ml/24 h | 15±2 | 17±4 | 14±1 | 25±2* |
| Ammonia, mmol/24 h | 0.53±0.13 | 0.58±0.09 | 0.51±0.10 | 0.34±0.14* |
| pH | 6.77±0.24 | 6.58±0.12 | 6.71±0.10 | 6.83±0.1 |
Values are means ± SE. n = Number of rats. VH = Vehicle-treated group; CsA = cyclosporine A.
p < 0.05 vs. VH.
Induction of Chronic CsA Nephropathy
Table 3 shows the body weight, blood pressure, renal function, CsA concentration and histopathological findings in rats treated with VH and CsA. One week CsA treatment did not affect the body weight, blood pressure, or renal function. Four weeks of CsA treatment decreased body weight and increased serum creatinine levels significantly compared with the VH group. Histological examination showed a significant increase in interstitial fibrosis in the CsA group compared with the VH group at 1 and 4 weeks.
Table 3.
Basic parameters in experimental groups
| Parameters | VH1 (n = 5) | CsA1 (n = 5) | VH4 (n = 5) | CsA4 (n = 5) |
|---|---|---|---|---|
| Body weight, g | 215±6 | 225±4 | 300±4 | 245±5* |
| Water intake, ml/24 h | 14±2 | 17±3 | 15±1 | 34±6* |
| Blood pressure, mm Hg | 125±4 | 128±5 | 122±3 | 124±3 |
| Urine osmolality, mosm/kg | 1,212±430 | 1,112±230 | 1,139±264 | 560±28* |
| FENa, % | 0.03±0.01 | 0.02±0.01 | 0.03±0.01 | 0.04±0.01 |
| TcW, ml/24 h | 48±4 | 41±7 | 43±3 | 25±3* |
| BUN, mg/dl | 8.0±0.6 | 18.5±22.1* | 10.0±0.9 | 33.0±2.0* |
| SCr, mg/dl | 0.56±0.02 | 0.62±0.02 | 0.58±0.02 | 1.90±0.08* |
| TIF, % | 0.0±0.0 | 3.2±1.4 | 0.0±0.0 | 28.5±3.0* |
Values are means ± SEM. n = Number of rats. VH = Vehicle-treated group; CsA = cyclosporine A; FENa = fractional excretion of sodium; TcW = free-water reabsorption; BUN = blood urea nitrogen; SCr = serum creatinine; TIF = tubulointerstitial fibrosis.
p < 0.05 vs. VH.
Rhbg and Rhcg Protein Expression
We quantified renal Rhbg and Rhcg protein expression using immunoblot analysis of the renal cortex and outer medulla. Figure 1 summarizes these results. One week of CsA treatment increased Rhbg protein, but did not increase Rhcg protein expression in the cortex and outer medulla. On the other hand, 4 weeks of CsA treatment significantly decreased Rhcg protein expression in proteins isolated from the cortex and outer medulla (p < 0.05, n = 5 in each group) but did not significantly alter Rhbg protein expression (fig. 1).
Fig. 1.
Western blot and densitometric analysis of Rhbg (A, C) and Rhcg (B, D) in the cortex (Co) and outer medulla (OM) of VH- (□)) or CsA-treated rat kidneys (■). Each lane was loaded with a sample from a different animal. Both of Rhbg and Rhcg are prominent with a size of 50 kDa. At 1 week, there was a significant increase in the protein level of Rhbg in the CsA group compared with the VH group. But Rhcg was not changed significantly between the 2 groups. At 4 weeks, there was no significant difference in the protein level of Rhbg between the experimental groups. However, the amount of Rhcg significantly decreased in the CsA group treated for 4 weeks compared with the VH group treated for 4 weeks. VH1 = Vehicle group treated for 1 week; CsA1 = cyclosporine group treated for 1 week; VH4 = vehicle group treated for 4 weeks; CsA4 = cyclosporine group treated for 4 weeks. Values are referred to VH as 100%. * p < 0.05 vs. VH.
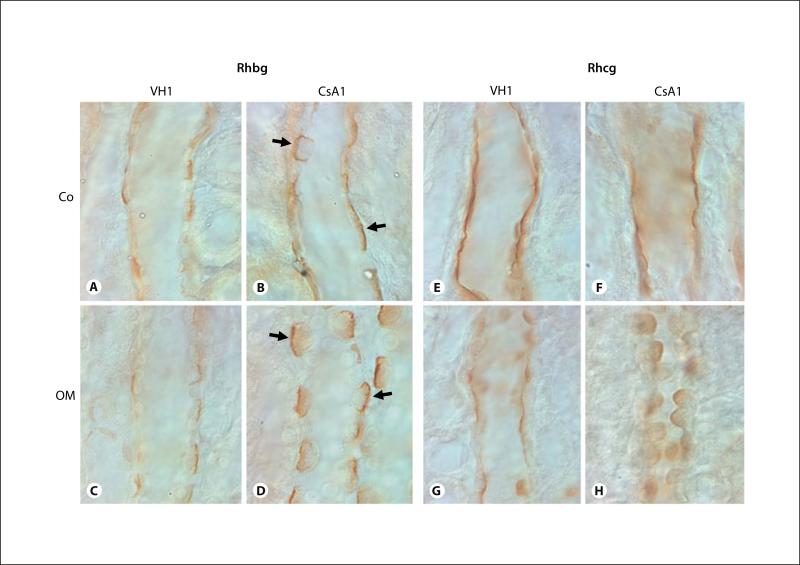
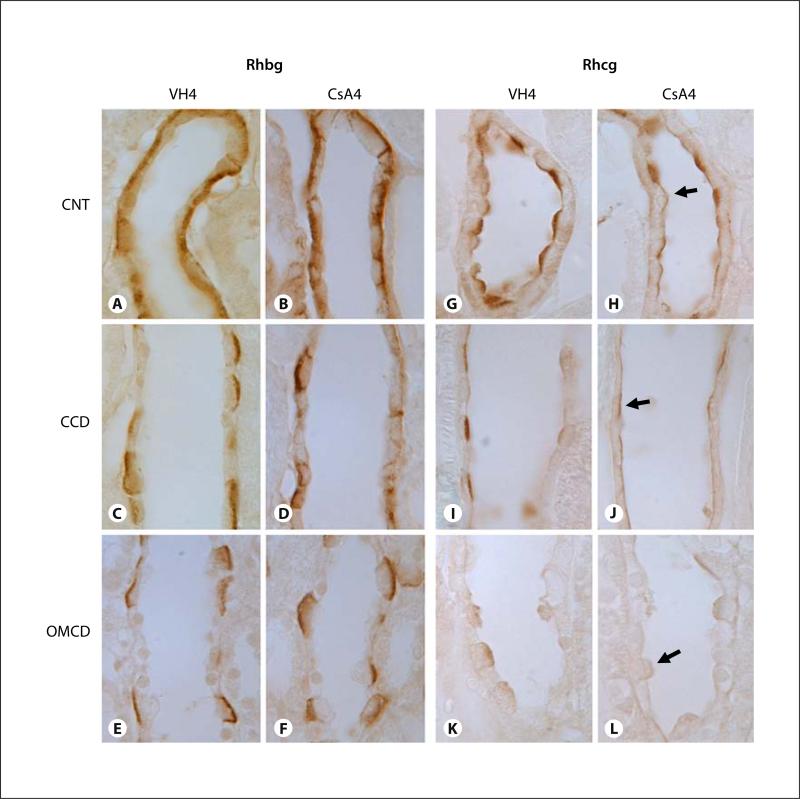
Figures 2 and 3 show representative pictures of Rhbg and Rhcg protein expression in VH- and CsA-treated rat kidneys. Immunohistochemical examination of Rhbg and Rhcg protein expression in rat kidneys with 1 week of CsA treatment revealed an increased intensity of Rhbg immunoreactivity, but there was no change in Rhcg immunoreactivity (fig. 2). On the other hand, in rat kidneys treated for 4 weeks with CsA, the intensity of Rhcg expression was reduced in the distal convoluted collecting duct, initial collecting duct, cortical collecting duct and outer medullary collecting duct of CsA-treated rat kidneys as compared with VH-treated rat kidneys (fig. 3). There were no identifiable changes in the cellular distribution of Rhcg expression. However, chronic CsA administration did not alter steady-state Rhbg protein expression in either the cortex or the outer medulla (p = not significant for each region, n = 5 in each group).
Fig. 2.
Representative micrographs of immunohistochemistry for Rhbg (A–D) and Rhcg (E–H) in the cortical collecting duct (CCD; A, B, E, F), outer medullary collecting duct (OMCD; C, D, G, H) of rat kidneys treat with VH (A, C, E, G) or CsA (B, D, F, and H) for 1 week. Predominant immunoreactivity for Rhbg was detected in the basolateral plasma membrane of the cortical collecting duct (CCD) and outer medullary collecting duct (OMCD). Rhbg expression was increased in rat kidneys treated with CsA for 1 week compared with VH-treated rat kidneys (A–D). Intense immuno-reactivity for Rhcg was detected in the apical plasma membrane of CCD and OMCD. Rhcg expression did not alter in CsA rat kidneys treated with CsA for 1 week compared with VH-treated rat kidneys (E–H). Arrows indicate a strong positive Rhbg reaction from the apical side of the collecting duct. Co = Cortex; OM = outer medulla. Magnification × 1,000.
Fig. 3.
Representative micrographs of immunohistochemistry for Rhbg (A–F) and Rhcg (G–L) in the connecting tubule (CNT; A, B, G, H), cortical collecting duct (CCD; C, D, I, J), and outer medullary collecting duct (OMCD; E, F, K, L) of rat kidneys treated with VH (A, C, E, G, I, K) or CsA (B, D, F, H, J, L) for 4 weeks. Predominant immunoreactivity for Rhbg was detected in the basolateral plasma membrane of CNT, CCD, and OMCD. Rhbg expression did not alter in rat kidneys treat with CsA for 4 weeks compared with VH-treated rat kidneys (A–F). Intense immunoreactivity for Rhcg was detected in the apical plasma membrane of CNT, CCD, and OMCD. Rhcg expression was reduced in those regions of the rat kidney treated with CsA for 4 weeks compared with VH-treated rat kidney (G–L). Arrows indicate a weak positive Rhcg reaction from the apical side of the collecting duct. VH4 = Vehicle group treated for 4 weeks; CsA4 = cyclosporine group treated for 4 weeks. Magnification × 1,000.
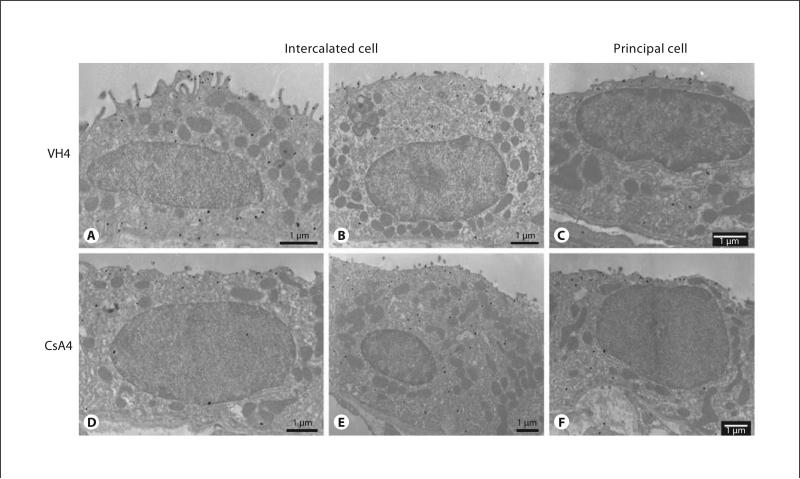
To confirm the subcellular changes in Rhcg in collecting duct cells from rat kidneys treated for 4 weeks with CsA, we used electron microscopic immunocyto-chemistry with a pre-embedding immunoperoxidase method. A large amount of Rhcg immunolabeling in type A intercalated cells was seen in the apical plasma membrane, subapical region, and basal plasma membrane in the VH-treated rat kidney, whereas a small amount of labeling of Rhcg was detected in the CsA-treated rat kidney. However, there was no difference in Rhcg-labeling in type B intercalated cells and principal cells between both groups (fig. 4). The subcellular distribution of Rhbg was not changed between the 2 groups, consistent with light microscopy and immunoblot observations (not shown).
Fig. 4.
Transmission electron microscopic localization of Rhcg in the intercalated cells (A, B, D, E) and principal cells (C, F) from the cortical collecting duct of rat kidneys treated with VH (A–C) and CsA (D–F) for 4 weeks using an immunogold method. In the VH-treated group, numerous amounts of Rhcg immunolabeling are shown in the apical membrane microprojection, subapical region, and basal membrane (A), whereas Rhcg labeling is lower the CsA-treated group with short apical microprojections (D). Rhcg immunolabeling is mainly present in the apical region in type B intercalated cell and principal cell. However, there is no difference in Rhcg labeling in between VH- and CsA-treated groups (B, C, E, F).
Rhbg and Rhcg mRNA Quantifications
After 1 week of CsA administration, both Rhbg and Rhcg mRNA expression were increased in the outer medulla but were not significantly altered in the cortex. In contrast, after 4 weeks of CsA treatment no significant differences in Rhcg mRNA expression were observed in any of these regions (p = not significant in the cortex and outer medulla; n = 5). Figure 5 summarizes these results.
Fig. 5.
mRNA expression of Rhbg and Rhcg using real-time PCR in the cortex and outer medulla (OM) from VH- (□)) or CsA-treated rat kidneys (■) for 1 week (A) and 4 weeks (B). CsA treatment did not alter mRNA expression of both Rhbg and Rhcg from cortex and OM. Relative Rhbg or Rhcg mRNA values are normalized to GAPDH mRNA. NS = No significance.
Discussion
The current studies examine the effect of chronic CsA nephropathy on the renal expression of the ammonia transporter family members, Rhbg and Rhcg. Long-term CsA treatment induced chronic metabolic acidosis and reduced urine ammonium excretion. This functional change was accompanied by decreased Rhcg in the cortex and outer medulla. These results suggest that cyclosporine decreases renal ammonia metabolism and that this occurs, at least in part, from parallel decreases in the ammonia transporter family member, Rhcg.
The results of our study revealed that CsA-induced renal injury significantly decreased Rhcg expression and immunoreactivity in the cortex and outer medulla. The correlation between changes in Rhcg expression and renal ammonia transport in conditions where ammonia metabolism is both increased, e.g. chronic metabolic acidosis [15], and decreased (current study) supports the emerging paradigm that Rhcg-mediated ammonia transport plays an important role in renal ammonia metabolism. Moreover, the observation that changes in Rhcg protein expression, both in response to CsA (current study) and in response to chronic metabolic acidosis [15], do not involve changes in steady-state Rhcg mRNA expression suggests that regulation of Rhcg protein expression through post-translational mechanisms may be a general phenomenon.
We further evaluated the subcellular localization of Rhcg using the immunogold method in rat kidneys treated with VH and CsA for 4 weeks. In the VH group, immunolabeling of Rhcg was observed in the apical and basolateral plasma membrane, and it was scattered throughout the cytoplasm in the intercalated cells. Principal cells were mainly present in the apical region with a low amount of immunolabeling of Rhcg compared to inter-calated cells. However, the CsA-treated group showed that only type A intercalated cells were inactivated with a decreased immunogold particle number. On the other hand, there was no change in type B intercalated cells and principal cells. This finding suggests that long-term treatment with CsA on Rhcg expression might influence specific type A intercalated cells.
In contrast to decreased Rhcg expression, there was no significant alteration in either Rhbg mRNA or protein expression in chronic CsA nephropathy. Similar findings were observed in chronic metabolic acidosis [15], and suggest either that an independent mechanism of protein expression regulates Rhbg-mediated ammonia transport or that there is a lack of change in Rhbg-mediated ammonia transport in response to CsA-induced renal injury and chronic metabolic acidosis. Another possibility is that Rhbg does not contribute to renal ammonia metabolism, which is supported by the observation that Rhbg knockout does not detectably alter basal or acidosis-stimulated renal ammonia metabolism [22] .
Renal ammonia metabolism involves a complex interplay between ammoniagenesis in the proximal tubule and specific transport events in the proximal tubule, thick ascending limb of the loop of Henle and the collecting duct. Besides the kidney, the liver is responsible for the metabolism of ammonia. Therefore, it is presumable that CsA may decrease urinary ammonia excretion by multiple factors, and we speculate about the possible mechanisms. First, CsA may decrease the proximal tubule ammonia-genesis by decreasing phosphoenolpyruvate kinase ammoniagenic activity and mRNA expression [23]. Second, CsA decreases medullary interstitial ammonia accumulation by decreasing the apical Na+ -K+ -2Cl– cotransporter, NKCC2, in the thick ascending limb of the loop of Henle in the CsA-treated rat kidney [24]. The current study suggests that CsA may decrease trans cellular ammonia secretion in the collecting duct by decreasing Rhbg expression. Furthermore, CsA-induced renal dysfunction may decrease ammonia excretion and CsA may affect ammonia metabolism in the liver. Therefore, we cautiously conclude that CsA affects ammoniagenesis and specific transporters, and they synergistically interact to alter renal ammonia metabolism and result in the development of metabolic acidosis in CsA-induced renal injury.
Compared to other animal models of CsA-induced renal injury which showed typical distal tubular acidosis [21, 25, 26], our model in the current studies showed normochloremic metabolic acidosis without respiratory compensation. The reason why our model did not show typical renal tubular acidosis is unclear, but it may be related to the low-salt diet. We and others found that at sodium depletion exacerbates CsA nephrotoxicity, and CsA treatment in rats on a low-salt diet induced a histological feature similar to that described in patients on long-term CsA therapy [27, 28]. This finding suggests that the reninangiotensin-aldosterone system is required in rats to induce CsA-related structural injury in the kidney. Indeed, angiotensin II receptor blockade and spironolactone are effective in decreasing CsA-induced renal injury [29]. With this model of chronic CsA nephropathy, we also found that CsA treatment of rats on a low-salt diet caused marked weight loss due to severe oxidative stress and polyuria [19]. Therefore, there is a possibility that volume depletion caused metabolic alkalosis and this may mask metabolic acidosis caused by CsA. This presumption may explain why the metabolic acidosis observed in the current study was mild without respiratory compensation and hyperchloremia.
In summary, the current studies are the first to examine the effects of chronic CsA nephropathy on the expression of the recently identified ammonia transporter family members, Rhbg and Rhcg. Changes in ammonia secretion by the kidney in response to chronic CsA nephropathy appear to involve, at least in part, specific changes in transcellular ammonia secretion mediated by Rhcg expression in the renal collecting duct.
Acknowledgments
The authors thank Gina Cowsert for secretarial assistance. These studies were supported by funds from the NIH (DK45788 and NS47624 to I.D.W.), the Department of Veterans Affairs Merit Review Program (to I.D.W.), the Korea Research Foundation (grant KRF-2005-003-E00006 to K.H.H.), an ISN Fellowship Award (to H.-Y.K.), Korea Science & Engineering Foundation (R13-2002-005-03001-0) through the Cell Death Disease Research Center at the Catholic University of Korea, and the postdoctoral Fellowship Program of Korea Science & Engineering Foundation (to H.-Y.K.).
References
- 1.Ling BN, Eaton DC. Cyclosporin A inhibits apical secretory K + channels in rabbit cortical collecting tubule principal cells. Kidney Int. 1993;44:974–984. doi: 10.1038/ki.1993.339. [DOI] [PubMed] [Google Scholar]
- 2.Thompson CB, June CH, Sullivan KM, Thomas ED. Association between cyclosporin neurotoxicity and hypomagnesaemia. Lancet. 1984;17:1116–1120. doi: 10.1016/s0140-6736(84)91556-3. [DOI] [PubMed] [Google Scholar]
- 3.Young BA, Burdmann EA, Johnson RJ, Alpers CE, Giachelli CM, Eng E, Andoh T, Bennett WM, Couser WG. Cellular proliferation and macrophage influx precede interstitial fibrosis in cyclosporine nephrotoxicity. Kidney Int. 1995;48:439–448. doi: 10.1038/ki.1995.312. [DOI] [PubMed] [Google Scholar]
- 4.Batlle DC, Gutterman C, Tarka J, Prasad R. Effect of short-term cyclosporine A administration on urinary acidification. Clin Nephrol. 1986;25(suppl 1):S62–S69. [PubMed] [Google Scholar]
- 5.DuBose TD, Jr, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: new physiological concepts and their clinical implications. J Am Soc Nephrol. 1991;1:1193–1203. doi: 10.1681/ASN.V1111193. [DOI] [PubMed] [Google Scholar]
- 6.Good DW, Caflisch CR, DuBose TD., Jr Transepithelial ammonia concentration gradients in inner medulla of the rat. Am J Physiol. 1987;252:F491–F500. doi: 10.1152/ajprenal.1987.252.3.F491. [DOI] [PubMed] [Google Scholar]
- 7.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol. 1987;253:F595–F605. doi: 10.1152/ajprenal.1987.253.4.F595. [DOI] [PubMed] [Google Scholar]
- 8.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol. 2007;17:317–340. doi: 10.1146/annurev.physiol.69.040705.142215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, Cherif-Zahar B, Planelles G. NH3 is involved in the NH4 + transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem. 2004;279:15975–15983. doi: 10.1074/jbc.M308528200. [DOI] [PubMed] [Google Scholar]
- 10.Ludewig U. Electroneutral ammonium transport by basolateral rhesus B glycoprotein. J Physiol. 2004;559:751–759. doi: 10.1113/jphysiol.2004.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mak DD, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol. 2006;290:F297–F305. doi: 10.1152/ajprenal.00147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zidi-Yahiaoui N, Mouro-Chanteloup I, D'Ambrosio AM, Lopez C, Gane P, Le van Kim C, Cartron JP, Colin Y, Ripoche P. Human rhesus B and rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J. 2005;391:33–40. doi: 10.1042/BJ20050657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol. 2005;289:F347–F358. doi: 10.1152/ajprenal.00253.2004. [DOI] [PubMed] [Google Scholar]
- 14.Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins Rh B glycoprotein and Rh C glycoprotein in the intestinal tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1036–G1047. doi: 10.1152/ajpgi.00418.2004. [DOI] [PubMed] [Google Scholar]
- 15.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol. 2006;290:F397–F408. doi: 10.1152/ajprenal.00162.2005. [DOI] [PubMed] [Google Scholar]
- 16.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol. 2006b;290:F1443–F1452. doi: 10.1152/ajprenal.00459.2005. [DOI] [PubMed] [Google Scholar]
- 17.Yang CW, Ahn HJ, Kim WY, Li C, Jung JY, Yoon SA, Kim YS, Cha JH, Kim J, Bang BK. Synergistic effects of mycophenolate mofetil and losartan in a model of chronic cyclosporine nephropathy. Transplantation. 2003;75:309–315. doi: 10.1097/01.TP.0000045034.48833.51. [DOI] [PubMed] [Google Scholar]
- 18.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol. 2004;287:F628–F638. doi: 10.1152/ajprenal.00363.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lim SW, Li C, Sun BK, Han KH, Kim WY, Oh YW, Lee JU, Kador PF, Knepper MA, Sands JM, Kim J, Yang CW. Long-term treatment with cyclosporine decreases aquaporins and urea transporters in the rat kidney. Am J Physiol Renal Physiol. 2004;287:F139–F151. doi: 10.1152/ajprenal.00240.2003. [DOI] [PubMed] [Google Scholar]
- 20.Lim SW, Han KH, Jung JY, Kim WY, Yang CW, Sands JM, Knepper MA, Madsen KM, Kim J. Ultrastructural localization of UT-A and UT-B in rat kidneys with different hydration status. Am J Physiol Regul Integr Comp Physiol. 2006;290:R479–R492. doi: 10.1152/ajpregu.00512.2005. [DOI] [PubMed] [Google Scholar]
- 21.Bertani T, Perico N, Abbate M, Battaglia C, Remuzzi G. Renal injury induced by long-term administration of cyclosporin A to rats. Am J Pathol. 1987;127:569–579. [PMC free article] [PubMed] [Google Scholar]
- 22.Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol. 2005;289:F1281–F1290. doi: 10.1152/ajprenal.00172.2005. [DOI] [PubMed] [Google Scholar]
- 23.Morris SM, Jr, Kepka-Lenhart D, McGill RL, Curthoys NP, Adler S. Specific disruption of renal function and gene transcription by cyclosporin A. J Biol Chem. 1992;267:13768–13771. [PubMed] [Google Scholar]
- 24.Lim SW, Ahn KO, Sheen MR, Jeon US, Kim J, Yang CW, Kwon HM. Downregulation of renal sodium transporters and TonEBP by long-term treatment with cyclosporine A. J Am Soc Nephrol. 2007;18:421–429. doi: 10.1681/ASN.2006060664. [DOI] [PubMed] [Google Scholar]
- 25.Wall SM, Truong AV, DuBose TD., Jr H+-K + - ATPase mediates net acid secretion in rat terminal inner medullary collecting duct. Am J Physiol. 1996;271:F1037–F1044. doi: 10.1152/ajprenal.1996.271.5.F1037. [DOI] [PubMed] [Google Scholar]
- 26.Tumlin JA, Sands JM. Nephron segment-specific inhibition of Na + /K + -ATPase activity by cyclosporin A. Kidney Int. 1993;43:246–251. doi: 10.1038/ki.1993.38. [DOI] [PubMed] [Google Scholar]
- 27.Elzinga LW, Rosen S, Bennett WM. Dissociation of glomerular filtration rate from tubulointerstitial fibrosis in experimental chronic cyclosporine nephropathy: role of sodium intake. J Am Soc Nephrol. 1993;4:214–221. doi: 10.1681/ASN.V42214. [DOI] [PubMed] [Google Scholar]
- 28.Yang CW, Kim YS, Kim J, Kim YO, Min SY, Choi EJ, Bang BK. Oral supplementation of L -arginine prevents chronic cyclosporine nephrotoxicityin rats. Exp Nephrol. 1998;6:50–56. doi: 10.1159/000020504. [DOI] [PubMed] [Google Scholar]
- 29.Bobadilla NA, Gamba G. New insights into the pathophysiology of cyclosporine nephrotoxicity: a role of aldosterone. Am J Physiol Renal Physiol. 2007;293:F2–F9. doi: 10.1152/ajprenal.00072.2007. [DOI] [PubMed] [Google Scholar]