Abstract
Objective
To compare a central analgesic mechanism known as diffuse noxious inhibitory controls (DNIC) using somatic test stimuli and somatic conditioning stimuli, (CS) in irritable bowel syndrome (IBS) patients and healthy controls.
Methods
Participants were 48 premenopausal females (27 with IBS), mean age of 29 years. The phasic heat test stimulus (peak temperature, 50°C) was applied to the left palm. The DNIC effect, which measured reductions in average pain ratings (APR) during counter irritation (submersion of the participant’s right hand in painful 12°C circulating water) compared with baseline, was compared between groups. In addition, a second, counterbalanced, CS protocol (right hand submerged in nonpainful 32°C circulating water) was performed. Differences in APR between the 2 counter-irritation protocols were compared between groups to control for nonspecific effects known to influence DNIC. Psychologic measures and cardiovascular reactivity were also assessed.
Results
IBS patients demonstrated smaller DNIC than controls (P=0.011, repeated measures analysis of variance), and greater state-anxiety, depression, catastrophizing, and anger-out expression (P<0.05). Group differences in DNIC were enhanced after controlling for nonspecific effects occurring during the nonpainful CS, and for psychologic measures (P=0.001, repeated measures analysis of covariance). There were no group differences in age, cardiovascular reactivity, APR, or pain ratings for the 12°C CS.
Discussion
These data demonstrate deficient DNIC in IBS. This is the first study to adequately control for alternative explanations of pain reduction during counterirritation. Only by controlling for nonspecific effects can evidence of deficient DNIC be attributed to dysregulation in endogenous analgesic mechanisms.
Keywords: diffuse noxious inhibitory controls, DNIC, pain, irritable bowel syndrome
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by recurring abdominal pain associated with altered bowel habits in patients who do not show signs or symptoms of an alternative disease to explain these symptoms.1 Many IBS patients have been found to be hypersensitive to balloon distension of the colon and rectum.2–4 Although not all IBS patients demonstrate this hypersensitivity, visceral hyperalgesia is so common in IBS patients that it is often considered to be a biologic marker for IBS.4,5 Contrary to earlier reports, which found evidence of visceral but not somatic hyperalgesia, most recent investigations have also demonstrated somatic hyperalgesia in IBS patients.6–9 These new data suggest the possibility that IBS patients may have a dysregulation in central pain processing similar to that seen in other chronic pain disorders such as fibromyalgia syndrome (FMS) and temporomandibular disorders (TMD).10
Investigations to explore central pain dysregulation in these and other chronic pain disorders have focused on two aspects, namely (1) exaggerations in temporal summation that facilitates ascending pain signals, and (2) deficits in diffuse noxious inhibitory controls (DNIC) that provide tonic descending anti-nociceptive signals. Research consistently shows enhanced temporal summation of afferent pain signals as well as deficits in DNIC in FMS11–13 and TMD patients14–17 compared to healthy controls (HCs), supporting the view that central alteration in pain signaling is an important contributor to the onset or worsening of chronic pain symptoms.10,18–21 According to Lautenbacher and Rollman, the nature of FMS, or any chronic pain condition, points to a deficient DNIC process reflecting decrements in endogenous analgesic systems.11
The counterirritation phenomenon known as DNIC occurs when pain perception from one nociceptive stimulus is substantially inhibited by a second nociceptive stimulus administered somewhere else in the body.22 This inhibition is potent, affecting all of the activities of the wide dynamic range neurons in the dorsal horn. DNIC mechanisms act like a barrier to prevent the spread of pain and keep it bearable by providing a tonic inhibitory influence.21 Deficits in DNIC are believed to play an important role in geriatric and chronic pain.10,18–21,23
Testing DNIC requires administering a phasic, noxious test stimulus (TS), before and concurrent with a tonic noxious conditioning stimulus (CS), typically, ischemic, cold pressure, or noxious heat pain, heteroptopically.11,13,23–25 The difference between the pain that is experienced with the first phasic pain stimulus, without the CS, compared with the second phasic stimulus of identical intensity and duration during the concurrent tonic pain is described as the DNIC effect.
Wilder-Smith and colleagues in 200426 reported deficits in DNIC in IBS patients compared with controls using a visceral TS of painful intrarectal distensions during application of a somatic CS (placing the foot in ice-water).26 Although these investigators report group differences in the activity of magnetic resonance imaging in pain regulatory centers of the brain during the DNIC procedure, the choice of rectal distensions as the TS and noxious cold applied to the foot as the CS present several questions that need to be addressed. (1) It cannot be determined if the reduction in median pain scores for the TS during concurrent noxious CS was due to distraction from the CS, which is known to effect DNIC measures11,13,18 or (2) whether the lack of DNIC in IBS patients was due to hypervigilance to the TS of rectal distensions.27 Using painful gut distensions for IBS patients as the TS may complicate the evaluation of DNIC. (3) Peripheral sensitization of gut afferent fibers due to a history of chronic abdominal pain may interfere with the assessment of the central pain regulatory process of DNIC on rectal pain perception. Peripheral sensitization occurs when polymodal C-fiber nociceptors and A-fiber mechanonociceptors increase their sensitivity after repeated noxious stimulation, which is unique to the nociceptive system. According to Bouin and colleagues,28 dysregulation in the neurobiology of visceral (gut) afferents and pain sensitivity control is believed to explain IBS symptoms. (4) Convergence of nociceptive afferent signals from rectal distension and from cold pain at the foot that share transmission pathways in lumbar dorsal horn neurons may further complicate the assessment of the effect of the CS on the TS. Also, Wilder-Smith et al did not control for psychologic factors29–34 or cardiovascular reactivity,35,36 which have been shown to influence pain measurements. The aim of the present investigation was to assess group differences in central analgesic mechanisms by comparing DNIC in IBS patients and HCs using a somatic TS and a somatic CS, while controlling for nonspecific effects on pain perception, such as distraction from the CS, psychologic symptoms, and cardiovascular reactivity.
METHODS
Participants
Premenopausal women, 18 years of age and older were recruited for this investigation. IBS patients met Rome II criteria37 and currently had painful symptoms of IBS. Exclusion criteria were status postmenopause, pregnant or nursing, major clinical depression or anxiety disorder, hypertension, heart disease, kidney disease, diabetes mellitus, seizure disorder, asthma, or thyroid disorder. Individuals taking analgesics, narcotics, or antidepressants were also excluded from participation. Healthy participants could not have a history of any chronic pain conditions. All IBS subtypes were recruited, as well as IBS patients reporting additional chronic pain conditions. Recruitment was by campus-wide e-mail, fliers, and from a registry of individuals interested in participation in research from the University of North Carolina Center for Functional Gastrointestinal and Motility Disorders. This investigation was approved by the University of North Carolina Medical Institutional Review Board.
Design
Somatic, rather than visceral pain stimuli were used for both the test and conditioning stimuli to avoid interference from peripheral sensitization in the gut that may be present in IBS patients. In addition, pain testing was performed on the upper extremities to avoid convergence of nociceptive signals at lumbar dorsal horn neurons, which may occur when measuring simultaneous pain signaling from the lower extremities and the gastrointestinal tract. Pain testing was performed during the follicular phase of the menstrual cycle (days 4 to 8) to minimize gonadal hormone influences on pain perception. To minimize sympatho-mimetic influences on pain perception, all participants were instructed to refrain from consuming caffeine and nicotine for at least 2 hours before testing and all participants were tested at approximately the same time in the afternoon to control for diurnal fluctuations in cortisol. A battery of questionnaires to identify group differences in mood, perceived stress, and stress coping strategies were administered. Blood pressure (BP) and heart rate (HR) measures were also taken at baseline and following each pain stimulus procedure.
Measures of psychologic status included the Spielberger State-Trait Anxiety Inventory,38 the Beck Anger Expression Inventory,39 and the Beck Depression Inventory. 40 The Perceived Stress Scale41 measured recent exposure and responses to stress, and pain coping style was assessed by the Catastrophizing subscale of the Coping Strategies Questionnaire (CSQ).42 Characterization of current clinical pain was obtained with the Irritable Bowel Syndrome Severity Scale43 and the Physical Symptoms Questionnaire,44 which measures the frequency and intensity of comorbid pain conditions.
After the completion of the informed consent and of the questionnaires, participants were given a brief exposure to the phasic heat stimuli TS apparatus using the TSA-II, NeuroSensory Analyzer (Medoc Medical Instruments Inc, Durham, NC) and the ice-water CS to minimize apprehension regarding these stimuli. This consisted of a practice trial for the phasic heat pain stimuli and a brief exposure to the CS by submerging their right hand in 12°C circulating water for 10 seconds.
After the brief exposures to the pain stimuli, the right arms of the participants were instrumented with HR and BP monitors (Acutracker, Suntech Instruments, Chapel Hill, NC). After a 10-minute rest, BP and HR were acquired as baseline measures. Immediately after each pain testing procedure, HR and BP were recorded.
Pain Testing Procedures
Baseline Thermode Pain Ratings
Phasic heat pain ratings were acquired from 2 locations on the proximal palmar surface of the left hand. Participants were randomly assigned to be tested at either the heel pad or thumb pad of the left hand. Pain ratings from all protocols were acquired from the same location for each participant from a series of 8 phasic thermal stimuli delivered using a 30 by 30mm contact thermode with peak temperatures of 50°C, baseline temperatures of 40°C, and an interstimulus interval of 3 seconds (peak to peak). Participants were instructed to rate the intensity of the pain from each phasic heat stimulus on a 0 to 100 scale as soon as they noticed the peak temperature with 0 representing no pain, and 100 representing the most intense pain imaginable. Average pain ratings (APR) were acquired from the series of 8 thermal stimuli and were compared between the groups. Participants were informed that the procedure would be terminated if they gave a rating of 100 or verbally requested that the stimuli be discontinued.
DNIC
DNIC effects were assessed in 2 protocols. Both provided precisely the same phasic heat stimuli for the TS as was used in the baseline protocol. For 1 DNIC test, the participant’s right hand was submerged to the wrist in circulating water at a noxious temperature (12°C), and for the second protocol, the procedure was duplicated except that the temperature of the water was 32°C. To control for order effects, the DNIC procedures using the 2 water temperatures were counter-balanced. Participants were instructed to focus their attention on the phasic heat pain TS throughout the DNIC procedure and not on the CS, to minimize the effects of distraction from the CS.
Noxious CS
Phasic heat pulses were administered in the same manner as in the baseline procedure. This commenced after the tonic CS (other hand submerged in 12°C water) had been applied for 20 seconds. The duration of phasic heat testing was 24 seconds (8 phasic stimuli), as in the baseline session. The CS exposure time was 44 seconds. Changes in APR from the phasic heat pain during baseline, to APR during counterirritation were assessed to compare DNIC between the groups.
Non-noxious CS
The DNIC procedure during non-noxious CS was identical to the procedure using noxious CS, except that the temperature of the water used for the CS was a neutral, non-noxious temperature (32°C). This non-noxious CS controls for nonspecific effects on pain perception and DNIC, such as distraction, by providing the same experience as was provided in the noxious counterirritation procedure except for the absence of nociception. For this investigation, the term “nonspecific effects” represents the effects on DNIC scores that are due to factors other than endogenous analgesia that DNIC protocols are designed to measure. Reductions in pain ratings during a non-noxious water bath CS cannot be due to the neurochemical process of DNIC, which requires counterirritation (pain) from the CS. The sequence of the noxious and non-noxious CS procedures was counterbalanced. Both the initial DNIC effect on APR and the DNIC effect based on the difference in APR during the noxious and the non-noxious conditions were compared between the groups.
Water Pain Ratings
Participants were also asked to rate the pain in their right hand caused by submersion in water for 44 seconds on the same 0 to 100 pain rating scale for both CS. This score was acquired immediately at the conclusion of the phasic heat pulses, to avoid distracting the participant from the TS, and before removing the hand from the water.
Data Analysis
Groups were compared by analysis of variance (ANOVA) with respect to all demographic, psychologic, and cardiovascular reactivity variables (systolic BP and HR). To compare sensitivity to somatic pain stimuli, 2 ANOVAs were performed with group as the between participants factor and (1) APR; and (2) water pain rating (WPR) as within factors, respectively. To compare the DNIC effect between groups, 2 repeated measures ANOVAs were performed with Group as the between factor and DNIC as a repeated factor: (1) assessing APR during baseline and during the concurrent noxious 12°C CS counterirritation sessions; and (2) assessing APR during baseline and during the concurrent non-noxious 32°C CS sessions. A repeated measures analysis of covariance was performed with Group as the between participants factor and DNIC as a repeated factor assessing APR during the noxious 12°C CS counterirritation sessions and during the concurrent non-noxious 32°C CS session. This was done to statistically control for nonspecific effects that occur during counterirritation protocols. Alpha was set at P<0.05 for all analyses (SPSS, 15.0).
RESULTS
Forty-eight female participants were recruited for this investigation. This included 21 HC and 27 patients with IBS. Five IBS patients also had another chronic pain condition (3 with migraine headaches, 2 with TMD). Within the IBS group, 10 were diarrhea predominant (IBS-D), 8 were constipation predominant (IBS-C), and 9 were identified as IBS-alternators (IBS-A, where symptoms alternate between constipation and diarrhea). There were no significant differences between the IBS and HC groups on any demographic variables. Eighty-five percent of the IBS patients and 81% of the HC participants were college graduates. The mean age for the IBS group was 28.9 years and it was 28.5 years for the HC group. The mean body mass index was also virtually the same across groups (HC=23.9 and IBS=24.1). IBS patients included 25 white, 1 African American, and 1 Asian participants. HCs included 14 white, 3 African American, 1 Asian, 2 Native American, and 1 African participants.
Psychologic Outcome Measures
All psychologic questionnaires were completed before any pain perception testing. IBS patients reported significantly greater psychologic distress than did HC (Table 1) as reflected in scores on the anger-out subscale on the Anger Expression Inventory (P=0.039, ANOVA), the catastrophizing pain coping style subscale of the CSQ (P=0.005, ANOVA), the state-anxiety index of the Spielberger State-Trait Anxiety Index (P=0.008, ANOVA), and the Beck Depression Inventory (P=0.019, ANOVA). There were no differences on any psychologic measures in IBS subgroups.
TABLE 1.
Group Mean (SEM) Scores for All Psychologic Questionnaires
| Questionnaires | Controls | IBS |
|---|---|---|
| State anxiety | 25.6 (1.6) | 32.2 (1.7)* |
| Trait anxiety | 29.4 (1.6) | 33.6 (2.0) |
| Depression | 1.9 (1.1) | 4.8 (0.9)† |
| Anger expression | 42.9 (0.75) | 45.0 (0.7) |
| Anger-in expression | 26.4 (0.56) | 25.3 (0.57) |
| Anger-out expression | 11.9 (0.64) | 13.6 (1.0)† |
| Perceived stress | 16.9 (1.5) | 21.5 (1.8) |
| Catastrophizing | 1.4 (1.8) | 2.0 (0.17)* |
P<0.01.
P<0.05.
IBS indicates irritable bowel syndrome.
Cardiovascular Reactivity Outcome Measures
There were no group differences on measures of systolic BP or HR at baseline, or after any of the pain testing procedures. In addition there were no group differences for changes in systolic BP or HR after any pain test.
Somatic Pain Sensitivity
There were no group differences in baseline APR from the phasic heat stimuli on the left hand (IBS=47.5 and HC=47.6) or WPRs from the right hand during 12°C water emersion (IBS=57.4 and HC=55.5). Mean WPRs were less than 1 during 32°C water emersion. The location of phasic heat stimuli on the palm did not affect pain ratings or the DNIC effect. All pain ratings were based on the scale ranging from 0 to 100.
IBS Clinical Pain Severity
IBS patients reported a mean symptom severity score of 251.9 on the Irritable Bowel Severity Scale (IBSS).43 This represents a moderate severity of IBS symptoms. This included 5 patients who rated the symptoms as mild, 13 as moderate, and 9 as severe. There were no differences on the IBSS scores, or on any demographic variable by IBS subtypes (IBS-D, IBS-C, and IBS-A).
Primary Outcome Measure–DNIC
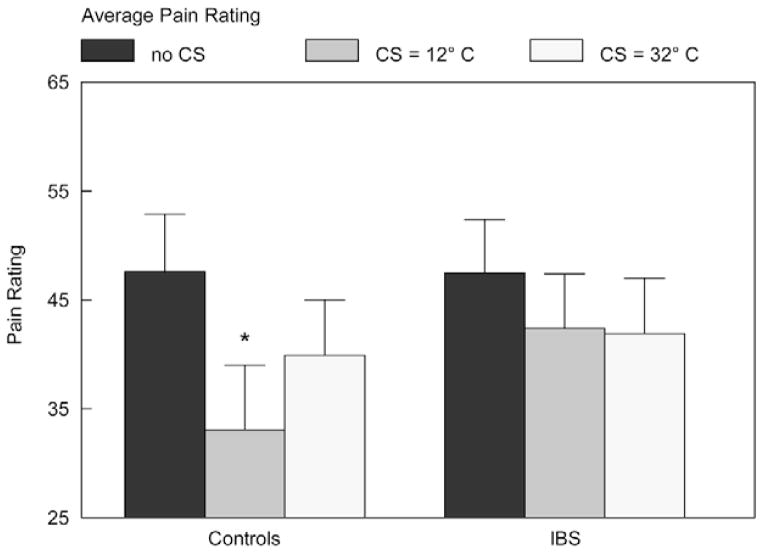
When comparing APR during baseline and during the painful CS of 12°C water, there was a significant main effect of DNIC (Fig. 1), indicating a DNIC effect across all participants [F=30.4 (1, 46), P<0.001, repeated measures ANOVA]. There was also a significant interaction effect (Fig. 1) between Group DNIC (APR with and without the CS) indicating a significant deficit in the DNIC effect for IBS patients compared with controls [F=6.97 (1, 46), P=0.011, repeated measures ANOVA]. A post hoc analysis was carried out to determine whether IBS participants who had additional chronic pain conditions may have explained the group difference seen in DNIC. After controlling for IBS participants who reported having a second chronic pain condition, group differences in APR reductions (DNIC effect) remained significant [F=4.85 (1, 41), P=0.033, repeated measures ANOVA].
FIGURE 1.
Average pain ratings of the phasic TS: Reductions in APR (the DNIC effect) from the baseline procedure (dark gray bar) to the APR during the concurrent tonic CS at 12°C (medium gray bar) demonstrate compromised DNIC in IBS participants compared with controls (F = 6.97, P = 0.011, repeated measures ANOVA). When nonspecific effects on group differences were controlled by comparing reductions in APR (DNIC effect) during the 2 counterirritation protocols (12°C and 32°C, represented by the medium and light gray bars, respectively), and by including psychologic measures that differed by group as covariates, compromised DNIC was demonstrated in IBS participants compared with controls (F = 11.1, P = 002, ANCOVA). ANCOVA indicates analysis of covariance; ANOVA, analysis of variance; APR, average pain rating; C, Celsius; CS, conditioning stimuli; DNIC, diffuse noxious inhibitory control; IBS, irritable bowel syndrome; TS, test stimuli.
There was also a significant main effect (reduction in APR for the TS for both groups) using the nonpainful (32°C water) CS [F=12.5 (1, 46), P=0.001, repeated measures, ANOVA] (Fig. 1). However, there was not a significant difference between groups in the DNIC effect for APR [F=0.29 (1, 46), P=0.6, repeated measures, ANOVA]. Reductions in pain ratings during the nonpainful CS are due to nonspecific effects and cannot be related to endogenous analgesic mechanisms responsible for DNIC, which requires counterirritation from a noxious CS.
A repeated measures analysis of covariance comparing APR during the noxious 12°C CS counterirritation session to APR during the non-noxious 32°C CS counterirritation session shows greater group differences in DNIC in favor of HC, demonstrated by the enhanced interaction effect between Group X and DNIC [F=12.5 (1, 42) P=0.001, analysis of covariance]. Psychologic measures that were significantly different between IBS and HC groups (state anxiety, depression, anger-out expression of anger, and catastrophizing) were included as covariates in this analysis to control for these influences on pain ratings and DNIC.
A substantial effect size was seen for this interaction effect of group membership and DNIC (η2=0.23), whereas the effect size for all 4 psychologic measures combined was less than 8% (depression η2=0.045, catastrophizing η2=0.015, anger-out expression η2=0.015, and state anxiety η2=0.001). This demonstrates an even greater deficit in the DNIC effect for IBS participants compared with HC participants and provides a measure of the true DNIC effect due to endogenous analgesic mechanisms after controlling for nonspecific effects, which are known to effect DNIC and pain measures.
Finally, in a post hoc analysis for IBS patients, the DNIC effect for APR was surprisingly not associated with symptom severity, as reported on the IBSS (r=0.2, P=0.31). However, symptom severity scores on the IBSS were associated with one of the psychologic measures, namely the catastrophizing subscale of the CSQ for IBS patients (r=0.46, P=0.016). There were no differences in the DNIC effect on APR within IBS subtypes (IBS-D, IBS-C, and IBS-A).
DISCUSSION
The results of the present investigation demonstrate deficits in endogenous analgesic mechanisms for participants with IBS. The pain inhibitory mechanism responsible for DNIC was significantly impaired during concurrent noxious counterirritation (opposite hand immersion in painful 12°C water) in IBS participants compared with controls. There were also decreases in pain ratings during concurrent nonpainful 32°C CS. However, this latter condition reflects non-specific effects that are not due to counterirritation. These pseudo-DNIC effects have rarely been accounted for in previous investigations of DNIC.18 Although some investigators have used similar counter-balanced conditioned stimuli procedures to compare DNIC effects in noxious and non-noxious CS,18,45 results in these investigations simply stated that the group differences seen in DNIC during the true counterirritation protocol were not found in the non-noxious CS protocol. Only one other investigation has attempted to account for the role of nonspecific effects in DNIC outcome measures.18
It has been suggested that distraction from the CS may have contributed to the DNIC effects seen in earlier studies.11,13,18 Although distraction does not completely explain DNIC,27,46,47 it has a major influence on the modulation of pain perception.48 Tracey and Dunckley (2004)49 suggested that brain regions that are involved in hypervigilance may connect to brainstem structures responsible for DNIC leading to dysregulation of DNIC. Further, Reinert et al27 speculated that hypervigilance to the TS during DNIC procedures may disrupt DNIC, especially if the TS is meaningful, such as rectal distension for IBS patients,27 as seen in the Wilder-Smith et al IBS investigation of DNIC.26
We have extended the findings of the Wilder-Smith et al study and improved on the design for assessing DNIC in IBS participants in several important ways. (1) Non-specific effects were controlled for by counterbalancing 2 CS procedures. One used a noxious CS (submersion of the hand in 12°C water), whereas the other provided the identical stimuli (circulating water) in a non-noxious form (32°C). In addition, group differences in psychologic factors as reported on questionnaires were statistically controlled for in secondary analyses. (2) The DNIC effect was assessed using somatic pain for both the test and conditioning stimuli to avoid interference from peripheral sensitization (example, due to inflammation) that may be present in the gut of IBS participants. By using somatic pain test and conditioning stimuli, the DNIC measures more specifically reflect dysregulation in central pain mechanisms. (3) The DNIC effect of IBS participants with additional chronic pain syndromes were excluded without affecting the outcome of the study. The present investigation of endogenous analgesic mechanisms in IBS is the first study to extensively control for alternative explanations known to contribute to DNIC effects.
Limitations of This Study
We expected that individuals with deficient DNIC would have higher baseline APR from the phasic heat stimulus and higher pain ratings from the noxious water condition, but this was not the case in this investigation. Edwards et al18 and others21 also failed to demonstrate any associations between DNIC scores and other pain testing measures, such as pain threshold and tolerance from noxious heat stimuli. Edwards et al suggested that endogenous pain modulating systems, such as DNIC, may be subserved by different mechanisms than those that govern less complex pain responses.18,50 Similarly, if deficits in DNIC play a role in IBS pain, it would be expected to correlate with pain severity (less DNIC would be associated with increased symptom severity). This was not the case with self-reported pain severity on the IBSS. One possible explanation is that the severity scores on the IBSS, at least in part, reflect psychologic factors like catastrophizing rather than, or in addition to pain symptoms. It may also be that psychologic factors play a larger role in simple pain measures, such as threshold, tolerance, than in the more complex DNIC process of pain modulation, but this requires further investigation.
Removing nonspecific effects from the DNIC effect is an important finding in this investigation; this was accomplished statistically by comparing 2 counterirritation protocols. Although this strategy differs from the traditional strategy, which compares pain ratings during a counterirritation protocol to pain ratings during a baseline protocol, it represents an important advancement in the study of DNIC in chronic pain disorders because it allows for the isolation of the endogenous analgesic mechanism effect on DNIC from nonspecific effects. Eliminating the confounds that can result from self-reported pain ratings can ideally be accomplished by direct observation of the physiologic mechanisms responsible for DNIC, such as alterations in functional magnetic resonance imaging activity of the caudal medulla,26 or alterations in the RIII reflex51 during pain testing. This would eliminate the subjectivity that results from pain reports and further improve upon our understanding of physiologic mechanisms that contribute to functional pain disorders.
In conclusion, the present study showed that IBS patients demonstrate compromised inhibitory regulation of phasic somatic pain stimuli. This disinhibition was independent of peripheral sensitization from the gut, cardiovascular reactivity, or psychologic mechanisms. Further investigation into central pain dysregulation in IBS may lead to improvements in diagnosis, and ultimately, to novel therapies for these patients.
References
- 1.Drossman D. Understanding the Irritable Gut: The Functional Gastrointestinal Disorders. McLean, VA: Degnon Associates, Inc; 2008. pp. 183–199. [Google Scholar]
- 2.Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14:125–132. doi: 10.1136/gut.14.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterol. 1990;98:1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 4.Mertz H, Naliboff B, Munakata J, et al. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterol. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 5.Chun A, Desautels S, Slivka A, et al. Visceral algesia in irritable bowel syndrome, fibromyalgia, and sphincter of oddi dysfunction, type III. Dig Dis Sci. 1999;44:631–636. doi: 10.1023/a:1026682113096. [DOI] [PubMed] [Google Scholar]
- 6.Bouin M, Delvaux M, Blanc C, et al. Intrarectal injection of glycerol induces hypersensitivity to rectal distension in healthy subjects without modifying rectal compliance. Eur J Gastroenterol Hepatol. 2001;13:573–580. doi: 10.1097/00042737-200105000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 8.Verne GN, Robinson ME, Vase L, et al. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues AC, Nicholas VG, Schmidt S, et al. Hypersensitivity to cutaneous thermal nociceptive stimuli in irritable bowel syndrome. Pain. 2005;115:5–11. doi: 10.1016/j.pain.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Diatchenko L, Nackley AG, Slade GD, et al. Idiopathic pain disorders—pathways of vulnerability. Pain. 2006;123:226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Staud R, Vierck CJ, Cannon RL, et al. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 13.Staud R, Robinson ME, Vierck CJ, Jr, et al. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–174. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 14.Maixner W, Fillingim R, Booker D, et al. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–351. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 15.Maixner W, Fillingim R, Sigurdsson A, et al. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 16.Kashima K, Rahman OI, Sakoda S, et al. Increased pain sensitivity of the upper extremities of TMD patients with myalgia to experimentally-evoked noxious stimulation: possibility of worsened endogenous opioid systems. Cranio. 1999;17:241–246. doi: 10.1080/08869634.1999.11746100. [DOI] [PubMed] [Google Scholar]
- 17.Sarlani E, Grace EG, Reynolds MA, et al. Evidence for upregulated central nociceptive processing in patients with masticatory myofascial pain. J Orofac Pain. 2004;18:41–55. [PubMed] [Google Scholar]
- 18.Edwards RR, Ness TJ, Weigent DA, et al. Individual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variables. Pain. 2003;106:427–437. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Staud R, Rodriguez ME. Mechanisms of disease: pain in fibromyalgia syndrome. Nat Clin Pract Rheumatol. 2006;2:90–98. doi: 10.1038/ncprheum0091. [DOI] [PubMed] [Google Scholar]
- 20.Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Curr Rheumatol Rep. 2002;4:322–328. doi: 10.1007/s11926-002-0041-x. [DOI] [PubMed] [Google Scholar]
- 21.Pielsticker A, Haag G, Zaudig M, et al. Impairment of pain inhibition in chronic tension-type headache. Pain. 2005;118:215–223. doi: 10.1016/j.pain.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Le Bars D, Villanueva L, Bouhassira D, et al. Diffuse noxious inhibitory controls (DNIC) in animals and in man. Patol Fiziol Eksp Ter. 1992;4:55–65. Review. [PubMed] [Google Scholar]
- 23.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 24.Guieu R, Serratrice G, Pouget J. Counter irritation test in primary fibromyalgia. Clin Rheumatol. 1994;13:605–610. doi: 10.1007/BF02243002. [DOI] [PubMed] [Google Scholar]
- 25.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 26.Wilder-Smith CH, Schindler D, Lovblad K, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinert A, Treede R, Bromm B. The pain inhibiting pain effect: an electrophysiological study in humans. Brain Res. 2000;862:103–110. doi: 10.1016/s0006-8993(00)02077-1. [DOI] [PubMed] [Google Scholar]
- 28.Bouin M, Lupien F, Riberdy M, et al. Intolerance to visceral distension in functional dyspepsia or irritable bowel syndrome: an organ specific defect or a pan intestinal dysregulation? Neurogastroenterol Motil. 2004;16:311–314. doi: 10.1111/j.1365-2982.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 29.Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterol. 1998;115:1263–1271. doi: 10.1016/s0016-5085(98)70099-x. [DOI] [PubMed] [Google Scholar]
- 30.Blomhoff S, Jacobsen MB, Spetalen S, et al. Perceptual hyperreactivity to auditory stimuli in patients with irritable bowel syndrome. Scand J Gastroenterol. 2000;35:583–589. doi: 10.1080/003655200750023534. [DOI] [PubMed] [Google Scholar]
- 31.Bruehl S, Chung OY, Burns JW, et al. The association between anger expression and chronic pain intensity: evidence for partial mediation by endogenous opioid dysfunction. Pain. 2003;106:317–324. doi: 10.1016/S0304-3959(03)00319-1. [DOI] [PubMed] [Google Scholar]
- 32.Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 33.France CR, France JL, al’Absi M, et al. Catastrophizing is related to pain ratings, but not nociceptive flexion reflex threshold. Pain. 2002;99:459–463. doi: 10.1016/s0304-3959(02)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granot M, Friedman M, Yarnitsky D, et al. Enhancement of the perception of systemic pain in women with vulvar vestibulitis. BJOG. 2002;109:863–866. doi: 10.1111/j.1471-0528.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 35.Fillingim RB, Maixner W. The influence of resting blood pressure and gender on pain responses. Psychosom Med. 1996;58:326–332. doi: 10.1097/00006842-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Maixner W, Fillingim R, Kincaid S, et al. Relationship between pain sensitivity and resting arterial blood pressure in patients with painful temporomandibular disorders. Psychosom Med. 1997;59:503–511. doi: 10.1097/00006842-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Drossman DA, Corazziari E, Talley NJ, et al. Rome II. The Functional Gastrointestinal Disorders. Diagnosis, Pathophysiology and Treatment: A Multinational Consensus. McLean, VA: Degnon Associates; 2000. [Google Scholar]
- 38.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 39.Spielberger CD, Reheiser EC, Sydeman SJ. Measuring the experience, expression, and control of anger. Issues Compr Pediatr Nurs. 1995;18:207–232. doi: 10.3109/01460869509087271. [DOI] [PubMed] [Google Scholar]
- 40.Beck AT. A systematic investigation of depression. Compr Psychiatry. 1961;2:163–170. doi: 10.1016/s0010-440x(61)80020-5. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 42.Hirsh AT, George SZ, Riley JL, III, et al. An evaluation of the measurement of pain catastrophizing by the coping strategies questionnaire. Eur J Pain. 2007;11:75–81. doi: 10.1016/j.ejpain.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 44.Palsson OS, Jones KR, Turner MJ, et al. Development of specific questionnaires to assess somatization and comorbid diagnoses in irritable bowel syndrome (IBS) Gastroenterology. 2002;122:A502. [Google Scholar]
- 45.Sigurdsson A, Maixner W. Effects of experimental and clinical noxious counterirritants on pain perception. Pain. 1994;57:265–275. doi: 10.1016/0304-3959(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 46.Willer JC, De BT, Le BD. Encoding of nociceptive thermal stimuli by diffuse noxious inhibitory controls in humans. J Neurophysiol. 1989;62:1028–1038. doi: 10.1152/jn.1989.62.5.1028. [DOI] [PubMed] [Google Scholar]
- 47.Willer JC, Bouhassira D, Le BD. Neurophysiological bases of the counterirritation phenomenon: diffuse control inhibitors induced by nociceptive stimulation. Neurophysiol Clin. 1999;29:379–400. doi: 10.1016/S0987-7053(00)87263-9. [DOI] [PubMed] [Google Scholar]
- 48.Longe SE, Wise R, Bantick S, et al. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. Neuroreport. 2001;12:2021–2025. doi: 10.1097/00001756-200107030-00047. [DOI] [PubMed] [Google Scholar]
- 49.Tracey I, Dunckley P. Importance of anti-and pro-nociceptive mechanisms in human disease. Gut. 2004;53:1553–1555. doi: 10.1136/gut.2004.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De BT, Cesaro P, Willer JC, et al. Diffuse noxious inhibitory controls in man. Involvement of the spinoreticular tract. Brain. 1990;113(Pt 4):1223–1234. doi: 10.1093/brain/113.4.1223. [DOI] [PubMed] [Google Scholar]
- 51.Sandrini G, Rossi P, Milanov I, et al. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia. 2006;26:782–789. doi: 10.1111/j.1468-2982.2006.01130.x. [DOI] [PubMed] [Google Scholar]