Abstract
Obesity is a common comorbidity in adults with mobility impairing neurological and musculoskeletal conditions, such as stroke and arthritis. The interaction between mobility impairments and environmental factors often compromises motivation and ability to engage in healthy behaviors. Such difficulties to engage in healthy behaviors can result in energy imbalance, weight gain, and a cycle of functional declines; i.e., obesity can exacerbate mobility impairments and symptoms and increase the likelihood of other comorbid conditions, all of which make it more difficult to engage in healthy behaviors. To help disrupt this cycle, there is a need to identify strategies to optimize energy balance. Thus, this review summarizes clinical trials of nutrition and weight loss interventions in adults with mobility impairing conditions. Although adults with osteoarthritis were represented in large rigorous clinical trials, adults with neurological conditions were typically represented in small feasibility studies characterized by a small number of participants, a short-term follow-up, and high attrition rates. Studies varied greatly in outcome measures, description and implementation of the interventions, and the strategies used to promote behavior change. Nutrition and weight loss research in adults with mobility impairing conditions is still in its formative stages and there is a substantial need to conduct randomized controlled trials.
Keywords: Disability, Nutrition, Nervous System Diseases, Musculoskeletal Diseases
Adults with mobility impairing neurological and musculoskeletal conditions, such as stroke and arthritis, are approximately two to four times more likely to be obese compared to adults without disabilities (1-3). The reciprocal relationship between obesity and impairments in neurological and musculoskeletal systems may help to explain the increased likelihood of obesity. Obesity is a risk factor in the diagnosis of several mobility impairing neurological and musculoskeletal conditions (4, 5). Furthermore, neurological and musculoskeletal impairments make it difficult to engage in healthy behaviors to achieve energy balance and prevent weight gain (1, 6). Regardless of whether obesity is a cause or a consequence of a neurological or musculoskeletal condition, it can initiate and accelerate a cycle of preventable functional declines; i.e., obesity can exacerbate mobility impairments and symptoms, as well as increase the likelihood of other comorbid conditions, such as diabetes and cardiovascular disease, all of which can make it more difficult to engage in healthy behaviors (7-10). Thus, there is a need to optimize energy balance in adults with mobility impairing neurological and musculoskeletal conditions to avoid and disrupt the cycle of preventable functional declines.
Obesity may accelerate disease processes and accentuate mobility impairments among adults with neurological and musculoskeletal conditions. Obesity is a pro-inflammatory state that might exacerbate inflammatory disease processes (11). For example, adipokines, which are cytokines secreted by adipose tissue, are associated with the pathogenesis of rheumatoid arthritis and multiple sclerosis (12-15). Obesity may also accentuate mobility impairments by increasing joint loads and pain as well as restricting range of motion (7). In a research sample of women with disabling neurological and musculoskeletal conditions, Nosek et al. (16) found that obesity was a prevalent problem and obesity was associated with disability severity and several comorbid conditions.
The interaction between obesity, mobility impairments, and environmental factors may also increase participation restrictions in life roles and can compromise confidence, motivation, and ability to engage in healthy behaviors (17). For example, the built environment, equipment, and polices of many recreational centers create accessibility barriers for adults with mobility impairments to engage in community-based exercise programs (18). Adults with mobility impairing conditions experience discrimination, restricted nutritional autonomy, and can have negative views about their body image, all of which may be compounded by obesity and lead to decreased confidence and ability to participate in life roles and healthy behaviors (19-24). In a qualitative study, Pain and Wiles (25) found that people with disabilities who were obese often felt discriminated against and experienced problems obtaining appropriate mobility devices and accessing community services.
Although adults with mobility impairing conditions need access to health promotion and wellness services, they often have limited opportunities to receive such services (17, 26-28). Physical, occupational, and dietary therapy is typically not a reimbursable healthcare service unless it is deemed medically necessary (29, 30). Healthcare services with the goal of tertiary prevention and health education are not readily accessible. Furthermore, adults with mobility impairing conditions often experience many barriers in accessing government programs that can disseminate relevant and appropriate health education (27, 31, 32). Difficulties in obtaining expert advice may make adults with mobility impairing conditions susceptible to misinformation claiming that a particular diet can cure or reduce symptom severity.
Given the need to support adults with mobility impairing conditions in achieving energy balance, it is important to review the existing research literature. Several recent review articles summarize health behavior interventions in adults with disabilities (33-37). However, these reviews typically focus on physical activity and/or the benefits of engaging in a particular health behavior; e.g., describing the benefits of exercise rather than strategies used to promote exercise adherence. Furthermore, many reviews are disease-specific and there are concerns about developing behavior change interventions independently of each other rather than building effective intervention strategies (38). Thus, there is a need to identify and describe empirically-tested behavior change techniques to support healthy eating habits and weight management across adults with different disabling conditions. Such a review may facilitate researchers and clinicians to utilize these techniques in clinical practice or to test and refine in research.
Therefore, the purpose of this review was to identify and summarize clinical trials of nutrition and weight loss interventions in adults with neurological and musculoskeletal conditions that characteristically result in mobility impairments. Specifically, we summarize the outcome measures used in these clinical trials and describe the delivery format and behavior change techniques used in the evaluated intervention. Describing the behavior change techniques used in nutrition and weight loss interventions is an important first step in understanding the “active ingredients” that produce the desired behavioral changes and resulting functional and quality of life outcomes. Because there are several reviews on physical activity interventions, we restricted our review to studies that evaluated behavior change interventions with a nutritional education component and included weight loss as an outcome measure.
Methods
Search strategies, study selection, and result reporting are described below according to PRISMA (39). We used Coventry Aberdeen LOndon – REfined (CALO-RE) taxonomy to describe the behavior change techniques used in the interventions (40). The taxonomy defines 40 behavior change techniques (e.g., goal-setting, using a role model, and enlisting social support) commonly used in intervention research (41). The CALO-RE is based on the Abraham and Michie's taxonomy, which was found to have good consistency between coders and between intervention manuals and research articles (41).
Eligibility criteria
We applied the following inclusion criteria: (a) clinical trials of interventions that incorporated educational topics on nutrition; (b) the study included community-dwelling adults with chronic neurological or musculoskeletal conditions that characteristically result in lower-extremity mobility impairments (e.g., multiple sclerosis, stroke, spinal cord injury, arthritis, lupus, cerebral palsy, and spina bifida); (c) body weight was used as an outcome measure; (d) the study was described in the English language and published between 1980 and 2013.
Exclusion criteria were: (a) case studies or studies with less than 11 research participants, conference proceedings and abstracts, review articles that described ongoing research, observational/secondary data analysis studies that identify risk factors for disease, or studies exploring body mass index as a moderator of an intervention not focused on nutrition or weight loss; (b) medications, supplements, gastric tube feeding, or surgical interventions; (c) interventions designed to promote weight gain or prevent sarcopenia; (d) patients living in a long-term care facility or the study had a primary inclusion criterion of childhood age or having a diagnosis of cardiovascular disease, idiopathic/non-specific signs and symptoms (e.g., chronic pain), developmental disability not characterized by mobility impairments (e.g., excluding autism, intellectual disability, and Down syndrome, while including spina bifida and cerebral palsy), cancer, endocrine disease, mental health disorders, epilepsy, or Alzheimer's disease; (e) inadequate description of how the intervention promoted behavior change (i.e., operationally defined as coding at least two behavior change techniques from the CALO-RE taxonomy, which was intended to exclude interventions that were coded as using only the behavior change technique of instruction).
Information sources & search
We used multiple search strategies to identify studies. We first searched PubMed, Scopus, CINHAL, and PsycINFO using the following MeSH and/or subject terms: disabilities, physical conditions, musculoskeletal conditions, and nervous system conditions. We then combined these terms with the following MeSH and/or subject terms: diet, overweight, nutrition, eating behavior, body weight, nutrition therapy, nutrition support, nutritional status, nutrition assessment, weight reduction programs, weight control, obesity, weight loss, weight gain, and appetite. The search was limited to English and human adults ≥ 18 years of age. An additional search in Google Scholar and PubMed was performed by using the terms nutrition, weight, intervention, or education and combing them with the following search phrases and names of specific conditions: mobility, autoimmune disease, neuromuscular, arthritis, cerebral palsy, amputation, fracture, fibromyalgia, spina bifida, spinal cord injury, traumatic brain injury, polio, stroke, lupus, muscular dystrophy, multiple sclerosis, or Parkinson. We also searched the reference list of relevant review articles and the following journals were hand-searched: Journal of Nutrition, Journal of the Academy of Nutrition and Dietetics, British Journal of Nutrition, Disability Studies Quarterly, Disability and Health Journal, and American Journal of Preventive Medicine.
Study selection
For all retrieved studies, citations and abstracts were downloaded to EndNote and duplications were removed. The search procedure was divided into two phases: (1) title and abstract review and (2) full-text article review. For the first phase, we scanned titles and abstracts in each database to identify any potential study that could meet inclusion-exclusion criteria. After this preliminary search, we scanned the abstracts of articles to exclude reviews, conference proceedings, case studies, studies published before 1980, and studies on children, healthy adults, and individuals who did not have mobility impairing conditions as defined above. For the second phase of review, we scanned the articles in detail, coded behavior change techniques, and excluded studies that did not meet any remaining criteria.
Data collection process, data items, & synthesis
For the remaining pool of articles, sample characteristics (e.g., gender, race, education, and disability level), type of research design, outcome measures, and intervention characteristics (e.g., length of intervention, number of contacts, delivery format, guiding intervention theory, and/or framework) were extracted from the articles. By using simple pooling across studies, mean amount of weight loss and other characteristics of the research sample and intervention were calculated (e.g., mean estimates were not weighted by sample size). The first author and a graduate assistant extracted all data and coded behavior change techniques independently. Behavior change techniques were compared and tallied across studies. Disagreements in coding were discussed until consensus was reached.
Results
Study selection
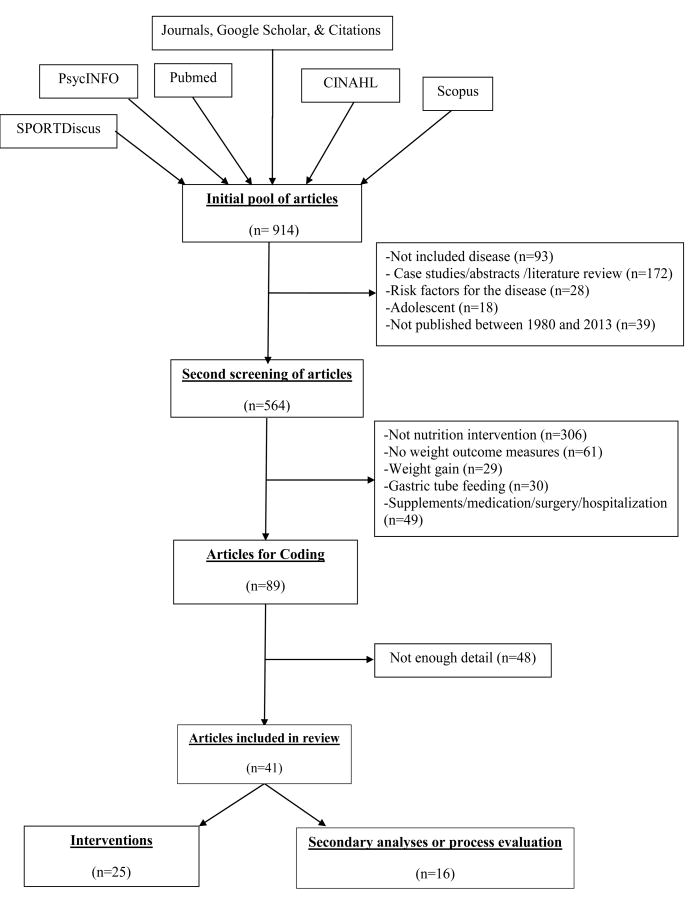
We had an initial pool of 914 articles. Of these, we excluded 350 articles during the first phase of review (see Figure 1). Many articles were excluded due to being literature reviews, observational studies on risk factors for diseases, or did not include adults with mobility impairing neurological and musculoskeletal conditions. In the second phase of review, we excluded 475 articles, as they did not describe the implementation of an intervention, or were medication, surgical, or supplementation interventions, or focused on gastric tube feeding. An additional 48 articles were excluded for not providing enough detail on how behavior change was encouraged. The remaining 41 articles described 25 interventions; 16 of the articles described a secondary/process evaluation of the intervention.
Figure 1. Flow of research articles.
Study characteristics
Table 1 provides a summary of the characteristics of the 25 research samples. The 25 research samples had 2490 community-dwelling adults with neurological and musculoskeletal conditions; 65% were female and the mean age was 56.5 years. Most studies had research samples (n=20) with a mean age of greater than 50 and five research samples had a mean age greater than 65. Twelve studies reported on the race/ethnicity (two studies > 80% racial minorities and eight studies < 30% racial minorities). There were 165 adults with neurological conditions (stroke, n=44; spinal cord injury, n=53; multiple sclerosis, n=37; polio, n=11; spina bifida, n=13; cerebral palsy, n=7) and 1643 adults with osteoarthritis, 505 adults with rheumatoid arthritis, 125 adults with fibromyalgia, and 41 adults with lupus.
Table 1. Summary of included studies and intervention evaluated.
| N | Condition | Age | Design | Intervention type | Intervention Format | #BCT | Main benefits/conclusions | |
|---|---|---|---|---|---|---|---|---|
| ADAPT trial (42-51) | 316 | OA | 69 | RCT | WL | Group, one-to-one, phone | 12 | Weight loss, physical function, OA symptoms, cost-effective |
| PACT trial (52-54) | 87 | OA | 70 | RCT | WL | Group, one-to-one | 9 | Weight loss, physical function |
| IDEA trial (55, 56) | 454 | OA | 66 | RCT | WL | Group, one-to-one, phone | 13 | Weight loss, reduced inflammation |
| Chen (57) | 16 | SCI | 44 | Pre/post | WL | Group | 11 | Weight loss |
| Davies (58) | 23 | Lupus | 46 | RCT | WL | Phone | 3 | Weight loss, fatigue |
| Hansen (59) | 109 | RA | 57 | RCT | Nutri: Anti-inflam | Face-to-face | 3 | Symptoms |
| Hoffman (60) | 11 | Polio | 54 | NRCT | Wellness | Group | 4 | Diet |
| John (61, 62) | 110 | RA | 61 | RCT | Risk reduction | Group | 17 | Reduce cardiovascular risk |
| Martin (63) | 48 | OA | 61 | Pre/post | WL | Group | 2 | Weight loss, physical function, pain |
| McDougal (64) | 24 | RA | 56 | Pre/post | Nutri: Vegan | Face-to-face | 3 | Symptoms, diet |
| McKellar (65) | 130 | RA | 54 | NRCT | Nutri: Med | Group | 3 | Symptoms, diet |
| Messier (66) | 24 | OA | 68 | RCT | WL | Group, one-to-one | 6 | Weight loss, symptoms, gait |
| Nenonen (67) | 43 | RA | 52 | RCT | Nutri: Vegan | Face-to-face | 4 | Symptoms |
| Paans (68) | 35 | OA | 57 | Pre/post | WL | Group, one-to-one, phone | 12 | Physical function, pain |
| Panush (69) | 33 | RA | 55 | RCT | Nutri: Anti-inflam | One-to-one | 5 | No effect |
| Radomski (70) | 13 | SCI | 53 | Pre/post | Wellness & WL | Group, one-to-one | 6 | Feasible, Goal Attainment Scaling |
| Ravaud (71) | 336 | OA | 64 | RCT | WL | One-to-one | 4 | Weight loss, symptoms |
| Rimmer (72) | 35 | Stroke | 53 | RCT | Wellness | Group | 9 | Weight loss, QOL, physical function |
| Rimmer (73) | 102 | Cross | 47 | RCT | WL | Phone | 11 | Weight loss |
| Senna (74) | 83 | Fibro | 46 | RCT | WL | Face-to-face | 3 | Weight loss, QOL, symptoms |
| Shah (75-77) | 17 | Lupus | 45 | RCT | WL | Group, phone | 12 | Weight loss, diet, and QOL |
| Shapiro (78) | 42 | Fibro | 54 | Pre/post | WL | Group | 4 | Weight loss, QOL, symptoms |
| Skoldstam (79, 80) | 56 | RA | 58 | RCT | Nutri: Med | Group, one-to-one, phone | 4 | Symptoms, physical function, vitality |
| Somers (81) | 232 | OA | 58 | RCT | WL | Group, phone | 13 | Weight loss, physical function, symptoms |
| Wolf (82) | 111 | OA | 68 | RCT | WL | Group | 8 | Weight loss |
Key: OA = osteoarthritis; RA = Rheumatoid arthritis; SCI = spinal cord injury; RCT = Randomized Controlled Trial; NRCT = Non-randomized Controlled Trial; WL= weight loss; Nutri = nutrition; Anti-inflam = Anti-inflammatory; Med = Mediterranean; #BCT = Number of behavior change techniques used in the intervention; QOL = quality of life
There was little consistency on how health and function were characterized among the different research samples. However, some characteristics can be derived from study criteria. For example, seven studies excluded individuals for not being able to walk, and nine studies excluded individuals because their disease status was assessed as being too severe. Twelve studies tried to avoid ceiling effects by excluding individuals whose disease status or symptom impact was assessed as being only minor. Fifteen research studies had a criterion of being overweight or obese, (i.e., body mass index ≥ 25).
Research design and outcomes
Seventeen interventions were evaluated using a randomized controlled trial, while two other trials used a control group but did not randomize participants. Six interventions were evaluated using a pre-posttest design without a control group. One intervention was reported as being assessed using a double-blind research design, and ten interventions were reported as being assessed with a single-blind research design. Ten studies conducted a power analysis to determine the appropriate sample size, and seven studies conducting an intent-to-treat analysis. Mean attrition rate across all studies was 17.3%, with six studies reporting attrition rates greater than 25%.
The timing of when outcome measures were administered in relation to the first pretest assessment varied greatly. The first posttest assessment ranged from 3 weeks to 36 weeks (average 15 weeks) after the first pretest assessment. Thirteen studies included more than two posttest assessments. The second posttest assessment ranged from 12 weeks to 104 weeks (average 38 weeks) after the first pretest assessment. Five studies included assessment time points after intervention contacts had completely ceased.
Synthesis of results
Mean weight loss across interventions (n=23) was − 4.11 kg (two studies reported changes in BMI only). Nine studies included measures of percent body fat using dual-energy x-ray absorptiometry, skin fold calipers, or air displacement plethysmography. Outcome measures used to examine the potential beneficial effects of weight loss or a particular type of diet included patient-reported physical function (n=18), biomarkers (n=18), pain (n=17), engagement in healthy behaviors (n=16), patient-reported mental health (n=13), and provider-reported/objective outcomes of physical function (n=11). Fewer studies used outcome measures of social function (n=8), fatigue (n=7), and objective cognitive assessments (n=1). Only one study examined cost-effectiveness.
Intervention effects included statistically significant improvements across time or between groups in weight (n=21), patient-reported physical function (n=17), pain (n=16), biomarkers (n=14), engagement in healthy behaviors (n=12), and provider-reported/objective measures of physical function (n=7). Fewer studies reported statistically significant improvements in mental function (n=7), fatigue (n=5), and social function (n=6). Three studies reported that participants experienced adverse events serious enough to cause them to withdraw from the study. Twelve studies conducted analyses to identify mechanisms of action (i.e., explaining changes in outcomes or identifying sub-groups that responded differently to the intervention); two studies explored mechanisms of behavior change (e.g., exploring changes in self-efficacy); 11 studies explored mechanisms for improving impairments in body functions and structures (e.g., symptoms, body weight, biomarkers, etc.), and three studies explored mechanisms for improving function in daily activities.
Description of intervention
Nine interventions included only topics on nutrition; sixteen interventions included topics on nutrition and physical activity, and seven interventions included topics on symptom self-management. The most common delivery formats were face-to-face contacts (n=23) either in a group (n=17) or one-to-one instruction (n=10), and some using both forms of contact (n=7). Two interventions primarily used distance education approaches, i.e., phone. Five interventions used a combination of distance education approaches and face-to-face contact. The length of intervention ranged from 4 to 72 weeks (including follow-up visits). The number of contacts the participants had with the interventionists ranged from 3 to 216 contacts. One intervention modified/tailored the number of contacts based on the participants' needs, and one intervention had an email/call in-service to answer participants' questions when needed. The most common health professionals that delivered the interventions were registered dieticians/nutritionists (n=15), followed by exercise physiologists (n=10), non-licensed education specialists (n=7), psychologists (n=4), and physicians (n=3).
Behavior change techniques
Table 2 summarizes the frequency counts of the behavior change techniques used across interventions. Six interventions were described as being based on a behavior change theory (e.g., social cognitive theory). The number of behavior change techniques used within a single intervention ranged from 2 to 17 techniques, with 14 interventions incorporating five or more behavior change techniques. The most common behavior change technique employed was presenting instructive information (n=25), followed by self-monitoring of behavior (n=21), modeling or demonstrating the behavior (n=13), presenting feedback about performance (n=13), modeling (n=13), problem solving/barrier identification (n=12), self-monitoring of outcomes (n=10), and restructuring the environment (n=9). Action planning (n=1), time management (n=0), prompting social comparisons (n=1), communication skills (n=2), and rewarding participants based on effort (n=0) or success (n=1) were infrequently applied behavior change techniques.
Table 2. Frequency of behavior change technique used across all included interventions.
| Behavior change technique | Frequency |
|---|---|
| Instruction | 25 |
| Self-monitoring of behavior | 21 |
| Modeling (showing) | 13 |
| Feedback about performance | 13 |
| Problem solving/barrier identification | 12 |
| Self-monitoring of outcomes | 10 |
| Environmental restructuring | 9 |
| Relapse prevention | 8 |
| Follow-up prompts | 7 |
| Outcome goal setting | 7 |
| Process goal setting | 7 |
| Training in emotional management | 7 |
| Information about general consequences | 6 |
| Galvanize social support | 6 |
Discussion
Summary of evidence
Although adults with knee osteoarthritis were represented in large rigorous clinical trials (42-56), adults with systemic musculoskeletal conditions and neurological conditions were represented in smaller feasibility studies. These studies were typically conducted in a small number of participants, had a short-term follow-up, and were limited by attrition rates or non-compliers. Only two studies explored mechanisms of behavior change, and intervention descriptions often lacked sufficient detail to implement the intervention within clinical practice or improve upon it in research. Thus, our review highlights a need for fully-powered randomized controlled trials that examine and identify clearly-described and theoretically-based nutrition and weight loss interventions in adults with mobility impairing neurological and musculoskeletal conditions.
Delivery formats, intervention topics, outcome measures, and the combination of behavior change techniques implemented varied across studies depending on the research paradigm used. Several included interventions (n=11) were developed and tested using a traditional biomedical research paradigm. In such biomedical studies, participants were asked to adhere to a particular diet and/or exercise program using two or three behavior change techniques, and the intervention was evaluated with outcomes of disease severity and physical function. Some interventions were developed and tested using a patient-centered, biopsychosocial paradigm (45, 62, 72, 73, 77). In such biopsychosocial studies, participants collaborated with the interventionist to develop individualized diet and/or exercise programs employing multiple behavior change techniques, and the intervention was evaluated with outcomes of disease severity as well as physical, mental, and/or social function. Below we summarize findings in weight loss outcomes and the similarities and differences across the included studies in their research design and intervention format and dose as well as provide suggestions for future research.
Weight loss outcomes
Our findings that 21 studies reported statistically significant improvement in weight loss across time or between groups and that average weight loss across interventions was -4.11 kg should be interpreted with caution. Small sample sizes, high attrition rates, failure to conduct intent-to-treat analyses, maturation, and selection effects could have all been possible threats to validity (83). Nonetheless, given the higher rates of inactivity and increased barriers for eating healthy among adults with disabling conditions, it is possible that small improvements in lifestyle behaviors could result in significant short-term weight loss. However, research in the general population indicates that including only short-term follow-ups in weight loss studies can be misleading and that weight gain is likely once intervention contacts cease (84, 85).
Few included studies incorporated comprehensive measures of body composition. Measurements of lean body mass and percent body fat might be particularly important to include in studies of weight loss interventions among adults with neurological and musculoskeletal conditions. Such measures are needed to help explain findings of the obesity paradox in observational studies (86) and further examine whether weight loss and associated declines in muscle mass results in decreased physical function among adults with disabling conditions who may already have problems with sarcopenia. Furthermore, using percent body fat as a study inclusion criterion rather than body mass index may be more suitable in populations with muscle atrophy (17). Whether it is appropriate to use weight loss as a primary outcome in clinical trials rather than the desired outcome of increased muscle mass and decreased percent body fat needs to be explored among adults with mobility impairing neurological and musculoskeletal conditions.
Research design
Participants
More studies in our review focused on adults with musculoskeletal conditions than on adults with neurological conditions. In particular, 86% of the included research samples (n= 2,490) were in adults with arthritis. Perhaps this is because several observational studies indicate that obesity is associated with pain severity (87), whereas the adverse effects of obesity in adults with neurological conditions are not as well documented. Nonetheless, adults with multiple sclerosis, for example, have a similar life expectancy as the general population and therefore have a similar need to reduce cardiovascular risks as the general population (88). Stroke survivors, for example, have a substantial risk for a secondary stroke and therefore have a need to lose weight to reduce that risk (89). Thus, there is a need to develop and test nutrition and weight loss interventions in adults with neurological conditions. We further note that only one study (74) in this review included participants with different disabling conditions, which may help facilitate the translation of the intervention into healthcare systems (90). Thus, there is a need to examine weight loss interventions that include adults with different disabling conditions.
Outcomes in response to diet and weight loss
The most common outcomes that were incorporated to examine the possible beneficial effects of weight loss or a particular diet were indicators of physical function and pain severity and biomarkers. Few studies incorporated comprehensive outcome measures of social function or participation in life roles, which is arguably the most important outcome for adults with disabling conditions (91). The effects of weight loss or a particular diet on symptom severity, physical function, and social function and underlying biological mechanisms of improvement are important relationships to identify and merit further research. Fortunately, there are a growing number of research studies that are using animal models to understand the biological mechanisms between nutrition and disease processes, which might facilitate translational nutrition and weight loss research in adults with neurological and musculoskeletal conditions (92-94).
Types of Interventions
Nutrition interventions
Nine studies included in our review explored the efficacy of a particular diet to reduce symptom severity and improve function. The specific diets examined varied based on the etiology and pathology of the disabling condition. For example, studies in patients with arthritic conditions focused on anti-inflammatory and vegetarian diets. These studies took a traditional biomedical approach to prescribing the diet program and often were vague in describing the strategies used to promote adherence (59, 64, 65, 67, 69, 79, 80). Furthermore, most of the studies that examined a particular type of diet were initially excluded for lack of detail on how behavior change was encouraged. To reduce attrition and encourage adherence, future research should use a biopsychosocial research paradigm to develop interventions that strike a balance between prescribing the diet as intended and accommodating food preferences and individual circumstances. Behavior change theories could be used to guide the selection of strategies to facilitate adherence. Such research is needed for generating evidence-based guidelines on recommending a specific diet to slow disease progression or reduce symptom severity.
Weight loss interventions
Studies in this review that evaluated weight loss interventions (n=16), often with the goal of examining the effects of weight loss on symptom severity and function, varied in degree of patient-centeredness and consideration for food preferences and individual circumstances. Some studies prescribed a very regimented/structured diet that focused on caloric restriction. Although some of these diets were reported as being efficacious on reducing symptom severity and/or improving mobility (52-54, 58, 63), it is unclear whether individuals can adhere to the diet in the long-term. Frequent intervention contacts over a long period is likely needed to promote adherence, which might be cost prohibitive.
Alternatively, there were patient-centered weight loss interventions that incorporated multiple behavior change techniques or were developed with the input of stakeholders (e.g., patients and caregivers) to reduce accessibility barriers (43, 73, 75, 78, 81). Research participants received guidelines to reduce caloric intake that took into account individual circumstances, such as food and physical activity preferences and functional status. Nonetheless, many of these studies appeared to take a “shotgun” approach to implementing behavior change techniques, i.e., implementing multiple techniques with the goal of addressing at least some of the participants' needs (95). Applying the multiphase optimization strategy (MOST) (96) could reduce the need for using the shotgun approach. MOST is a framework to develop multicomponent behavior change interventions by comparing each component of the intervention in a randomized controlled factorial research design.
Health promotion and wellness interventions
Three of the included studies were framed as evaluating health promotion or wellness interventions (60, 70, 72). Rimmer et al. (72) described implementing a health-promotion intervention that included a range of topics from nutrition and physical activity to self-care and stress management. Radomski et al. (70) described implementing both a wellness and weight management program for adults with spinal cord injury. Future research will need to explore how to best frame interventions for adults with disabling conditions. Although theoretical differences between weight loss, wellness, and self-management interventions exist, these interventions typically encourage engagement in healthy behaviors associated with achieving energy balance. Nonetheless, how the intervention is framed may have implications for the types of participants that are enrolled in the study and how many respond positively to the intervention. For example, an individual might be inclined to participate in a study with an emphasis on achieving wellness or promoting the idea of being “healthy at every size” but not in a study with emphasis on achieving weight loss (97). Future research should compare the effectiveness of how interventions are framed in relation to participants' psychosocial characteristics.
Intervention format and strategies
Dosing
The lengths of the intervention and number of contacts with the interventionist varied greatly across the studies. Thus, research will need to establish optimal dosing that is cost-effective and promotes long-term behavior change. Optimal dosing might be dependent on the characteristics of the participants. For instance, those with more functional limitations and/or who experience more barriers to healthy behavior engagement might benefit from more frequent contacts with the interventionist. A systematic review of weight loss interventions in the general population indicates that a greater number of contacts are associated with better outcomes, but the identification of an optimal, cost-effective number of contacts remains elusive (98).
Delivery format
Interventions were typically delivered by a licensed health professional face-to-face either in-group or one-to-one visits. A group format might offer opportunities for participants to interact with each other and provide each other with support and advice, while a one-to-one format could afford opportunities to better tailor information to the participants' needs, preferences, and unique barriers (99). Some studies included both one-to-one and group instruction (43, 52, 79), which provides opportunities for both group interactions and individual tailoring of information. Only two of the interventions focused on using distance learning strategies (e.g., phone) to promote behavior change (58, 73). Given that people with disabling conditions often cite costs and transportation difficulties as major barriers to accessing health and wellness services (100), future research should examine low-cost distance learning approaches.
Behavior change techniques
The most common combination of behavior change techniques applied in the included interventions was instruction and self-monitoring. Dombrowski et al. (101) found in a review of weight loss interventions among adults with heart failure, breast cancer, binge eating disorder, coronary heart disease, and cardiovascular disease that instruction and self-monitoring were associated with better adherence outcomes. Instruction and self-monitoring might be two techniques to address the “how to adhere” question that adults with disabling conditions frequently ask. Research indicates that adults with disabling conditions often express concerns about not knowing how to engage in healthy behaviors on a regular basis (20, 100). Thus, instruction and self-monitoring might be strategies for initiating behavior change.
Surprisingly, few of the included interventions incorporated the behavior change techniques of training in communication skills, emotional regulation, time management, and action planning. These behavior change techniques seem to be particularly relevant to adults with disabling conditions. Lorig et al. (102) note that communication skills, emotional regulation, and action planning (i.e., setting goals and anticipating and planning to overcome barriers) are necessary skills to develop to effectively self-manage chronic conditions. Being unable to communicate needs to caregivers, ineffectively coping with emotions, and poor problem-solving skills may also contribute to obesity (103, 104). Thus, the utility of incorporating Lorig et al.'s framework into weight loss interventions for adults with disabling conditions should be explored.
Limitations
Limitations to this review include the inability to code interventions using the actual intervention manuals of each study, and not conducting a meta-analysis. Because we felt that it would not be possible to obtain all intervention manuals for the included studies (e.g., authors may not have developed a manual or their unwillingness to share their manual), we decided not to code intervention manuals. We felt there would have been inconsistencies between coding of interventions that had a manual and those that did not, which would make comparisons across interventions difficult. Because we did not code intervention manuals, there may have been strategies implemented in the interventions that we did not code, or the authors may not have been explicit. We decided not to conduct a meta-analysis because many of the included studies were not randomized controlled trials and had high attrition rates, leading to biases or inflations in effect size calculations. Conducting a meta-analysis with only the randomized controlled trials that had minimal risk of bias would have restricted our review primarily to adults with arthritis, which was not the intended purpose of this review. Furthermore, we excluded many articles for not providing enough detail about the intervention. An un-biased estimate of weight loss across randomized controlled trials in adults with mobility impairing conditions would need to consider these articles for inclusion. We also decided not to conduct a quantitative or qualitative assessment of risk in accordance with PRISMA guidelines because it was clear that many of the included articles described pilot studies to demonstrate feasibility for subsequent studies that focus on minimizing threats to validity.
Conclusions
In order to address the obesity epidemic, it will be important to target those that are most at risk for obesity - adults with disabling conditions. The aging baby boomer generation and the increasing rates of obesity in young adulthood will substantially escalate the proportion of the population with disabling conditions (1-3, 24). Thus, there is an urgent need not only to support healthy young adults in achieving energy balance (i.e., primary prevention), but also support adults with disabling conditions to achieve energy balance and maintain healthy lifestyle habits (i.e., tertiary prevention). However, compared to research on weight loss interventions in the general population, research on weight loss interventions in adults with mobility impairing neurological and musculoskeletal conditions is still in its formative stages.
Indeed, there is a substantial need to draw upon strategies developed for weight loss interventions in the general public and examine their effectiveness and relevance in adults with mobility impairing conditions. Too often, rehabilitation research in adults with mobility impairing conditions focuses only on exercise and increasing physical activity levels, which is consistent with the medical model of disability. However, preventing and treating obesity will require changing nutritional habits. Rehabilitation professionals frequently interact with adults with mobility impairing conditions and thus have many opportunities to not only promote exercise adherence, but also, at the very least, begin the discussion about eating healthy while living with a mobility impairment.
Acknowledgments
This work was supported through the National Institute of Nursing Research of the National Institutes of Health (NIH) under award number K01NR012975. The information presented in this article does not necessarily reflect the position, ideas, or opinions of NIH.
Footnotes
Conflict of interests: none
Contributor Information
Matthew A. Plow, Frances Payne Bolton School of Nursing Case Western Reserve University.
Shirley Moore, Frances Payne Bolton School of Nursing Case Western Reserve University.
Elaine Husni, Department of Rheumatic and Immunologic Diseases, Cleveland Clinic.
John P. Kirwan, Department of Pathobiology, Cleveland Clinic Lerner Research Institute.
References
- 1.Liou TH, Pi-Sunyer FX, Laferrere B. Physical disability and obesity. Nutr Rev. 2005;63:321–331. doi: 10.1111/j.1753-4887.2005.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 2.Rimmer JH, Wang E. Obesity prevalence among a group of Chicago residents with disabilities. Arch Phys Med Rehabil. 2005;86:1461–1464. doi: 10.1016/j.apmr.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Weil E, Wachterman M, McCarthy EP, et al. Obesity among adults with disabling conditions. JAMA. 2002;288:1265–1268. doi: 10.1001/jama.288.10.1265. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Farooqui AA, Farooqui T, Panza F, Frisardi V. Metabolic syndrome as a risk factor for neurological disorders. Cell Mol Life Sci. 2012;69:741–762. doi: 10.1007/s00018-011-0840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rimmer J, Braddock D. Health promotion for people with physical, cognitive, and sensory disabilities: an emerging national priority. Am J Health Promot. 2002;16:220–224. doi: 10.4278/0890-1171-16.4.220. [DOI] [PubMed] [Google Scholar]
- 7.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes rev. 2010;11:568–579. doi: 10.1111/j.1467-789X.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 8.Rimmer JH. Exercise and physical activity in persons aging with a physical disability. Phys Med Rehabil Clin N Am. 2005;16:41–56. doi: 10.1016/j.pmr.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Gariepy G, Wang J, Lesage A, Schmitz N. The interaction of obesity and psychological distress on disability. Soc Psychiatry Psychiatr Epidemiol. 2010;45:531–540. doi: 10.1007/s00127-009-0090-9. [DOI] [PubMed] [Google Scholar]
- 10.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 11.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 12.Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheum. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 13.Matarese G, Procaccini C, De Rosa V. The intricate interface between immune and metabolic regulation: a role for leptin in the pathogenesis of multiple sclerosis? J Leukoc Biol. 2008;84:893–899. doi: 10.1189/jlb.0108022. [DOI] [PubMed] [Google Scholar]
- 14.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Kitas GD. Obesity in rheumatoid arthritis. Rheumatol. 2011;50:450–462. doi: 10.1093/rheumatology/keq266. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira SR, Colado Simão AN, Kallaur AP, et al. Disability in patients with multiple sclerosis: influence of insulin resistance, adiposity, and oxidative stress. Nutr. 2014;30:268–273. doi: 10.1016/j.nut.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Nosek MA, Robinson-Whelen S, Hughes RB, et al. Overweight and obesity in women with physical disabilities: associations with demographic and disability characteristics and secondary conditions. Disabil Health J. 2008;1:89–98. doi: 10.1016/j.dhjo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Rimmer J, Wang E, Yamaki K, Davis B. Documenting disparities in obesity and disability. Focus: A Publication of the National Center for the Dissemination of Disability Research. 2010;24 [Google Scholar]
- 18.Rimmer JH, Riley B, Wang E, Rauworth A. Accessibility of health clubs for people with mobility disabilities and visual impairments. Am J Public Health. 2005;95:2022–2028. doi: 10.2105/AJPH.2004.051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17:941–964. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- 20.Plow M, Finlayson M. A qualitative study of nutritional behaviors in adults with multiple sclerosis. J Neurosci Nurs. 2012;44:337–350. doi: 10.1097/JNN.0b013e3182682f9b. [DOI] [PubMed] [Google Scholar]
- 21.Payette H. Nutrition as a determinant of functional autonomy and quality of life in aging: a research program. Can J Physiol Pharmacol. 2005;83:1061–1070. doi: 10.1139/y05-086. [DOI] [PubMed] [Google Scholar]
- 22.Webber CB, Sobal J, Dollahite JS. Physical disabilities and food access among limited resource households. Disabil Stud Q. 2007;27 [Google Scholar]
- 23.Carr D, Friedman MA. Is obesity stigmatizing? body weight, perceived discrimination, and psychological well-being in the united states. J Health Soc Behav. 2005;46:244–259. doi: 10.1177/002214650504600303. [DOI] [PubMed] [Google Scholar]
- 24.Iezzoni LI, Freedman VA. Turning the disability tide: the importance of definitions. JAMA. 2008;299:332–334. doi: 10.1001/jama.299.3.332. [DOI] [PubMed] [Google Scholar]
- 25.Pain H, Wiles R. The experience of being disabled and obese. Disabil Rehabil. 2006;28:1211–1220. doi: 10.1080/09638280600554561. [DOI] [PubMed] [Google Scholar]
- 26.Kroll T, Jones GC, Kehn M, Neri MT. Barriers and strategies affecting the utilisation of primary preventive services for people with physical disabilities: a qualitative inquiry. Health & Soc Care Comm. 2006;14:284–293. doi: 10.1111/j.1365-2524.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Sinnett S, Bengle R, Johnson MA, Brown A. Unmet needs for the Older Americans Act nutrition program. J Appl Gerontol. 2011;30:587–606. [Google Scholar]
- 28.Plow M, Cho C, Finlayson M. Utilization of health promotion and wellness services among middle-aged and older adults with multiple sclerosis in the mid-west US. Health Prom Int. 2010;25:318–330. doi: 10.1093/heapro/daq023. [DOI] [PubMed] [Google Scholar]
- 29.Nelms M, Sucher K, Roth SL. Nutrition therapy and pathophysiology. 2nd. Belmont, CA: Cengage Learning; 2011. [Google Scholar]
- 30.Clohan DB, Durkin EM, Hammel J, et al. Postacute rehabilitation research and policy recommendations. Arch Phys Med Rehabil. 2007;88:1535–1541. doi: 10.1016/j.apmr.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Odette F, Israel P, Li A, et al. Barriers to wellness activities for Canadian women with physical disabilities. Health Care Women Int. 2003;24:125–134. doi: 10.1080/07399330390170105. [DOI] [PubMed] [Google Scholar]
- 32.Hall L, Colantonio A, Yoshida K. Barriers to nutrition as a health promotion practice for women with disabilities. Int J Rehabil Res. 2003;26:245–247. doi: 10.1097/00004356-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Conn VS, Hafdahl AR, Minor MA, Nielsen PJ. Physical activity interventions among adults with arthritis: meta-analysis of outcomes. Semin Arthritis Rheum. 2008;37:307–316. doi: 10.1016/j.semarthrit.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 34.English C, Manns PJ, Tucak C, Bernhardt J. Physical activity and sedentary behaviors in community-dwelling stroke survivors: a systematic review. Phys Ther. 2013 doi: 10.2522/ptj.20130175. [DOI] [PubMed] [Google Scholar]
- 35.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11:459–463. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- 36.Hicks A, Ginis KM, Pelletier C, Ditor D, Foulon B, Wolfe D. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic review. Spinal Cord. 2011;49:1103–1127. doi: 10.1038/sc.2011.62. [DOI] [PubMed] [Google Scholar]
- 37.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 38.Stuifbergen AK. Building health promotion interventions for persons with chronic disabling conditions. Fam Community Health. 2006;29:28S–34S. doi: 10.1097/00003727-200601001-00006. [DOI] [PubMed] [Google Scholar]
- 39.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS medicine. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011;26:1479–1498. doi: 10.1080/08870446.2010.540664. [DOI] [PubMed] [Google Scholar]
- 41.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008;27:379–387. doi: 10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- 42.Rejeski WJ, Focht BC, Messier SP, Morgan T, Pahor M, Penninx B. Obese, older adults with knee osteoarthritis: weight loss, exercise, and quality of life. Health Psychol. 2002;21:419–426. doi: 10.1037//0278-6133.21.5.419. [DOI] [PubMed] [Google Scholar]
- 43.Miller GD, Rejeski WJ, Williamson JD, et al. The Arthritis, Diet and Activity Promotion Trial (ADAPT): design, rationale, and baseline results. Control Clin Trials. 2003;24:462–480. doi: 10.1016/s0197-2456(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 44.Penninx BW, Abbas H, Ambrosius W, et al. Inflammatory markers and physical function among older adults with knee osteoarthritis. J Rheumatol. 2004;31:2027–2031. [PubMed] [Google Scholar]
- 45.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 46.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 47.Focht BC, Rejeski WJ, Ambrosius WT, Katula JA, Messier SP. Exercise, self-efficacy, and mobility performance in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;53:659–665. doi: 10.1002/art.21466. [DOI] [PubMed] [Google Scholar]
- 48.Chua SD, Jr, Messier SP, Legault C, Lenz ME, Thonar EJ, Loeser RF. Effect of an exercise and dietary intervention on serum biomarkers in overweight and obese adults with osteoarthritis of the knee. Osteoarthr Cartilage. 2008;16:1047–1053. doi: 10.1016/j.joca.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sevick MA, Miller GD, Loeser RF, Williamson JD, Messier SP. Cost-effectiveness of exercise and diet in overweight and obese adults with knee osteoarthritis. Med Sci Sports Exerc. 2009;41:1167–1174. doi: 10.1249/MSS.0b013e318197ece7. [DOI] [PubMed] [Google Scholar]
- 50.Messier SP, Legault C, Loeser RF, et al. Does high weight loss in older adults with knee osteoarthritis affect bone-on-bone joint loads and muscle forces during walking? Osteoarthr Cartilage. 2011;19:272–280. doi: 10.1016/j.joca.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller GD, Nicklas BJ, Davis CC, Ambrosius WT, Loeser RF, Messier SP. Is serum leptin related to physical function and is it modifiable through weight loss and exercise in older adults with knee osteoarthritis? Int J Obes Relat Metab Disord. 2004;28:1383–1390. doi: 10.1038/sj.ijo.0802737. [DOI] [PubMed] [Google Scholar]
- 52.Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity (Silver Spring) 2006;14:1219–1230. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 53.Miller GD, Jenks MZ, Vendela M, Norris JL, Muday GK. Influence of weight loss, body composition, and lifestyle behaviors on plasma adipokines: a randomized weight loss trial in older men and women with symptomatic knee osteoarthritis. J Obes. 2012:708505. doi: 10.1155/2012/708505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller GD, Nicklas BJ, Loeser RF. Inflammatory biomarkers and physical function in older, obese adults with knee pain and self-reported osteoarthritis after intensive weight-loss therapy. J Am Geriatr Soc. 2008;56:644–651. doi: 10.1111/j.1532-5415.2007.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Messier SP, Legault C, Mihalko S, et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: design and rationale. BMC Musculoskelet Disord. 2009;10:93. doi: 10.1186/1471-2474-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Henson S, Jackson AB, Richards JS. Obesity intervention in persons with spinal cord injury. Spinal Cord. 2006;44:82–91. doi: 10.1038/sj.sc.3101818. [DOI] [PubMed] [Google Scholar]
- 58.Davies RJ, Lomer MC, Yeo SI, Avloniti K, Sangle SR, D'Cruz DP. Weight loss and improvements in fatigue in systemic lupus erythematosus: a controlled trial of a low glycaemic index diet versus a calorie restricted diet in patients treated with corticosteroids. Lupus. 2012;21:649–655. doi: 10.1177/0961203312436854. [DOI] [PubMed] [Google Scholar]
- 59.Hansen GV, Nielsen L, Kluger E, Thysen M, Emmertsen H, Stengaard-Pedersen K, Hansen EL, Unger B, Andersen PW. Nutritional status of Danish rheumatoid arthritis patients and effects of a diet adjusted in energy intake, fish-meal, and antioxidants. Scand J of Rheumatol. 1996;25:325–333. doi: 10.3109/03009749609104066. [DOI] [PubMed] [Google Scholar]
- 60.Hoffman CJ, Maynard FM. A pilot program of nutrition education and exercise for polio survivors: a community-based model for secondary disability prevention. Top Clin Nutr. 1992;7:69–80. [Google Scholar]
- 61.John H, Hale ED, Treharne GJ, Kitas GD, Carroll D. A randomized controlled trial of a cognitive behavioural patient education intervention vs a traditional information leaflet to address the cardiovascular aspects of rheumatoid disease. Rheumatology (Oxford) 2013;52:81–90. doi: 10.1093/rheumatology/kes237. [DOI] [PubMed] [Google Scholar]
- 62.John H, Hale ED, Bennett P, Treharne GJ, Carroll D, Kitas GD. Translating patient education theory into practice: developing material to address the cardiovascular education needs of people with rheumatoid arthritis. Patient Educ Couns. 2011;84:123–127. doi: 10.1016/j.pec.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin K, Fontaine KR, Nicklas BJ, Dennis KE, Goldberg AP, Hochberg MC. Weight loss and exercise walking reduce pain and improve physical functioning in overweight postmenopausal women with knee osteoarthritis. J Clin Rheumatol. 2001;7:219–223. doi: 10.1097/00124743-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 64.McDougall J, Bruce B, Spiller G, Westerdahl J, McDougall M. Effects of a very low-fat, vegan diet in subjects with rheumatoid arthritis. J Altern Complement Med. 2002;8:71–75. doi: 10.1089/107555302753507195. [DOI] [PubMed] [Google Scholar]
- 65.McKellar G, Morrison E, McEntegart A, et al. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann Rheum Dis. 2007;66:1239–1243. doi: 10.1136/ard.2006.065151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48:1062–1072. doi: 10.1111/j.1532-5415.2000.tb04781.x. [DOI] [PubMed] [Google Scholar]
- 67.Nenonen MT, Helve TA, Rauma AL, Hanninen OO. Uncooked, lactobacilli-rich, vegan food and rheumatoid arthritis. Br J Rheumatol. 1998;37:274–281. doi: 10.1093/rheumatology/37.3.274. [DOI] [PubMed] [Google Scholar]
- 68.Paans N, van den Akker-Scheek I, Dilling RG, et al. Effect of exercise and weight loss in people who have hip osteoarthritis and are overweight or obese: a prospective cohort study. Phys Ther. 2013;93:137–146. doi: 10.2522/ptj.20110418. [DOI] [PubMed] [Google Scholar]
- 69.Panush RS, Carter RL, Katz P, Kowsari B, Longley S, Finnie S. Diet therapy for rheumatoid arthritis. Arthritis Rheum. 1983;26:462–471. doi: 10.1002/art.1780260403. [DOI] [PubMed] [Google Scholar]
- 70.Radomski MV, Finkelstein M, Hagel S, Masemer S, Theis J, Thompson M. A Pilot Wellness and weight management program for individuals with spinal cord injury: participants' goals and outcomes. Top Spinal Cord Inj Rehabil. 2011;17:59–69. [Google Scholar]
- 71.Ravaud P, Flipo RM, Boutron I, et al. ARTIST (osteoarthritis intervention standardized) study of standardised consultation versus usual care for patients with osteoarthritis of the knee in primary care in France: pragmatic randomised controlled trial. BMJ. 2009;338:b421. doi: 10.1136/bmj.b421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rimmer JH, Braunschweig C, Silverman K, Riley B, Creviston T, Nicola T. Effects of a short-term health promotion intervention for a predominantly African-American group of stroke survivors. Am J Prev Med. 2000;18:332–338. doi: 10.1016/s0749-3797(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 73.Rimmer JH, Wang E, Pellegrini CA, Lullo C, Gerber BS. Telehealth weight management intervention for adults with physical disabilities: a randomized controlled trial. Am J Phys Med Rehabil. 2013;92:1084–1094. doi: 10.1097/PHM.0b013e31829e780e. [DOI] [PubMed] [Google Scholar]
- 74.Senna MK, Sallam RA, Ashour HS, Elarman M. Effect of weight reduction on the quality of life in obese patients with fibromyalgia syndrome: a randomized controlled trial. Clin Rheumatol. 2012;31:1591–1597. doi: 10.1007/s10067-012-2053-x. [DOI] [PubMed] [Google Scholar]
- 75.Shah M, Coyle Y, Kavanaugh A, Adams-Huet B, Lipsky PE. Development and initial evaluation of a culturally sensitive cholesterol-lowering diet program for Mexican and African American patients with systemic lupus erythematosus. Arthritis Care Res. 2000;13:205–212. doi: 10.1002/1529-0131(200008)13:4<205::aid-anr5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 76.Shah M, Kavanaugh A, Coyle Y, Adams-Huet B, Lipsky PE. Effect of a culturally sensitive cholesterol lowering diet program on lipid and lipoproteins, body weight, nutrient intakes, and quality of life in patients with systemic lupus erythematosus. J Rheumatol. 2002;29:2122–2128. [PubMed] [Google Scholar]
- 77.Shah M, Adams-Huet B, Kavanaugh A, Coyle Y, Lipsky P. Nutrient intake and diet quality in patients with systemic lupus erythematosus on a culturally sensitive cholesterol lowering dietary program. J Rheumatol. 2004;31:71–75. [PubMed] [Google Scholar]
- 78.Shapiro JR, Anderson DA, Danoff-Burg S. A pilot study of the effects of behavioral weight loss treatment on fibromyalgia symptoms. J Psychosom Res. 2005;59:275–282. doi: 10.1016/j.jpsychores.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 79.Skoldstam L, Hagfors L, Johansson G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:208–214. doi: 10.1136/ard.62.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagfors L, Nilsson I, Sköldstam L, Johansson G. Fat intake and composition of fatty acids in serum phospholipids in a randomized, controlled, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr Metab (Lond) 2005;10:2–26. doi: 10.1186/1743-7075-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Somers TJ, Blumenthal JA, Guilak F, et al. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: a randomized controlled study. Pain. 2012;153:1199–1209. doi: 10.1016/j.pain.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolf S, Foley S, Budiman-Mak E, et al. Predictors of weight loss in overweight veterans with knee osteoarthritis who participated in a clinical trial. J Rehabil Res Dev. 2010;47:171–181. doi: 10.1682/jrrd.2009.08.0136. [DOI] [PubMed] [Google Scholar]
- 83.Moroshko I, Brennan L, O'Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obes Rev. 2011;12:912–934. doi: 10.1111/j.1467-789X.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 84.Curioni C, Lourenco P. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond) 2005;29:1168–1174. doi: 10.1038/sj.ijo.0803015. [DOI] [PubMed] [Google Scholar]
- 85.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 86.Lainscak M, Haehling S, Doehner W, Anker S. The obesity paradox in chronic disease: facts and numbers. J Cachexia Sarcopenia Muscle. 2012;3:1–4. doi: 10.1007/s13539-012-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stone AA, Broderick JE. Obesity and pain are associated in the United States. Obesity (Silver Spring) 2012;20:1491–1495. doi: 10.1038/oby.2011.397. [DOI] [PubMed] [Google Scholar]
- 88.Ragonese P, Aridon P, Salemi G, D'Amelio M, Savettieri G. Mortality in multiple sclerosis: a review. Eur J Neurol. 2008;15:123–127. doi: 10.1111/j.1468-1331.2007.02019.x. [DOI] [PubMed] [Google Scholar]
- 89.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 90.Ory MG, Ahn S, Jiang L, et al. Successes of a national study of the Chronic Disease Self-Management Program: meeting the triple aim of health care reform. Med Care. 2013;51:992–998. doi: 10.1097/MLR.0b013e3182a95dd1. [DOI] [PubMed] [Google Scholar]
- 91.Cardol M, De Jong BA, Ward CD. On autonomy and participation in rehabilitation. Disabil Rehabil. 2002;24:970–974. doi: 10.1080/09638280210151996. [DOI] [PubMed] [Google Scholar]
- 92.Paoli A, Rubini A, Volek J, Grimaldi K. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789–796. doi: 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teeple E, Jay G, Elsaid K, Fleming B. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J. 2013;15:438–446. doi: 10.1208/s12248-013-9454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schiff M, Bénit P, Coulibaly A, Loublier S, EI-Khoury R, Rustin P. Mitochondrial response to controlled nutrition in health and disease. Nutr Rev. 2011;69:65–75. doi: 10.1111/j.1753-4887.2010.00363.x. [DOI] [PubMed] [Google Scholar]
- 95.Leeman J, Chang Y, Voils CI, Crandell JL, Sandelowski M. A mixed-methods approach to synthesizing evidence on mediators of intervention effects. West J Nurs Res. 2011;33:870–900. doi: 10.1177/0193945911402365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32:S112–118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bacon L, Aphramor L. Weight science: evaluating the evidence for a paradigm shift. Nutr J. 2011;10:9. doi: 10.1186/1475-2891-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greaves C, Sheppard K, Abraham C, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health. 2011;11:119. doi: 10.1186/1471-2458-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Plow M, Mathiowetz V, Lowe D. Comparing individualized rehabilitation to a group wellness intervention for persons with multiple sclerosis. Am J Health Promot. 2009;24:23–26. doi: 10.4278/ajhp.071211128. [DOI] [PubMed] [Google Scholar]
- 100.Rimmer JH, Riley B, Wang E, Rauworth A, Jurkowski J. Physical activity participation among persons with disabilities: barriers and facilitators. Am J Prev Med. 2004;26:419–425. doi: 10.1016/j.amepre.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 101.Dombrowski SU, Sniehotta FF, Avenell A, Johnston M, MacLennan G, Araújo-Soares V. Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: a systematic review. Health Psychol Rev. 2010;6:7–32. [Google Scholar]
- 102.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 103.Brennan L, Murphy K. The role of psychology in overweight and obesity management. Oxford: Wiley-Blackwell; 2012. [Google Scholar]
- 104.Plow M, Finlayson M, Cho C. Correlates of nutritional behavior in individuals with multiple sclerosis. Disabil Health J. 2012;5:284–392. doi: 10.1016/j.dhjo.2012.05.007. [DOI] [PubMed] [Google Scholar]