Abstract
Signal Transducers and Activators of Transcription (STATs) have been studied extensively and have been associated with virtually every biochemical pathway. Until recently, however, they were thought to exert these effects solely as a nuclear transcription factor. The finding that STAT3 localizes to the mitochondria and modulates respiration has opened up a new avenue through which STATs may regulate the cell. Recently, other members of the STAT family (STAT1, STAT2, STAT5, and STAT6) have also been shown to be present in the mitochondria. Coordinate regulation at the nucleus and mitochondria by these proteins places them in a unique position to drive cellular processes to achieve a specific response. This review summarizes recent findings that have led to our current understanding of how STATs influence mitochondrial function in health and disease.
Keywords: STATs, STAT3, Mitochondria, Review, Cancer, Ischemia/reperfusion, Metabolism
1. Introduction
Since their discovery twenty years ago, Signal Transducers and Activators of Transcription (STATs) have been studied extensively, and are now well-characterized transcription factors responsible for controlling a diverse array of biological functions. These proteins have been linked to immune regulation, development, metabolism, cell death, and tumorigenesis, amongst other cellular roles. There are seven known mammalian STAT family members (STAT1, 2, 3, 4, 5a, 5b, and 6), and while they have overlapping functions in some instances, in many cases they have divergent and often opposing roles. Classically, cytokine activated Janus Kinases (JAKs) tyrosine phosphorylate STATs allowing them to form homo- or hetero-dimers, which translocate to the nucleus to drive expression of early response genes. However, non-canonical roles of these transcription factors are emerging that suggests they play a much broader role in cellular homeostasis than strictly mediating nuclear gene expression. The discovery that a pool of STAT3 is in the mitochondria, where it exerts an effect on respiration and Ras transformation [1,2], suggests a role of STATs distinct from their nuclear actions. This review will summarize our current understanding of how the STAT family of transcription factors regulates mitochondrial function.
2. Signal Transducer and Activator of Transcription 3 (STAT3)
During the past 15 years there have been a number of reports suggesting that a variety of nuclear transcription factors (TFs) reside in the mitochondria, including NFκB, p53, AP-1, CREB, MEF2D, and IRF-3 [3,4]. In most cases, with the exception of p53, the role of these TFs in mitochondrial function has been limited. Using biochemical fractionation we identified a small pool of STAT3 (5–10% of total) that is located in the mitochondria of many tissues as well as cultured cells, where it exerts an effect on Complexes I and II of the electron transport chain (ETC) [1]. In Ras transformed cells, ATP levels are decreased in the absence of STAT3 and the activity of Complexes II and V of the ETC are diminished [2]. Through the use of mutants, it was established that serine 727 of STAT3 is crucial for its mitochondrial function, whereas known domains required for its nuclear action (tyrosine 705, DNA binding domain, etc.) are not sufficient to drive STAT3’s mitochondrial action [1,2]. Phosphorylation at S727 in STAT3 may also be required for its mitochondrial import, as a serine to alanine mutation decreased mitochondrial STAT3 levels in an in vitro mitochondrial import assay [5]. Though there is a much larger fraction of serine phosphorylated STAT3 in the mitochondria as compared to the cytosol, it is not known whether the entire mitochondrial pool of STAT3 is constitutively phosphorylated. It is also currently unclear which kinase is responsible for serine phosphorylation of mitochondrial STAT3. However, a recent report suggests that mitochondrial serine phosphorylation of STAT3 may be linked to the MEK–ERK pathway [6]. Additional studies have confirmed and added to the importance of STAT3’s mitochondrial localization, which may have important physiological consequences in the following areas: metabolism, cancer, and defense against cell stress.
2.1. Mitochondrial STAT3 as a modulator of cell metabolism
STAT3’s contribution to optimal activity of the ETC (see Box 1) places this transcription factor in an ideal position to modulate the energy status of the cell. A number of reports have evaluated the effect that STAT3 has on the activities of the complexes of the ETC [1,2,7–9], which are summarized in Table 1. Like the other TFs shown to be present in the mitochondria, the mitochondrial targeting sequence for STAT3 is likely cryptic and as such, it has yet to be determined. Therefore, in some studies, in order to better examine its mitochondrial function, the mitochondrial localization/targeting sequence (MLS/MTS) of human cytochrome c oxidase subunit VIII (an integral mitochondrial protein) has been fused to the N-terminus of STAT3. The impact of STAT3 on the ETC varies according to the model used and the context of the study, but most studies observe an increase in activity in the complexes in the presence of STAT3. Interestingly, over-expression of a DNA binding domain mutant, STAT3E, targeted to the mitochondria (MLS-STAT3E) suppresses activities of Complexes I and II [9]. STAT3E contains two point mutations in the DNA binding domain (E434A/E435A) that are important for both its recognition and binding of DNA response elements [10], further excluding nuclear contributions of STAT3. Under conditions of ischemia, however, over expression of MLS-STAT3E protects the activity of the ETC. This suggests that optimal effects of STAT3 on the ETC are concentration and stimulus dependent. Perhaps, over-expression of mitochondrial STAT3 alters its protein–protein interactions such that its actions on the ETC become more protective under conditions of stress and less effective in regulating the activity of the ETC under basal conditions. This idea would be consistent with the observations of Phillips and colleagues that the stoichiometry of mitochondrial STAT3 to components of the ETC is not 1:1 [11], which implies that mitochondrial STAT3 may not be modulating the ETC directly. If this is the case, then it must be doing so via an unknown mechanism whereby the overall levels of STAT3 in the mitochondria would be crucial for determining its binding partners and therefore, its regulation of mitochondrial function.
Box 1: The Electron Transport Chain (ETC).
The electron transport chain in the inner mitochondrial membrane consists of four complexes (I–IV). Complexes I–IV (I: NADH:ubiquinone oxidoreductase, II: Succinate:ubiquinone oxidoreductase, III: Ubiquinol:ferricytochrome c oxidoreductase, IV: Ferrocytochrome c: oxygen oxidoreductase) are responsible for driving the transport of electrons down the chain by pairing electron donors with specific electron acceptors. As a result of this transfer of energy, protons (H+ ions) are pumped across the inner mitochondrial membrane from the mitochondrial matrix side into the inter-membrane space, thereby generating an electrochemical gradient across the inner mitochondrial membrane. Complex V, also known as ATP Synthase, is then able to dissipate this proton gradient and couple the resulting energy release with ATP production. However, the generation of fuel for the cell is not without cost as the alternative reaction of proximal complexes I–III directly with molecular oxygen can lead to the formation of reactive oxygen species (ROS) that may be detrimental to the cell. In order to measure the activity of the electron transport chain a few widely used techniques can be employed. In general, purified intact mitochondria that have been detergent solubilized or permeabilized mitochondria are incubated in the presence of specific substrates for each of the complexes with the readout of the activity depending upon the assay being employed (i.e. oxygen consumption, spectrometric analysis, blue native gel staining, etc.). Following measurement of the activity of the respective complex in the presence of its substrate, the specificity of the reaction for the complex can be verified through the addition of a known inhibitor to that complex (i.e. rotenone for Complex I, antimycin A for Complex III, azide or cyanide for Complex IV). Coupling these analyses with other mitochondrial measures performed in intact mitochondria, including oxidative phosphorylation and mitochondrial membrane potential, provide an indication of the functional status of the mitochondria.
Table 1.
STAT3 affects the activities of the ETC complexes. Summary of the published work on mitochondrial STAT3’s regulation of the electron transport chain. The results presented in this table showcase the effect that STAT3 has when present in the mitochondria as some studies have utilized STAT3 null backgrounds as the basis for their comparison.  : increase in activity;
: increase in activity;  : decrease in activity; – no change in activity; blank: complex activity not reported on; °MLS-STAT3E: cardiac specific overexpression of mitochondrial targeted STAT3 containing mutations in the DNA binding domain.
: decrease in activity; – no change in activity; blank: complex activity not reported on; °MLS-STAT3E: cardiac specific overexpression of mitochondrial targeted STAT3 containing mutations in the DNA binding domain.
| Cell/tissue type | STAT3 | Complex I | Complex II | Complex III | Complex IV | Complex V | Reference |
|---|---|---|---|---|---|---|---|
| Pro-B Cells | Endogenous | – | – | [1] | |||
| Murine Heart Mitochondria | Endogenous | – | – | [1] | |||
| MEFs (+ HRasV12) | Endogenous | – | – | – | [2] | ||
| MEFs (SIRT1 KO) | Endogenous | [7] | |||||
| HL-1 (murine cardiomyocyte) | Endogenous | [8] | |||||
| Murine Heart Mitochondria | MLS-STAT3E° | – | – | [9] |
As mentioned, mitochondrial STAT3 is also closely associated with cellular ATP levels. In Ras transformation of mouse embryonic fibroblasts (MEFs), Gough et al. showed that the activity of Complex V (ATP Synthase) of the ETC was dramatically reduced in the absence of STAT3, thereby limiting ATP production [2]. While not directly linked to Complex V function, a study using a conditional knockout of STAT3 in astrocytes also observed a considerable reduction in ATP levels compared to cells expressing STAT3 [12]. Similarly, blockade of STAT3 activity using the STAT3 inhibitor Stattic has been shown to decrease ATP production in purified rat heart mitochondria [13], and in Stattic treated human spermatozoa [14]. Stattic is a small molecule inhibitor of STAT3 that binds to its SH2 domain that prevents its activation and dimerization, and also blocks its nuclear translocation [15]. Though the effects of Stattic on mitochondrial STAT3 have not been carefully investigated, its use on isolated mitochondria as in the study above [13] suggests that it can also inhibit STAT3’s mitochondrial action. Further investigation is needed to confirm whether or not STAT3’s association with ATP is the result of mitochondrial STAT3 affecting Complex V activity, or if it is merely a reflection of upstream effects on the ETC. Those upstream effects may partly explain the lower mitochondrial membrane potential reported in the absence of STAT3 [12,14], which would impact ATP production by reducing the proton gradient across the inner mitochondrial membrane. It is possible that a nuclear component of STAT3 can also regulate mitochondrial metabolism because expression of a constitutively active form of STAT3 (STAT3c/c) resulted in decreased mitochondrial membrane potential and ATP generation, likely due to down regulation of nuclear encoded mitochondrial ETC component mRNA’s [16]. The functional significance is a metabolic switch toward a more glycolytic phenotype, which is in contrast to the enhancement of oxidative phosphorylation driven by mitochondrial STAT3. It is interesting to note the apparent discrepancy between nuclear and mitochondrial-localized STAT3 in terms of regulating mitochondrial function, and while speculative, may point to dual regulation by these pools of STAT3 to control cellular energy.
While a mechanism regulating STAT3’s ability to monitor and modify the energy status of the cell is still elusive, there are some indications of signaling cascades operating here. In Sirtuin 1 knockout (KO) MEFs, there are selective increases in mitochondrial phospho-S727 STAT3 that was found to be downstream of NFκB activation [7]. The kinase responsible for this increased phosphorylation for serine 727 remains to be determined. Increased activity of mitochondrial STAT3 was correlated with elevated ETC activity, respiration, and cellular ATP levels. Sirtuins represent a family of deacetylase proteins that sense the metabolic status of the cell and regulate the acetylation status of their targets to maintain energy homeostasis, among other things [17]. It is notable that Sirtuin 1 has been reported to regulate acetylation of STAT3 that plays an important role in gluconeogenesis [18]. Though Sirtuin1 is localized to the nucleus, Sirtuin 3 is found exclusively in the mitochondria. As Sirtuin 3 affects mitochondrial metabolism and function by altering the acetylation state of mitochondrial proteins [19–21], it is plausible that it exerts a similar effect on STAT3.
Due to the link between cellular metabolism and physiology, STAT3’s presence or absence from mitochondria likely has pathological implications. One arena where this may be the case is in the development of metabolic syndrome, which has close ties with mitochondrial dysfunction. STAT3 has been shown to be involved in the pathogenesis of obesity and insulin resistance [22], and as such, it is an intriguing possibility that part of the pathology seen in metabolic disorders is influenced by STAT3’s mitochondrial actions. Indirect evidence for this is presented in a study examining liver specific deletion of src homology phosphatase 2 (Shp2) [23]. Deletion of Shp2, which plays a role in insulin signaling, prevented the development of metabolic syndrome in mice exposed to a high fat diet. Interestingly, in the absence of Shp2, the authors also saw increased activation (phospho-S727) of mitochondrial STAT3, which was correlated with increased mitochondrial respiration and energy expenditure that limited metabolic dysfunction. As activation of Shp2 and its phosphatase activity plays a role in negatively regulating cytokine mediated signal transduction [24], Shp2’s deletion likely leads to increased activation of downstream pathways, including the STATs. This may better explain STAT3’s increased activation, as opposed to impaired insulin signaling, especially when one considers the positive correlation between high fat diet and circulating cytokine levels such as IL-6. Further, a study on polymorphisms in the STAT3 gene in human subjects were closely linked to mtDNA copy number and insulin resistance, whereas variants of known regulators of the mitochondrial genome, such as TFAM or PPARγ, did not show the same association [25]. Additional studies are needed to characterize the interplay of mitochondrial and nuclear STAT3 effects in mediating metabolic disease.
2.2. Cancer and mitochondrial STAT3
STAT3’s mitochondrial localization and influence on energy stores makes it ideally situated to modify cell growth. As an example, mitochondrial STAT3 is required to drive neurite outgrowth in response to a variety of ligands including nerve growth factor [26,27]. Coupled with the fact that the mitochondrion is the central player in mediating cell death and survival pathways, mitochondrial STAT3 may play a role in cancer biology. This idea was first investigated in the context of Ras transformation. HRasV12 expressing MEFs required mitochondrial STAT3 to drive colony formation in vitro and tumor growth in mice [2]. Expression of mutant forms of STAT3 in a STAT3 null background demonstrated that serine 727 phosphorylation of STAT3 was necessary to mediate these effects. The serine phosphorylation of STAT3 via oncogenic Ras was later found to be partially dependent upon Ras activation of the MEK–ERK pathway as MEK inhibitors were able to interfere in part with mitochondrial STAT3’s role in Ras tumorigenesis [6]. In contrast, sites crucial for the nuclear actions of STAT3 (SH2 domain, tyrosine 705 site, DNA binding domain) were not required to drive Ras transformation. Expression of mitochondrial targeted STAT3 (MTS-STAT3) drove Ras transformation as efficiently as wild-type STAT3 suggesting that mitochondrial localized STAT3 is sufficient to drive these oncogenic effects. The necessity of mitochondrial STAT3 in Ras transformation extended to other mutant forms of Ras (KRas and NRas), but not necessarily to other oncogenes such as vSrc. Since activation of the downstream targets of Ras (Raf/MEK/Erk and PI3K) were largely unaffected in the absence of STAT3, the mechanism regulating Ras dependence on mitochondrial STAT3 likely resides in STAT3’s ability to regulate the ETC to facilitate adequate production of ATP to drive continued cell growth.
These results were extended by recent work demonstrating that mitochondrial STAT3 is also important in the growth of already transformed cells [28]. Expression of a mitochondrial targeted STAT3 (MLS-STAT3) in 4T1 cells, a murine breast adenocarcinoma model, showed that S727 of STAT3 is also required for optimal cancer cell growth. Mutation of S727 to alanine leads to decreased colony formation in soft agar, which is mirrored by the development and progression of tumors in immunocompetent Balbc mice. Tumors expressing MLS-S727A STAT3 were smaller than their wild-type counterparts and also displayed decreases in the number of liver and lung metastases. In contrast, expression of the phospho-mimetic mutant MLS-S727D STAT3 resulted in increased colony formation in vitro, and larger tumors in mice with a greater metastatic burden. The nuclear effects of STAT3 could largely be excluded as these constructs contained mutations in the nuclear localization sequence, SH2 domain, DNA binding domain, and tyrosine 705 sites. The mechanism behind mitochondrial STAT3’s regulation of breast cancer growth was attributed to optimization of the ETC and control of reactive oxygen species (ROS) production in a hypoxic environment. Hypoxia features prominently in the transformation and growth of solid tumors and is tightly coupled to elevations in mitochondrial ROS production [29]. The relevance of ROS in driving tumorigenesis and cellular growth has been widely studied, and is thought to be a potential therapeutic target in cancer therapy [30,31]. In line with this, cells harboring MLS-S727D STAT3 displayed elevated Complex I activity of the ETC and decreased ROS production when cultured in 1% oxygen. However, those cells expressing the S727A mutant had reduced ETC activity and higher levels of ROS under hypoxic conditions. Interestingly, only tumors from mice expressing MLS-S727A STAT3 showed a selective growth advantage when mice were injected with the anti-oxidant Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP).
These results point to a role of mitochondrial STAT3 in regulating ROS levels as a determinant of cancer cell growth and proliferation. This makes the mitochondrial pool of STAT3 a potentially interesting therapeutic target, especially when considering the number of cancers that display constitutive STAT3 activation. Through the use of a wide array of targeted inhibitors, numerous studies have demonstrated that blockade of STAT3’s nuclear prosurvival program is efficacious in promoting cancer cell death [32] Despite this, these compounds have had little therapeutic value thus far. However, few reports have evaluated how these compounds affect the mitochondrial program of STAT3. A recent study by Mackenzie and colleagues demonstrated the importance of targeting mitochondrial STAT3 in pancreatic cancer [33]. They showed that the anti-tumor activity of their novel compound, phosphovalproic acid (P-V), depended solely on inhibition of the actions of mitochondrial STAT3, despite also blocking the STAT3 driven increased expression of the anti-apoptotic proteins Bcl-xl, Mcl-1, and survivin. Human pancreatic cancer cell lines that expressed a mitochondrial targeted STAT3 (MLS-STAT3) that were orthotopically implanted into nude mice were completely insensitive to the tumor growth limiting effects of P-V. Over-expression of the dominant negative STAT3 mutant Y705F also blocked the pro-apoptotic program of P-V thereby lending support to the idea that it is the mitochondrial pool of STAT3, rather than the nuclear pool, that is sustaining the cells’ oncogenic program. P-V exerted these effects by preventing the mitochondrial localization of STAT3, which in turn led to a depressed mitochondrial membrane potential, elevated ROS production, and increased mitochondrial-mediated apoptosis. Targeting mitochondrial STAT3 may therefore, be just as important in limiting cancer growth and survival as blockade of its transcriptional activity. The importance of mitochondrial STAT3 in pancreatic cancer is not without precedent as a study by Kang et al. showed that STAT3 localized to the mitochondria plays a role in advanced glycation end product-specific receptor (RAGE) mediated autophagy [34]. Autophagy is upregulated in pancreatic cancers basally, which makes the mechanism of autophagy an attractive therapeutic target [35]. In this study [34], autophagy triggered IL-6 induced serine 727 phosphorylation of STAT3, which was sufficient to increase mitochondrial STAT3 levels and elevate overall ATP production, likely leading to enhanced tumorigenesis and cellular proliferation. The absence of RAGE in a KRAS-induced neoplastic model significantly increased time to tumor development, which was coupled by decreases in mitochondrial STAT3 and its activity with subsequent increased apoptosis and decreased ATP production. Again, these studies suggest that mitochondrial STAT3 is important in the growth and maintenance of tumors. While the study by Mackenzie et al. implicated JAK2, Src, and HSP-90 with decreases in phospho-STAT3 and an overall decrease in mitochondrial STAT3 levels, further investigation is needed to characterize the specific effects and binding partners of mitochondrial STAT3 that drive these pro-oncogenic processes [33].
Another study investigating the involvement of STAT3 in pituitary growth and tumorigenesis also determined that Src may be an upstream regulator of mitochondrial STAT3 activity. In this report, a polymorphism in the fibroblast growth factor receptor led to enhanced activation of Src, which was correlated with increased levels and activation of STAT3 in the mitochondria that led to elevated mitochondrial activity as assessed by cytochrome c oxidase activity [36]. Whether Src’s involvement here is merely an indicator of overall cellular activation or is specific to initiating a signaling cascade to control STAT3’s actions in the mitochondria remains unknown.
A recent study had demonstrated that STAT3 interacts with Cyclophilin D (CypD) to regulate the mitochondrial permeability transition pore (MPTP) [37]. While the structural components of the permeability transition pore are debated [38–40], the only protein known to be absolutely required for pore functioning is Cyclophilin D (CypD), which is thought to be activated in the mitochondrial matrix and translocate to the inner mitochondrial membrane to facilitate pore opening [41]. Under non-stressed conditions the pore transiently opens and closes to maintain calcium concentration gradients and mitochondrial membrane potential. Sustained opening of the pore due to certain stimuli (ROS, misfolded mitochondrial proteins, excess Ca2+, etc.) leads to mitochondrial swelling, dysfunction, and ultimately, apoptosis or necrosis [42]. Other TFs, such as p53, can also control cell viability by modulating MPTP opening through interactions with CypD [43]. Interestingly, the permeability transition pore more readily opens in the absence of STAT3 or in the presence of the STAT3 inhibitor, Stattic, thereby implicating STAT3 in MPTP regulation [13,37]. This may serve as a cytoprotective mechanism to mitigate cell death and hence be advantageous for cancer cells. This idea is in line with the work by Mantel et al., who also suggest that STAT3’s regulation of the MPTP may have pathophysiological consequences in myleoproliferative disorders [44].
Though it would appear that STAT3 is playing a pro-survival role here, there is one report that demonstrates that translocation of STAT3 to the mitochondria is actually required for tumor necrosis factor (TNF) induced necroptosis [45]. As STAT3’s effect on the MPTP was not investigated in this study, further work is needed to clarify the mechanism through which STAT3 controls cell viability at the mitochondria.
2.3. Defense against cellular stress
A large body of work on STAT3 and its mitochondrial function lies in the area of ischemia/reperfusion injury, and the importance that STAT3 may play in preserving cell viability and function following myocardial injury, and though not studied as extensively, in the case of cerebrovascular accident. The importance of STAT3 in this context has been thoroughly reviewed elsewhere [3,46], and hence, will only be briefly discussed. Ischemia triggers dysfunction of the electron transport chain, which upon reperfusion drives the production of excess reactive oxygen and nitrogen species (ROS/RNS) that damage mitochondria and the cell. Therefore, better understanding of the molecular pathways regulating ROS/RNS production affords the opportunity of maximizing cardio- or neuroprotection. STAT3 is recruited to mitochondria following ischemic injury [9], while the pool of mitochondrial STAT3 may be activated as early as seven minutes post-reperfusion in mouse hearts subjected to ischemia and reperfusion [47]. Mitochondrial localized STAT3 preserves Complex I activity during ischemia and reduces ROS production, which is sufficient to limit cytochrome c release, likely preserving cell viability [9]. Similar results were obtained in a model of ischemic post-conditioning in porcine hearts [48]. In these studies increases in phospho-Y705 STAT3 in the mitochondria were directly linked to decreases in infarct size, maintenance of Complex I activity, and decreased MPTP opening. These protective effects were diminished in the presence of the STAT3 inhibitor, Stattic. The role of mitochondrial STAT3 in preserving cardiac function may be more relevant in the context of ischemic post-conditioning, which uses brief, repeated bouts of ischemia prior to reperfusion in an attempt to limit injury via ROS/RNS induced activation of pro-survival kinases. In line with this, mice harboring a cardiomyocyte specific deletion of STAT3 showed no difference in infarct size following ischemia and reperfusion [37]. There was however a statistically significant difference in infarct size following ischemic postconditioning, in which STAT3 knockout hearts had larger infarcts than their wild-type counterparts [49]. In fact, in a rat model, mitochondrial STAT3 activation was not observed with ischemia/reperfusion, but only with ischemic postconditioning [50]. While not studied in the context of ischemia, other studies have demonstrated that cardiac disease and injury is modulated by mitochondrial STAT3. Decreased activation of mitochondrial STAT3 (pS727) is permissive to the development of cardiac hypertrophy driven by catecholamine injury [51]. Likewise, decreases in total and phosphorylated mitochondrial STAT3 were correlated with the pathology seen in a model of dilated cardiomyopathy [52]. In contrast, increased STAT3 activation in mitochondria correlates with improved mitochondrial activity and optimal left ventricular functioning in a rat model [53]. Additional studies are needed to fully understand the mechanism and timing behind which mitochondrial STAT3 exerts these protective effects.
The upstream and downstream signaling components that mediate STAT3’s functional significance in the mitochondria in reperfusion injury/cell stress have remained elusive, though there are potential targets. The finding that STAT3 may regulate the mitochondrial permeability transition pore (MPTP) [13,37] has broad implications in ischemia/reperfusion injuries, as the MPTP is a major player in cellular injury following this insult [54,55]. As the MPTP is activated in response to a number of cellular stressors including ROS and elevated intra-mitochondrial Ca2+ concentrations (the latter likely due to the tight connection between Ca2+ homeostasis and ROS production), it is possible that oxidative modification of STAT3 in the mitochondria regulates its association and control of the MPTP. STAT3 has been shown to be S-glutathionylated [56–58] and S-nitrosylated [59] in the cytosol, and it is capable of forming multimers following an oxidative insult that impinges on its ability to bind DNA [60]. Whether similar events are modifying STAT3 in the mitochondria and if this has a functional significance remains to be determined [61]. Under these circumstances, recruitment of STAT3 to the mitochondria may involve both redox-dependent and redox-independent mechanisms [50], and thus, the mechanism by which STAT3 translocates to the mitochondria is likely stimulus dependent. More classical signaling cascades have also been linked to mitochondrial STAT3’s activation and protective effects in the heart. Pre-treatment with the JAK inhibitor AG490 has been shown to block the reduction in infarct size mediated by STAT3 in ischemia/reperfusion models [47,48,50]. IL-6 mediated signaling may also play a role as it is linked to STAT3 activation during ischemia and is important for cardioprotection [62]. In the absence of heat shock protein H11 kinase/Hsp22 (Hsp22), a stress response protein that is important for responses to cardiac overload, both the mitochondrial and nuclear actions of STAT3 were diminished [63]. Hsp22 was determined to be necessary for STAT3 activation, which was dependent on NFκB driven IL-6 production, again pointing to IL-6 activation as potentially upstream of STAT3’s mitochondrial function. Further studies are required to adequately separate the importance of these signaling cascades in mitochondrial STAT3 function apart from their effects on STAT3 dependent gene transcription.
Aside from its clear role in the heart, mitochondrial STAT3 may play a role in other systems in minimizing oxidant cellular stress. A study using a mouse model with a hematopoietic cell specific deletion of STAT3 demonstrated that the absence of STAT3 leads to mitochondrial dysfunction and significantly elevated ROS production [44]. The authors believed that this was likely due to defects in the ETC that led to electron leak and subsequent elevated mitochondrial and cellular ROS levels. The mitochondrial dysfunction observed correlated with decreased hematopoietic stem cell reserves, and a shift toward a hematological makeup that mimicked human myeloproliferative disorders. As these myeloproliferative diseases are generally seen in older individuals, it is notable that these mice displayed an accelerated aging phenotype as evidenced by lymphoid-myeloid markers and their dramatically reduced lifespan. While not previously addressed, perhaps the absence of mitochondrial STAT3 contributes to the aging process. In fact, mitochondrial STAT3 is reduced in the hearts of older mice [37]. Considering the link between aging and mitochondrial dysfunction, it is plausible that reduction in mitochondrial STAT3 levels over time plays a part in this phenomenon. Therefore, therapeutic strategies designed to maintain STAT3 in mitochondria could prove fruitful in combating age related disorders.
The similarities between ischemia/reperfusion injuries in the heart and the nervous system, makes it likely that mitochondrial STAT3 may play an equally important role in cerebrovascular accidents. STAT3 normally regulates the expression of the manganese superoxide dismutase (Mn-SOD) gene, a protein that is important in reducing mitochondrial oxidative species, but that regulation is lost during cerebral ischemia and reperfusion [64]. However, inactivation of STAT3 with Stattic during ischemia and reperfusion still significantly increased neuronal cell death and infarct size. While it is clear that the nuclear actions of STAT3 regulate the anti-oxidant status of the cell basally, it is possible that STAT3 translocates to the mitochondria following oxidative injury to regulate mitochondrial function directly to limit ROS production and maintain cell viability. Perhaps then, inactivation of STAT3 in this model limits its mitochondrial translocation, and may explain in part the increase in neuronal cell death observed following Stattic treatment. STAT3 is also required to maintain mitochondrial membrane potential and limit cell death in lung epithelial cells exposed to hyperoxia [65]. Again, while this study did not directly investigate mitochondrial STAT3’s actions, it suggests that STAT3 may protect the cell from oxidative stress by its actions in both the nucleus and the mitochondria.
2.4. Summary
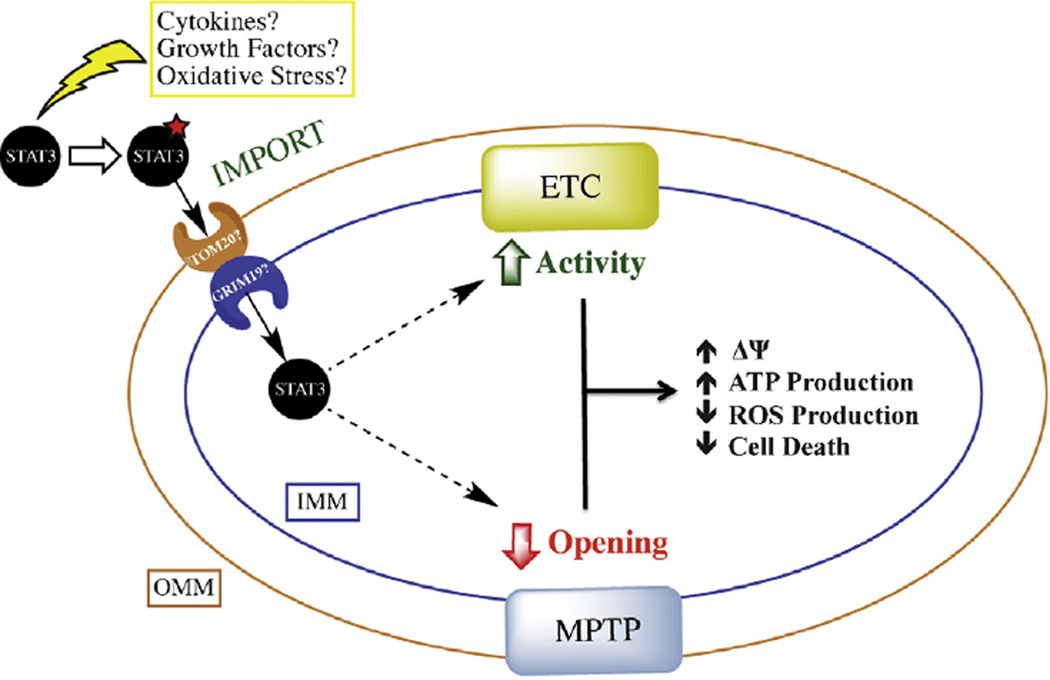
The role of STAT3 in regulating mitochondrial function is summarized in Fig. 1. Though not currently known, it is likely that upstream activation of signaling cascades by cytokines, growth factors, and/or oxidative stress is involved in STAT3’s targeting to the mitochondria. Due to the association of S727 phosphorylation with STAT3’s mitochondrial function, a number of signaling cascades known to activate STAT3 at this residue (including ERK, JNK, p38 MAPK, Protein Kinase C, mTOR, etc.) [66] also probably play a role in STAT3’s mitochondrial action with the particular kinase involved being stimulus and cell-type specific. Within the mitochondria STAT3 modulates two major players in mitochondrial physiology: the ETC and MPTP. This tends to result in increased mitochondrial membrane potential (ΔΨ), increased ATP production, decreased ROS levels, and decreased cell death.
Fig. 1.
Model of STAT3’s mitochondrial action. Upstream activation by cytokines, growth factors, or oxidative stress likely post-translationally modifies STAT3 to target it to the mitochondria. Though still unknown, mitochondrial import of STAT3 may rely on translocases of the outer membrane (TOM20 potentially) and GRIM-19 in the inner mitochondrial membrane. Once imported, STAT3 modulates both the electron transport chain (ETC) and the mitochondrial permeability transition pore (MPTP) to regulate mitochondrial membrane potential (ΔΨ), ATP production, ROS production, and cell death. OMM: outer mitochondrial membrane; IMM: inner mitochondrial membrane.
3. Other STATs with known mitochondrial function
Although STAT3’s actions in the mitochondria have been the most studied, there is evidence from the literature and unpublished results that points to the importance of other STAT family members in mitochondrial function. Aside from STAT3, other STATs (STAT1, STAT2, STAT5, and STAT6) have been shown to localize to the mitochondria. Their mitochondrial actions are discussed below and are summarized in Table 2.
Table 2.
Role of STATs in mitochondrial function. Members of the STAT family of proteins with known mitochondrial actions.
| Mitochondrial function | Reference | |
|---|---|---|
| STAT1 | • Regulation of mitochondrial biogenesis | Sisler JD et al., unpublished |
| • Negative regulation of mitochondrial encoded transcripts | Sisler JD and M Morgan et al., unpublished | |
| STAT2 | • Negative regulation of mitochondrial encoded transcripts | Sisler JD et al., unpublished |
| • Mitochondrial translocation as a target of viral NS degradasome | [70] | |
| STAT3 | • Regulation of the ETC, cellular respiration, and mitochondrial membrane potential | [1,2,7–9,12–14,16,23,33,37,44,48,53] |
| • Modulation of mitochondrial ROS production | [9,28,33,36,44] | |
| • Association with ATP production | [2,12,13,14,16,34] | |
| • Transformation and cellular growth | [2,6,26,27,28,33,34,36] | |
| • Inhibition of mitochondrial permeability transition pore | [13,37,48] | |
| • Protection against ischemia/reperfusion and cardiac injury | [9,13,37,47–49,51–53,63] | |
| • TNF induced necroptosis | [45] | |
| STAT4 | • No published data toward mitochondrial function as of yet | |
| STAT5 | • Cytokine mediated mitochondrial translocation and binding of the D-loop of mitochondrial DNA | [72] |
| STAT6 | • Shown to co-localize with mitochondria using both fluorescence and electron microscopy | [73] |
3.1. Signal Transducer and Activator of Transcription 1 (STAT1)
In contrast to STAT3, which is a well-known negative regulator of the mitochondrial apoptotic program (based on its control of Bcl-2 family member protein expression), STAT1 is a prominent player in pro-apoptotic signaling [67–69]. While most of the actions of STAT1 are attributed to its role as a TF, STAT1 has been reported to localize to the mitochondria [37]. Unpublished results from our lab (Sisler JD and M Morgan) have confirmed that STAT1 is present in the mitochondria from a variety of tissues and cell types, but its exact functional significance there is still unclear. As a key mediator of interferon (IFN) signaling, our results suggest that mitochondrial localized STAT1 may play a role in downregulating the transcription of mitochondrial encoded RNA’s, while also negatively controlling nuclear encoded transcripts of the electron transport chain in response to IFNβ but not IFNγ. The reduction of mitochondrial encoded RNA’s in the presence of IFNβ occurs even in platelets, which lack nuclei, and was abrogated in STAT1 null platelets. In vitro transcription assays demonstrated that STAT1 could inhibit mitochondrial transcription on both the heavy and light strands of the mitochondrial genome, albeit to a lesser extent on the light strand (Sisler JD et al., unpublished results). Interestingly, starvation reduces STAT1 protein levels in tissues that leads to increased mitochondrial biogenesis, which correlates with data obtained from STAT1 knockout mice that display elevated levels of mitochondrial biogenesis compared to wild type mice (Sisler JD et al., unpublished results). Further work is ongoing to clarify the role that mitochondrial STAT1 plays in regulation of mitochondrial content.
3.2. Signal Transducer and Activator of Transcription 2 (STAT2)
A study by Goswami and colleagues demonstrated that translocation of STAT2 to mitochondria could be mediated by viruses in an attempt to alleviate the cells anti-viral response [70]. STAT2 activation features prominently in interferon induced anti-viral cell defense. In this context, they examined the role of viral targeting of the mitochondrial antiviral signaling adaptor (MAVS) in suppressing innate immunity. The viral non-structural (NS) proteins NS1 and NS2, with the help of MAVS, drive the formation of a degradasome at the mitochondria, which was responsible for targeting the cell’s anti-viral protein repertoire to the mitochondria for degradation, which included STAT2. Though the authors focused on the pool of STAT2 that was degraded in the mitochondria by the formation of the viral degradasome, there was a small fraction of STAT2 that basally resided in the mitochondria of the lung epithelial adenocarcinoma A549 cell line. With the emergence of mitochondria as an important player in innate immunity by controlling the cell’s energy and oxidation status [71] and STAT2’s role in immune defense, it is possible that mitochondrial localized STAT2 could also contribute by priming mitochondria for an immune response. Unpublished results from our lab also indicate that a small pool of STAT2 basally resides in the mitochondria (Sisler JD et al.). Here it may selectively negatively regulate the transcription of mitochondrial RNAs. Mitochondrial encoded mRNA transcripts are increased in the absence of STAT2, but unlike STAT1, no effect was observed on nuclear encoded mitochondrial mRNA’s or on mitochondrial biogenesis (Sisler JD et al., unpublished results). The importance of these findings remains to be determined. Nevertheless, it points to a much broader regulation of cellular homeostasis by these transcription factors.
3.3. Signal Transducer and Activator of Transcription 5 (STAT5)
There is limited knowledge about the role of STAT5 in mitochondrial function. One report has shown its localization to the mitochondria in a murine T lymphoma cell line, where it associates with the E2 component of pyruvate dehydrogenase [72]. The authors also reported STAT5 translocation to the mitochondria following cytokine stimulation (IL-2 treatment of the human leukemia cell line CLL-20 and IL-3 treatment of the murine pro-B cell line BaF3). Cytokine induced targeting of STAT5 to the mitochondria was specific as neither STAT1 nor STAT3 was recruited to the mitochondria under these conditions. STAT5 was shown to bind to the D loop of mitochondrial DNA, but the actions of STAT5 on mitochondrial gene expression have not been delineated. Due to the switch to aerobic glycolysis that was observed in these cell lines upon mitochondrial STAT5 translocation, the authors speculated that STAT5 might be playing a role here in regulating mitochondrial metabolism in cancer cells. Considering the role that STAT5 plays in cancer, this study is consistent with an analogous role for STAT5 as STAT3 in driving tumorigenesis via its actions in the mitochondria. As this study did not differentiate between STAT5a and STAT5b, which have both redundant and unique biological actions, more work is needed to fully understand mitochondrial localized STAT5’s role and function.
3.4. Signal Transducer and Activator of Transcription 6 (STAT6)
To date only one report indicates that STAT6 localizes to the mitochondria. Khan and colleagues demonstrate the association of STAT6 with mitochondria in a variety of cells using immunofluorescence [73]. The use of immunogold electron microscopy in human pulmonary artery endothelial cells showed the constitutive presence of STAT6 in the mitochondria. This was further validated using live-cell imaging. Interestingly, only the N-terminal half of STAT6 was required for its mitochondrial localization indicating that the SH2 domain and tyrosine 641 phosphorylation site are not needed for its targeting to this organelle. Though this work provides compelling evidence for STAT6 in the mitochondria, the functional significance of STAT6’s localization here was not explored and warrants further investigation.
4. Conclusions and future directions
The increasing number of reports that link STATs and other TFs with the mitochondria demonstrate both the relevance and importance of a careful investigation of their function in this subcellular organelle. Though new actions of these proteins in the mitochondria are being unveiled, fundamental questions still remain unanswered. When one considers their relative low abundance in the mitochondria and the limitations of cell fractionation, delineation of the mitochondrial targets of STATs is challenging. There are also likely inherent differences in the amounts of these proteins present in the mitochondria depending upon the tissue or cell type studied and whether or not the cells are stressed. These factors likely contribute to the discrepancies in detection of STAT3 in the mitochondria. This has been particularly the case with fluorescent-based approaches [73,74]. Similar differences in STAT5’s mitochondrial localization have also been reported [37,72].
The general mechanism by which these proteins are differentially trafficked to intracellular compartments and organelles remains unknown, despite their broad intracellular distribution. STATs, like the other nuclear TFs with roles in the mitochondria, lack a classical mitochondrial localization sequence. Most studies of STAT3 demonstrate selective accumulation of phospho-S727 STAT3 in the mitochondria, though reports of tyrosine phosphorylated STAT3 also exist, suggesting other regulatory modifications might be involved. It has not been carefully explored whether or not tyrosine phosphorylation of STAT3 in the mitochondria occurs in the absence of serine phosphorylation or if there is any difference in STAT3’s mitochondrial action depending upon its phosphorylation status. Based on studies where the serine site in STAT3 has been mutated to an alanine to render it phospho-null [1,2,6,28] it would appear that phosphorylation of S727 in STAT3 is required for its mitochondrial action. Though the role of tyrosine phosphorylated STAT3 in mitochondrial function is unclear, it is known to not be required for STAT3 to regulate respiration, Ras-dependent transformation, or for tumor growth of breast cancer cells [1,2,28]. Phosphorylation of STAT5 [72] and STAT1 (Sisler JD et al., unpublished results) might also be required for their mitochondrial targeting. However, phosphorylation of STAT6 was not required for its accumulation in the mitochondria [73]. In the case of p53, a nuclear transcription factor with distinct roles in the mitochondria [3], its trafficking has been suggested to be dependent on Pin1, a cytoplasmic peptidyl-prolyl cis-trans isomerase. Isomerization of proline bonds by Pin1 has been implicated as being important for regulation of protein function and intracellular localization [75], and absence of Pin1 attenuates the recruitment of p53 to the mitochondria [76]. STAT3 has been shown to interact with Pin1 [77,78], suggesting that Pin1 may represent one mechanism through which STAT3 is transported to the mitochondria.
Once at the mitochondria, the import of STATs represents another crucial regulatory step, as most proteins that enter the mitochondria must be first unfolded. Typically, the re-folding and maturation into functional proteins depends on the mitochondrial family of heat shock proteins (HSPs). Other nuclear transcription factors have been shown to interact with these HSPs presumably as part of their mitochondrial targeting and maturation following import [3]. It remains to be determined whether HSPs play a similar role in determining STAT mitochondrial function, though considering STAT3’s localization to the inner mitochondrial membrane and matrix it is likely that some chaperone protein is required. GRIM-19, a component of Complex I of the ETC, has been shown to facilitate import of STAT3 into mitochondria [5], but it is probable that other proteins are involved, such as translocases of the outer membrane (TOM), of which STAT3 is known to interact with TOM20 (see Fig. 1) [37]. In order to better understand the function of STATs in the mitochondria the mechanism behind their mitochondrial targeting and import needs to be more fully addressed.
Lastly, how STAT protein levels are regulated in the mitochondria is also not clear. The idea of inter-organelle communication may feature prominently in this regulation. Notably, knockdown of the mitochondrial matrix protein CypD leads to constitutive activation of cytosolic STAT3 leading to its nuclear localization [79], and recent results from our lab demonstrated that expression of a mitochondrial targeted STAT3 construct led to nuclear activation of endogenous STAT3 protein [28]. Whether nuclear localized STAT3 could be exerting a similar effect on the mitochondrial pool of STAT3 requires further investigation. Even if this intracellular signaling is not responsible for determining levels of STATs in the mitochondria, it points to a much broader network of communication. Coordinate regulation and communication between the nucleus and mitochondria would serve a clear adaptive advantage, especially in the context of cellular proliferation and survival. As more information becomes available, we will be better equipped to understand the emerging role that the STAT family of transcription factors has on mitochondrial function.
Acknowledgements
We would like to thank Marc Cantwell for his critical reading of the manuscript and for his suggestions. We also thank Dr. Ed Lesnefsky and Dr. Jennifer Sisler for their valuable advice. This work was supported by R01 1GM101677, “The Jak/Stat Pathway and Mitochondrial Function,” to ACL.
Abbreviations
- STAT
Signal Transducer and Activator of Transcription
- JAK
Janus kinase
- TFs
transcription factors
- ETC
electron transport chain
- MLS
mitochondrial localization sequence
- MTS
mitochondrial targeting sequence
- MEFs
mouse embryonic fibroblasts
- KO
knockout
- ROS
reactive oxygen species
- MPTP
mitochondrial permeability transition pore
- CypD
cyclophilin D
- RNS
reactive nitrogen species
- ΔΨ
mitochondrial membrane potential
- IFN
interferon
- NS
non-structural
- HSPs
heat shock proteins
- TOM
translocase of the outer membrane
References
- 1.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szczepanek K, Lesnefsky EJ, Larner AC. Multi-tasking: nuclear transcription factors with novel roles in the mitochondria. Trends Cell Biol. 2012;22:429–437. doi: 10.1016/j.tcb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Psarra AM, Sekeris CE. Glucocorticoid receptors and other nuclear transcription factors in mitochondria and possible functions. Biochim Biophys Acta. 2009;1787:431–436. doi: 10.1016/j.bbabio.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Tammineni P, Anugula C, Mohammed F, Anjaneyulu M, Larner AC, Sepuri NB. The import of the transcription factor STAT3 into mitochondria depends on GRIM-19, a component of the electron transport chain. J Biol Chem. 2013;288:4723–4732. doi: 10.1074/jbc.M112.378984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gough DJ, Koetz L, Levy DE. The MEK-ERK pathway is necessary for serine phosphorylation of mitochondrial STAT3 and ras-mediated transformation. PLoS One. 2013;8:e83395. doi: 10.1371/journal.pone.0083395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernier M, Paul RK, Martin-Montalvo A, Scheibye-Knudsen M, Song S, He HJ, et al. Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J Biol Chem. 2011;286:19270–19279. doi: 10.1074/jbc.M110.200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elschami M, Scherr M, Philippens B, Gerardy-Schahn R. Reduction of STAT3 expression induces mitochondrial dysfunction and autophagy in cardiac HL-1 cells. Eur J Cell Biol. 2013;92:21–29. doi: 10.1016/j.ejcb.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, et al. Mitochondrial-targeted signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem. 2011;286:29610–29620. doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath CM, Wen Z, Darnell JE., Jr A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 1995;9:984–994. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- 11.Phillips D, Reilley MJ, Aponte AM, Wang G, Boja E, Gucek M, et al. Stoichiometry of STAT3 and mitochondrial proteins: Implications for the regulation of oxidative phosphorylation by protein-protein interactions. J Biol Chem. 2010;285:23532–23536. doi: 10.1074/jbc.C110.152652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarafian TA, Montes C, Imura T, Qi J, Coppola G, Geschwind DH, et al. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS One. 2010;5:e9532. doi: 10.1371/journal.pone.0009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boengler K, Ungefug E, Heusch G, Schulz R. The STAT3 inhibitor stattic impairs cardiomyocyte mitochondrial function through increased reactive oxygen species formation. Curr Pharm Des. 2013;19:6890–6895. doi: 10.2174/138161281939131127115940. [DOI] [PubMed] [Google Scholar]
- 14.Lachance C, Goupil S, Leclerc P, Stattic V. a STAT3 inhibitor, affects human spermatozoa through regulation of mitochondrial activity. J Cell Physiol. 2013;228:704–713. doi: 10.1002/jcp.24215. [DOI] [PubMed] [Google Scholar]
- 15.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Demaria M, Giorgi C, Lebiedzinska M, Esposito G, D’Angeli L, Bartoli A, et al. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging (Albany NY) 2010;2:823–842. doi: 10.18632/aging.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, et al. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol Rev. 2012;92:1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie Y, Erion DM, Yuan Z, Dietrich M, Shulman GI, Horvath TL, et al. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol. 2009;11:492–500. doi: 10.1038/ncb1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wunderlich CM, Hovelmeyer N, Wunderlich FT. Mechanisms of chronic JAKSTAT3-SOCS3 signaling in obesity. JAKSTAT. 2013;2:e23878. doi: 10.4161/jkst.23878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata N, Matsuo K, Bettaieb A, Bakke J, Matsuo I, Graham J, et al. Hepatic src homology phosphatase 2 regulates energy balance in mice. Endocrinology. 2012;153:3158–3169. doi: 10.1210/en.2012-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaper F, Gendo C, Eck M, Schmitz J, Grimm C, Anhuf D, et al. Activation of the protein tyrosine phosphatase SHP2 via the interleukin-6 signal transducing receptor protein gp130 requires tyrosine kinase Jak1 and limits acute-phase protein expression. Biochem J. 1998;335(Pt 3):557–565. doi: 10.1042/bj3350557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianotti TF, Castano G, Gemma C, Burgueno AL, Rosselli MS, Pirola CJ, et al. Mitochondrial DNA copy number is modulated by genetic variation in the signal transducer and activator of transcription 3 (STAT3) Metabolism. 2011;60:1142–1149. doi: 10.1016/j.metabol.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Zhou L, Too HP. Mitochondrial localized STAT3 is involved in NGF induced neurite outgrowth. PLoS One. 2011;6:e21680. doi: 10.1371/journal.pone.0021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L, Too HP. GDNF family ligand dependent STAT3 activation is mediated by specific alternatively spliced isoforms of GFRalpha2 and RET. Biochim Biophys Acta. 2013;1833:2789–2802. doi: 10.1016/j.bbamcr.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Raje V, Yakovlev VA, Yacoub A, Szczepanek K, Meier J, et al. Mitochondrial-localized Stat3 promotes breast cancer growth via phosphorylation of serine 727. J Biol Chem. 2013;288:31280–31288. doi: 10.1074/jbc.M113.505057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ralph SJ, Rodríguez-Enríquez S, Neuzil J, Saavedra E, Moreno-Sánchez R. The causes of cancer revisited: “Mitochondrial malignancy” and ROS-induced oncogenic transformation-why mitochondria are targets for cancer therapy. Mol Aspects Med. 2010;31:145–170. doi: 10.1016/j.mam.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 31.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Crowe PJ, Goldstein D, Yang JL. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (review) Int J Oncol. 2012;41:1181–1191. doi: 10.3892/ijo.2012.1568. [DOI] [PubMed] [Google Scholar]
- 33.Mackenzie GG, Huang L, Alston N, Ouyang N, Vrankova K, Mattheolabakis G, et al. Targeting mitochondrial STAT3 with the novel phospho-valproic acid (MDC-1112) inhibits pancreatic cancer growth in mice. PLoS One. 2013;8:e61532. doi: 10.1371/journal.pone.0061532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang R, Tang D, Lotze MT, Zeh HJ., 3rd AGER/RAGE-mediated autophagy promotes pancreatic tumorigenesis and bioenergetics through the IL6-pSTAT3 pathway. Autophagy. 2012;8:989–991. doi: 10.4161/auto.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang R, Tang D. Autophagy in pancreatic cancer pathogenesis and treatment. Am J Cancer Res. 2012;2:383–396. [PMC free article] [PubMed] [Google Scholar]
- 36.Tateno T, Asa SL, Zheng L, Mayr T, Ullrich A, Ezzat S. The FGFR4-G388R polymorphism promotes mitochondrial STAT3 serine phosphorylation to facilitate pituitary growth hormone cell tumorigenesis. PLoS Genet. 2011;7:e1002400. doi: 10.1371/journal.pgen.1002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 42.Halestrap AP, Doran E, Gillespie JP, O’Toole A. Mitochondria and cell death. Biochem Soc Trans. 2000;28:170–177. doi: 10.1042/bst0280170. [DOI] [PubMed] [Google Scholar]
- 43.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. P53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantel C, Messina-Graham S, Moh A, Cooper S, Hangoc G, Fu XY, et al. Mouse hematopoietic cell-targeted STAT3 deletion: stem/progenitor cell defects, mitochondrial dysfunction, ROS overproduction, and a rapid aging-like phenotype. Blood. 2012;120:2589–2599. doi: 10.1182/blood-2012-01-404004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shulga N, Pastorino JG. GRIM-19-mediated translocation of STAT3 to mitochondria is necessary for TNF-induced necroptosis. J Cell Sci. 2012;125:2995–3003. doi: 10.1242/jcs.103093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Szczepanek K, Chen Q, Larner AC, Lesnefsky EJ. Cytoprotection by the modulation of mitochondrial electron transport chain: the emerging role of mitochondrial STAT3. Mitochondrion. 2012;12:180–189. doi: 10.1016/j.mito.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somers SJ, Frias M, Lacerda L, Opie LH, Lecour S. Interplay between SAFE and RISK pathways in sphingosine-1-phosphate-induced cardioprotection. Cardiovasc Drugs Ther. 2012;26:227–237. doi: 10.1007/s10557-012-6376-2. [DOI] [PubMed] [Google Scholar]
- 48.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 49.Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- 50.Penna C, Perrelli MG, Tullio F, Angotti C, Camporeale A, Poli V, et al. Diazoxide postconditioning induces mitochondrial protein S-nitrosylation and a redox-sensitive mitochondrial phosphorylation/translocation of RISK elements: no role for SAFE. Basic Res Cardiol. 2013;108 doi: 10.1007/s00395-013-0371-z. 371-013-0371-z. Epub 2013 Jul 20. [DOI] [PubMed] [Google Scholar]
- 51.Jeong K, Kwon H, Min C, Pak Y. Modulation of the caveolin-3 localization to caveolae and STAT3 to mitochondria by catecholamine-induced cardiac hypertrophy in H9c2 cardiomyoblasts. Exp Mol Med. 2009;41:226–235. doi: 10.3858/emm.2009.41.4.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M, Wei J, Shan H, Wang H, Zhu Y, Xue J, et al. Calreticulin-STAT3 signaling pathway modulates mitochondrial function in a rat model of furazolidone-induced dilated cardiomyopathy. PLoS One. 2013;8:e66779. doi: 10.1371/journal.pone.0066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang M, Wei J, Pan X, Shan H, Yan R, Xue J, et al. Change of cardiac mitochondrial STAT3 activity in rats with selenium deficiency and its relation with myocardial injury. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:967–971. [PubMed] [Google Scholar]
- 54.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 55.Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol. 2005;289:H237–H242. doi: 10.1152/ajpheart.01192.2004. [DOI] [PubMed] [Google Scholar]
- 56.Xie Y, Kole S, Precht P, Pazin MJ, Bernier M. S-glutathionylation impairs signal transducer and activator of transcription 3 activation and signaling. Endocrinology. 2009;150:1122–1131. doi: 10.1210/en.2008-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zgheib C, Kurdi M, Zouein FA, Gunter BW, Stanley BA, Zgheib J, et al. Acyloxy nitroso compounds inhibit LIF signaling in endothelial cells and cardiac myocytes: Evidence that STAT3 signaling is redox-sensitive. PLoS One. 2012;7:e43313. doi: 10.1371/journal.pone.0043313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butturini E, de Prati AC, Chiavegato G, Rigo A, Cavalieri E, Darra E, et al. Mild oxidative stress induces S-glutathionylation of STAT3 and enhances chemosensitivity of tumoral cells to chemotherapeutic drugs. Free Radic Biol Med. 2013;65:1322–1330. doi: 10.1016/j.freeradbiomed.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 59.Kim J, Won JS, Singh AK, Sharma AK, Singh I. STAT3 regulation by Snitrosylation: implication for inflammatory disease. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5223. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Cheung SH, Evans EL, Shaw PE. Modulation of gene expression and tumor cell growth by redox modification of STAT3. Cancer Res. 2010;70:8222–8232. doi: 10.1158/0008-5472.CAN-10-0894. [DOI] [PubMed] [Google Scholar]
- 61.Shaw PE. Could STAT3 provide a link between respiration and cell cycle progression? Cell Cycle. 2010;9:4294–4296. doi: 10.4161/cc.9.21.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: From protection to failure. Pharmacol Ther. 2008;120:172–185. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Qiu H, Lizano P, Laure L, Sui X, Rashed E, Park JY, et al. H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of STAT3 and accelerates the transition into heart failure on cardiac overload. Circulation. 2011;124:406–415. doi: 10.1161/CIRCULATIONAHA.110.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci. 2009;29:7003–7014. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ao X, Fang F, Xu F. Vasoactive intestinal peptide protects alveolar epithelial cells against hyperoxia via promoting the activation of STAT3. Regul Pept. 2011;168:1–4. doi: 10.1016/j.regpep.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 67.Lee HJ, Oh YK, Rhee M, Lim JY, Hwang JY, Park YS, et al. The role of STAT1/IRF-1 on synergistic ROS production and loss of mitochondrial transmembrane potential during hepatic cell death induced by LPS/d-GalN. J Mol Biol. 2007;369:967–984. doi: 10.1016/j.jmb.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 68.DeVries TA, Kalkofen RL, Matassa AA, Reyland ME. Protein kinase cdelta regulates apoptosis via activation of STAT1. J Biol Chem. 2004;279:45603–45612. doi: 10.1074/jbc.M407448200. [DOI] [PubMed] [Google Scholar]
- 69.Balasubramanian A, Ganju RK, Groopman JE. Signal transducer and activator of transcription factor 1 mediates apoptosis induced by hepatitis C virus and HIV envelope proteins in hepatocytes. J Infect Dis. 2006;194:670–681. doi: 10.1086/505708. [DOI] [PubMed] [Google Scholar]
- 70.Goswami R, Majumdar T, Dhar J, Chattopadhyay S, Bandyopadhyay SK, Verbovetskaya V, et al. Viral degradasome hijacks mitochondria to suppress innate immunity. Cell Res. 2013;23:1025–1042. doi: 10.1038/cr.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lartigue L, Faustin B. Mitochondria: metabolic regulators of innate immune responses to pathogens and cell stress. Int J Biochem Cell Biol. 2013;45:2052–2056. doi: 10.1016/j.biocel.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 72.Chueh FY, Leong KF, Yu CL. Mitochondrial translocation of signal transducer and activator of transcription 5 (STAT5) in leukemic T cells and cytokine-stimulated cells. Biochem Biophys Res Commun. 2010;402:778–783. doi: 10.1016/j.bbrc.2010.10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan R, Lee JE, Yang YM, Liang FX, Sehgal PB. Live-cell imaging of the association of STAT6-GFP with mitochondria. PLoS One. 2013;8:e55426. doi: 10.1371/journal.pone.0055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cimica V, Chen HC, Iyer JK, Reich NC. Dynamics of the STAT3 transcription factor: Nuclear import dependent on ran and importin-beta1. PLoS One. 2011;6:e20188. doi: 10.1371/journal.pone.0020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu KP, Zhou XZ. The prolyl isomerase PIN1: A pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 76.Sorrentino G, Mioni M, Giorgi C, Ruggeri N, Pinton P, Moll U, et al. The prolyl-isomerase Pin1 activates the mitochondrial death program of p53. Cell Death Differ. 2013;20:198–208. doi: 10.1038/cdd.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lufei C, Koh TH, Uchida T, Cao X. Pin1 is required for the Ser727 phosphorylation-dependent Stat3 activity. Oncogene. 2007;26:7656–7664. doi: 10.1038/sj.onc.1210567. [DOI] [PubMed] [Google Scholar]
- 78.Lv L, Zhang J, Zhang L, Xue G, Wang P, Meng Q, et al. Essential role of Pin1 via STAT3 signalling and mitochondria-dependent pathways in restenosis in type 2 diabetes. J Cell Mol Med. 2013;17:989–1005. doi: 10.1111/jcmm.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tavecchio M, Lisanti S, Lam A, Ghosh JC, Martin NM, O’Connell M, et al. Cyclophilin D extramitochondrial signaling controls cell cycle progression and chemokine-directed cell motility. J Biol Chem. 2013;288:5553–5561. doi: 10.1074/jbc.M112.433045. [DOI] [PMC free article] [PubMed] [Google Scholar]