Abstract
Executive function and working memory deficits are not only present in ADHD, but also in Reading Disorder (RD). Here, high-density ERPs were recorded during the Stop Signal Task in 53 children and adolescents: An ADHD-combined type group, a group with RD, and a healthy control group. The ADHD-C group displayed unique abnormalities of the frontal N200. Both healthy controls and RD groups showed a success-related right frontal N200 modulation, which was absent in the ADHD group. Second, for Success Inhibition trials, the ADHD-C had smaller right frontal N200 waves relative to healthy controls, while the RD group didn't. In contrast, NoGo-P3 abnormalities were present both in the ADHD-C and RD groups. Impaired early response inhibition mechanisms, indexed by the frontal N200, appear to be limited to ADHD-C. In contrast, deficits in later cognitive control and error monitoring mechanisms, indexed by the NoGo-P3, appear to be present in both conditions.
Keywords: ADHD, Reading Disorder, Event-Related Potentials, Stop Signal Task, Inhibitory Control, N200, NoGo-P3
Introduction
Attention Deficit-Hyperactivity Disorder (ADHD) is a common behavioral syndrome, characterized by low levels of attention and concentration and high levels of activity, distractibility and impulsivity (APA, 1994). There is a high rate of co-morbidity of ADHD with learning disabilities. ADHD has been found to be associated with reading disorder (RD) in at least 20% of the cases (Semrud-Clikeman et al, 1992). In the last twenty years several theoretical models have been formulated, and empirical research has been gathered, on what constitute the main core of cognitive symptoms in ADHD. However, only more recently research has attempted to characterize and separate the core cognitive features of ADHD and RD (e.g., Purvis et al, 2000; Burgio-Murphy et al, 2007; Tiffin-Richards et al, 2008).
One of the most influential theoretical models of ADHD posits that deficits in inhibitory control are the core symptoms in ADHD (Barkley, 1997). Other theoretical models emphasize deficits in cognitive control mechanisms, including both conflict monitoring and error processing (Nieuwenhuius, Yeung, van den Wildenberg & Ridderinkhof, 2003), a dysfunction in the regulation of motivation and reward, with a preference for immediate versus delayed rewards (delay aversion, e.g., Sonuga-Barke, 2005) or deficits in state/arousal regulation (cognitive-energetic model, e.g. Sergeant, 2000). In support of the inhibitory control model, children with Attention Deficit-Hyperactivity Disorder (ADHD) are impaired in laboratory tests that tap into response inhibition, such as go-NoGo tasks (see a review in Nichols & Waschbusch, 2004).
Neural mechanisms underlying inhibitory processes can be studied with a high degree of temporal resolution by recording event-related potentials (ERPs) from the scalp. In ERP studies using go-NoGo tasks, the frontally-maximal N200 wave, peaking around 200 ms, has been shown to have greater amplitude for NoGo relative to Go trials (e.g., Kok, 1986; Falkenstein et al, 1999; Smith et al, 2004). It has been proposed that the NoGo N200 indexes an early mechanism of inhibitory control that is a reflection of a “red flag” signal generated in prefrontal cortex to trigger the inhibitory process (Kok, 1986; Jodo & Kayama, 1992). In studies using the Stop Signal Task (SST, Logan et al, 1984) a frontal NoGo-N200 has been reported as being abnormally reduced in children with ADHD relative to control children (Pliszka et al, 2000; Dimoska et al, 2003; Albrecht et al, 2005; Liotti et al, 2007). Furthermore, a right frontal N200 is greater for Success than Failed inhibition trials, and this modulation is absent in ADHD children (Liotti et al, 2007). Likely source generators of the frontal N200 effects are suggested by fMRI studies of the SST in healthy subjects, pointing to the right middle/inferior frontal gyrus as critically involved in inhibitory control (Konishi et al, 1999; Rubia et al, 2005). Critically, ADHD adolescents have been found to display reduced right middle/inferior frontal gyrus activation in response to Stop Signals, particularly in response to Successful Inhibitions (Rubia et al, 1999; 2005). In summary, the available evidence point to a specific right PFC abnormality associated to the early triggering of inhibitory responses in ADHD-C adolescents and children.
A second ERP component associated to response inhibition in go-NoGo tasks is the NoGo-P3, (peaking around 300 msec), which displays greater amplitude over the frontocentral region for NoGo than Go trials (e.g., Falkenstein et al, 2002). ADHD children have been shown to display reduced No-Go-P3 waves in response to Stop Signals (particularly Failed inhibitions, Overtoom et al, 2002; Fallgatter et al, 2004; Liotti et al, 2005). fMRI studies of the SST and Stroop task in ADHD adolescents and children showed less activity in dorsal Anterior Cingulate cortex (dACC), particularly in response to Failed Inhibitions (Pliszka et al, 2006; Rubia et al, 2005). Recent models of the role of ACC emphasize a general role in conflict monitoring and error processing (Botvinick et al, 2001; Nieuwenhuius et al, 2003). For this reason, the Nogo-P3 has been associated to a late stage of monitoring of the outcome of the inhibitory process (e.g., Nieuwenhuius et al, 2003). The combined ERP and fMRI evidence to-date in ADHD points to a deficit in cognitive monitoring operations depending on dACC function (Nieuwenhuius et al, 2003; Liotti et al, 2005). A simple account in terms of inhibitory control (Barkley, 1997) may therefore be insufficient to capture the spectrum of cognitive operations impaired in ADHD (see Banaschewski et al, 2004, for a similar conclusion). A multi-dimensional account appears to be necessary, also including a deficit in cognitive control operations orchestrated by the dACC.
Recent research is starting to address the issue of characterizing and separating cognitive symptoms which are unique to ADHD or shared by other developmental disorders, and Reading Disorder (RD) in particular. Developmental dyslexia or RD is among the most prevalent of learning disabilities with estimates ranging from 5% to 12% (e.g., Shaywitz, 1998). There is a high rate of co-morbidity with other developmental conditions. In particular, ADHD has been found to be associated with RD in at least 20% of the cases (Semrud-Clikeman et al, 1992; Barkley, 1997). Children with a reading disability have been found to show a higher rate of attentional difficulties (Shaywitz et al, 1994). In addition, studies have shown impaired performance in attentional tasks requiring greater levels of selection and conflict, such as in the Stroop Task and the Wisconsin card sorting task (WCST, Bednarek et al, 2004; Helland & Asbjornsen, 2000; Tiffin-Richards et al, 2008).
Two recent studies have attempted to identify underlying mechanisms of impaired executive function in RD using ERPs. The first study employed the Continuous Performance Task (CPT) in RD and control adolescents. They reported reduced amplitude and increased latency of the NoGo-P3 in the RD group. Furthermore, the NoGo-P3 was greater on the right in controls, but symmetric in the RD group (Taroyan et al, 2007). A second study explored error processing in children with ADHD combined subtype (ADHD-C), RD, RD+Math disorder and control children. They found that the ADHD-C group had greater amplitude of the error related negativity (ERN) relative to the healthy control group, while the Error Positivity (Pe) was reduced in children with RD+Math disorder relative to the RD only and control groups (Burgio-Murphy et al, 2007). No ERP studies to-date have directly compared ADHD and RD groups in tasks directly tapping into response inhibition.
As an attempt to further characterize and possibly separate the cognitive core symptoms in ADHD and RD, the present study explored electrophysiological mechanisms of inhibitory control and cognitive monitoring (indexed by the N200 and the NoGo-P3) in three age and IQ matched groups of children: ADHD-C without RD, RD without ADHD, and a healthy comparison group. The ADHD and healthy comparison groups were part of a larger cohort of ADHD and healthy children recruited for an ERP and neuroimaging study of inhibitory control in ADHD-C (Pliszka et al, 2006; Liotti et al, 2007). To control for co-morbidity, the ADHD-C children did not meet criteria for a learning disability, and RD in particular and conversely, the RD children did not meet criteria for ADHD (any subtype).
Our first prediction is that only the ADHD-C children would show impaired behavioral measures of response inhibition in the SST, while the RD group would perform the task within normal limits. Concerning our ERP measures, following the reasoning that NoGo-N200 wave would directly reflect inhibitory control, and therefore relate to hyperactivity and impulsivity symptoms selectively present in children with ADHD, a second prediction was that right frontal N200 reduction and the absence of success-related N200 amplitude modulation reported previously would be only present in the ADHD-C group. In contrast, if the noGo-P3 reflects other executive control mechanisms, and in particular monitoring of the successful and unsuccessful outcome (errors) of the inhibitory process, our third prediction was that a NoGo-P3 reduction may not be restricted to ADHD-C, but also present in RD, where inattention, executive function and working memory deficits have been demonstrated.
Methods
Participants and diagnostic instruments
Subjects were right-handed children and adolescents aged 9 to 15 years of both genders. Participants' handedness was established by writing, throwing, demonstrating how they brushed their teeth, and show how they would kick a ball. All skills needed to be executed in front of the experimenter using each hand/foot. The study groups were subjects meeting criteria for ADHD-Combined Type (ADHD-C: n=16; 11 males), children meeting criteria for Reading Disorder (n=14; 10 males); and healthy controls (n=22; 14 males). ADHD-C and control subjects were part of larger cohorts whose ERP and fMRI findings in the SST have been published elsewhere (Liotti et al, 2007; Pliszka et al, 2006). Written informed consent from a parent and assent from the child were obtained according to the Institutional Review Board of the Health Science Center at San Antonio.
Individuals with ADHD met Diagnostic Interview for Children-Version IV-Parent version (DISC-IV-P) criteria for ADHD-C, could meet criteria for oppositional defiant disorder, but not meet criteria for conduct disorder or any anxiety, tic or affective disorder. All ADHD-C subjects in the present study had no history of psychotropic medication treatment, i.e., they were treatment naïve. Reading Disorder subjects and healthy controls could not meet criteria for any psychiatric disorders or any history of past treatment with psychotropic medication. No subjects in any group had history of neurological conditions or symptoms, such as head injury, loss of consciousness, motor or sensory loss, nor had they a history of substance or alcohol abuse. Children in the three groups were not taking any medication for a chronic condition on a daily basis for the last 3 months prior to the study.
Children with Reading Disorder (RD) were initially referred from the Texas school system. These children showed significant difficulties beyond what is required for a clinical diagnosis of RD in the state of Texas, including neuropsychological deficits in phonological analysis. All RD children were receiving special education services through the school in the area. Diagnosis of Reading Disorder was confirmed based on discrepancies between a standard measure of reading achievement (see below) and their General Conceptual Ability (GCA) score of the Differential Abilities Scales (DAS). The Broad Reading Score from the Wechsler Individual Achievement Test, Second Edition (WIAT-II) is a commonly used measure to evaluate a child's reading skills. It combines the scores from the Word Reading and Reading Comprehension tasks. All RD children had Broad Reading scores that were at least 20 standard score points below their GCA score.
RD children did not meet criteria for ADHD (either combined or inattentive) on the DISC-IV-P (see below). They were within 1 SD of the mean for their age and sex on the Cognitive Problems/Inattention and Hyperactivity factors of both the Connors teacher's rating scale-revised (CTRS-R) and the Connors parent's rating scale-revised (CPRS-R).
In addition to the DISC-IV-P, the Conners Global Index was used. Criteria for inclusion were based on parent and teacher ratings of the Restless/Impulsive score (RI). For the Reading Disorder and the healthy group, inclusion criteria were an RI equal or less than one standard deviation (SD) of the mean for child's age and gender. For the group of children with ADHD, inclusion criteria were an RI equal or greater than 1.5 SD of the mean for child's age and gender.
Current cognitive functioning was measured with the DAS. All subjects in the 3 groups were required to have a general conceptual ability (GCA) score > 85. The WIAT-II was administered to assess reading, writing and mathematics in all subjects. To rule out learning disorders in the ADHD and healthy groups, all subjects had to have reading and mathematic standard scores on the WIAT that were within one standard deviation of their full scale IQ on the Differential Abilities Scale.
Stimuli and Task
Participants sat with their eyes 50 cm from a computer monitor presenting a visual version of the Stop Signal Task (Pliszka et al, 2000). They discriminated between the letter “A” or “B” by responding with the index finger of the left or right hand. Stimuli were flashed for 150 ms slightly above a fixation dot. In 25% of the trials the A or B (Go stimuli) were followed by the letter “S” (stop signal) appearing slightly below fixation for 150 ms. Letters size was 1° × 0.7°. The interval between Go stimuli and the stop signal varied randomly (200-600 ms). Subjects were instructed to inhibit their response when seeing an S. Each trial ended with a random intertrial interval (1.5-1.8 sec). Each experimental run included 72 Go and 24 stop signal trials; there was a total of 10 runs, each lasting about 3 min. After each run, subjects were given about 30 sec to rest. After that, they were asked if they needed additional time, which was allowed as requested until they were ready to resume the task. In addition, a longer resting pause (about 5 min) was provided half way through the session. Subjects received 1-3 practice runs before the acquisition of data to ensure they understood the task and were capable of performing above chance. Subjects were told that it was important both to respond accurately to the GO signal (and not miss very many) and not to slow down excessively. To discourage strategic slowing, an on-line adjustment of difficulty of inhibitory performance was implemented. For each subject and run, if the mean Go RT in a run was longer than 600 msec (for example, 670 msec), all Stop Signal intervals in the following run were increased by the amount of time over 600 msec (+70 msec, see also Pliszka et al, 2000). This prevented the subjects from deliberately slowing down to catch all the stop signals.
Behavioral Analysis
The following behavioral parameters were measured: RT and percent error in the Go trials, probability of inhibition [P(I)] for each of the four 100-ms SOA subranges, Stop signal reaction time (SSRT) for each SOA subrange (Logan et al, 1984; Pliszka et al, 2000). The probability of inihibition [P(I)] was calculated for each of the four SOAs. For example, if a child successfully inhibited 25 of the 30 STOP signals with an SOA of 250 msec, the P(I) for that SOA would be .83. It is generally easy to inhibit a response when the stop signal occurs close to the Go stimulus, and more difficult (resulting in a lower P[(I)]) when the stop signal occurs far from the Go signal. A slope was then calculated using the P[(I)] values for the four stop signal SOAs. The stop signal reaction time (SSRT), which provides a measure of speed of the inhibitory process, was then determined according to the method of Logan et al (1984). The SSRT is presumed to begin when the stop signal occurs. It is calculated from the P[(I)] and the distribution of reaction times (RT) to the Go signal. The subject's reaction times to the Go signal are normally distributed. If a subject inhibits successfully 80% of the time at the 250 msec SOA, it is assumed that the stop process is fast enough to interrupt all the Go responses between the onset of the stop signal and the Go RT that is 80% of the distance from the longest RT. The RTs are rank-ordered from the longest to the shortest, and the RT that is 80% down the list is selected. The SSRT is calculated by subtracting that SOA (250 msec) from that RT; this is done for each of the four stop signal delays. The mean of these four values is the final SSRT for the subject.
Differences between diagnostic groups in mean SSRT and the slope of the inhibitory function can be confounded if there are group differences in the mean RT to the Go stimulus and/or variability of RT. This can be controlled by calculating the “relative finishing time” or ZRFT (Logan & al, 2004; Band, van der Molen & Logan, 2003). ZRFT is obtained for each of the four delay times using the formula: ZRFT= (mean Go RT − SOA − SSRT)/SD Go RT. The four values are again plotted and the slope calculated. If the differences in mean slope of the ZRFT remain significant after this correction, it can be concluded that the groups differ in ability to inhibit.
EEG recording and ERP analysis
Brain electrical activity was recorded using a customized 64-channel cap (Electrocap Inc., Eaton, OH) including four eye movement electrodes (two at the external canthi and two infraorbital) and a left mastoid electrode, all referenced to the right mastoid (as used in Pliszka et al, 2000; Liotti et al, 2000; 2007). The customized cap incorporated evenly distributed as well more ventral coverage of the scalp surface. In such modified configuration, electrode nomenclature is by reference to the electrode location of the traditional 10-20 system. The suffixes a, p, s, i stand, respectively, for anterior, posterior, superior, inferior to standard 10-20 recording sites (See Figure 1 for a selection of the modified sites). Amplifier settings were: bandpass= 0.01-100 Hz, gain= 104, sampling rate= 400 Hz, impedances <5kΩ. Epochs of the EEG contaminated by eye blinks were rejected off-line using an individually adjusted semi-automatic routine based on EEG amplitude in the frontopolar sites above the eyes (Fp1-Fp2) and the infraorbital electrodes below the eyes. Individual subject ERP averages were obtained for successful inhibitions (SI) and failed inhibitions (FI), time-locking to stop signal onset. Only FI trials where a button press followed the Stop signal were included. After eye-blink rejection, one participant in the RD group was eliminated for insufficient number of trials in one condition (<20, leaving an n=13 for that group). Mean number of blink-free trials and Standard Errors were as follow. For Successful Inhibitions: Controls= 101.2±7.2; RD= 85.8±9.3; ADHD= 74.8±8.4. For Failed Inhibitions: Controls= 43.8±4.0; RD= 46.6±5.2; ADHD= 42.7±4.7. Controls had significantly more SI trials than ADHD children, t(36)=2.6, p=0.012. The number of FI trials was similar across groups.
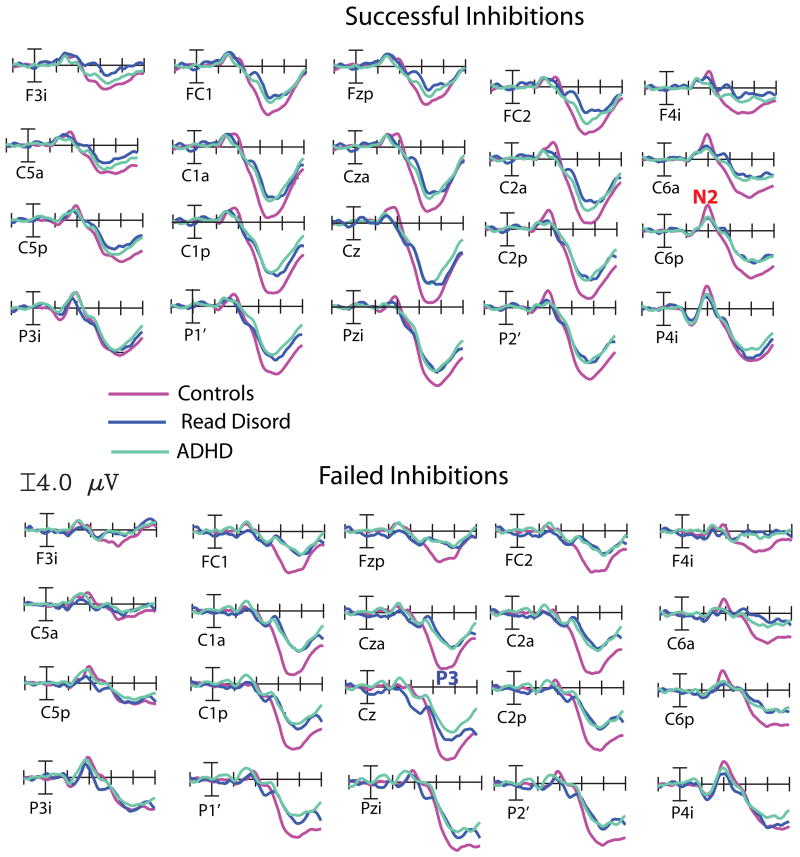
Figure 1.
Grandaverage ERP waveforms time-locked to the Stop Signal for Successful Inhibitions (top) and Failed Inhibitions (bottom) at 20 representative scalp sites over frontal, central and parietal scalp sites for the Control Group (in purple), the ADHD Group (in green) and the Reading Disorder Group (in blue).
ERP averages were re-referenced to the average of the two mastoid electrodes, and smoothed with a 9-point running average filter. Grand averages were calculated across subjects for each trial type and group. To help isolating effects of interest, within-group and between groups difference waves were calculated. To facilitate visualization, scalp voltage topographic distributions were obtained using spherical spline interpolation (Perrin et al, 1987).
Based on previous findings on N200 and NoGo-P3 in the Stop Signal Task (Pliszka et al, 2000; Overtoom et al, 2002; Liotti et al, 2007) after inspection of grand-average waveforms and scalp topography distributions for each trial type and various difference waves, time windows were selected around the peaks of the N200 (175-225 ms), and the NoGo-P3 (300-400 ms) for detailed analysis. Regions of interest (ROIs) were selected, by averaging together neighbour electrode sites. For the N200 group analyses, four ROIs were chosen, a left and right anterior frontal ROI (sites F7p/F8p, C3a/C4a, T3′/T4′ and PA1a/PA2a) and a left and right posterior temporo-parietal ROI (sites P3i/P4i, P3a/P4a, C5p/C6p and T35i/T46i). For the N200 within group analysis, the frontal ROIs were adjusted to reflect a more dorsal scalp distribution (sites FC1/FC2, F7a/F8a, C3a/C4a and F7p/F8p). For the NoGo-P3 window, four ROIs were chosen, two frontocentral (F3S/F4S, FC1/FC2, C1a/C2a and C3a/C4a), while the posterior ROI collapsed four parieto-occipital scalp sites (C1p/C2p, P1′/P2′, P3a/P4a and PO1/PO2).
Given our previous ERP findings of focal reductions of N200 and NoGo-P3 in ADHD relative control children using the SST, and success-related modulations of the same components (Pliszka et al, 2000; Liotti et al, 2005; Liotti et al, 2007), within group and between group analyses of Variance were restricted to a-priori scalp regions. For all analyses, the critical p-value was set at .05 (degrees of freedom were corrected with the Greenhouse-Geisser epsilon method (Greenhouse & Geisser, 1954).
Because of the short interval between the Go and Stop stimuli, the elicited ERP responses overlapped in time, distorting the final ERP averages. As predicted by previous behavioral studies, control children had steeper response inhibition functions (more SIs for shorter delay intervals and more FIs for longer intervals) than did the ADHD children. To correct for this differential overlap distortion problem across groups, subaverages of the SI and FI ERPs were obtained for each of the four time-delay subranges for each subject. These four subaverages were then collapsed together in an equally weighted way (i.e., 25% weighting for each subaverage), thereby equalizing the overlap from the Go-event ERPs, both between group and between trial type (Pliszka et al, 2000).
Results
Clinical and Demographic Variables
The groups were similar in age, verbal IQ or non-verbal IQ, but there was a marginally significant effect of GCA, F(2,49)=3.2, p =.051. Although the ADHD-C mean GCA score was lower, post-hoc contrasts (Tukey method) did not reveal significant group differences. As expected, there were main effects of diagnosis on WIAT Reading, F(2,49)=22.7, p<.001, on WIAT Writing, F(2, 49)=22.4, p< .001, and on WIAT Math, F (2,49)= 4.2, p =.022. For Reading and Writing subtests, all three groups significantly differed from each other, with the Control group performing the best, and the RD group scoring the worse. For the Math subtest, the Control group performed better than the ADHD-C and RD groups, which did not differ from each other (see Table 1).
Table 1.
Demographic characteristics, neuropsychological measures, and performance in SST
Demographic, clinical and stop signal performance variables
| ADHD | RD | Controls | p value | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||
| Age (years) | 12.3 (1.7) | 11.5 (1.6) | 12.4 (1.9) | ns |
| DAS- Verbal | 102.4 (12.4) | 108.6 (13.8) | 110.3 (15.2) | ns |
| DAS-Non Verbal | 105.3 (12.0) | 108.6 (9.3) | 114.9 (14.1) | ns |
| Gen. Cognitive Ability1 | 103.8 (12.9) | 112.0 (10.9) | 114.0 (13.5) | .051 |
| WIAT Reading2 | 100.4 (9.1) | 85.4 (10.0) | 108.4 (10.6) | .001 |
| WIAT Writing2 | 96.3 (8.0) | 85.1 (10.4) | 109.0 (12.2) | .001 |
| WIAT Math3 | 100.5 (13.9) | 101.2 (11.7) | 111.4 (13.5) | .022 |
| Parent Conners R/I4 | 83.0 (6.2) | 52.7 (8.9) | 48.6 (6.3) | .001 |
| Parent Conners Global4 | 82.7 (6.9) | 52.3 (9.2) | 47.3 (5.5) | .001 |
| Teacher Conners R/I4 | 80.1 (7.3) | 51.6 (6.8) | 48.4 (7.5) | .001 |
| Teacher Conners Global4 | 81.1 (8.9) | 50.1 (5.3) | 45.3 (9.6) | .001 |
| Accuray on Go (%) | 0.9 (0.1) | 0.8 (0.1) | 0.9 (0.1) | ns |
| RT on Go Task1 | 839.6 (175.9) | 834.3 (106.6) | 944.9 (157.2) | .049 |
| RT SD on Go Task | 198.3 (46.3) | 230.7 (34.8) | 174.0 (49.0) | .017 |
| Slope P(I) | - 0.8 (0.5) | -1.0 (0.7) | -1.3 (0.8) | .064 |
| SSRT (msec)4 | 321.6 (128.3) | 243.5 (94.3) | 221.9 (109.7) | .028 |
| ZRFT | - 5.4 (1.7) | - 4.45 (0.82) | - 6.38 (2.6) | .026 |
: Main effect of diagnosis, no multiple comparisons significant
: All three groups different from each other (RD<ADHD<Control)
: Both ADHD and RD different from control group(ADHD=RD<Control)
: ADHD group different from two other groups. RD and control not different from each other (ADHD<RD=Control).
There were main effects of diagnosis on the Parent Conners R/I scale F(2,49)=121.0, p < .001, and Global scales, F(2,49)=126.3, p < .001; for both scales, multiple comparisons showed that the control group was significantly lower than the ADHD and RD groups. For the teacher ratings, there was a main effect of diagnosis on both the R/I, F(2,48) = 97.7, p < .001 and the Global scale, F(2,49)= 89.9, p< .001, with Tukey tests showing that the ADHD-C group was higher than the other two groups. A summary of clinical and demographic variables is presented in Table 1.
Stop Signal Task Performance
The groups were not different in accuracy to the Go task (percent errors) (See table 1). There was a main effect of diagnosis on mean RT to the Go signal, with the control group being slower than the other two groups, F(2,49)=3.2, p =.049, but the multiple comparisons were not significant. There was also a significant effect of diagnosis on RT variability to the Go trials, F(2,49)=6.2, p =.004, with the RD group being more variable than the ADHD and control groups. There was a trend for the slope of the inhibitory function to be different between groups, F(2,49) =2.9, p=.064, with the ADHD group being the worse and the control group the best. Importantly, there was a main effect of diagnosis for the SSRT, F(2,49)=3.83, p=.028. As predicted, the ADHD group had the slower inhibitory process, as shown by the significantly longer SSRT in that group relative to the other two groups, who were not significantly different from each other. Because the groups were significantly different from each other in both mean Go RT and Go RT variability, ZFRTs were calculated for each stop signal delay and the slope of the inhibitory function was recalculated. As can be seen in Table 1, the group differences in slope remained significant after correcting for differences in the primary Go task, F(2,49)=4.46, p=.026. Indeed, controlling for Go RT differences enhanced the differences between groups. A summary of behavioural findings in the Stop Signal Task is provided in the bottom of Table 1.
ERP results
R Frontal N200 wave to the Stop signal- Between Group contrasts
Successful Inhibitions
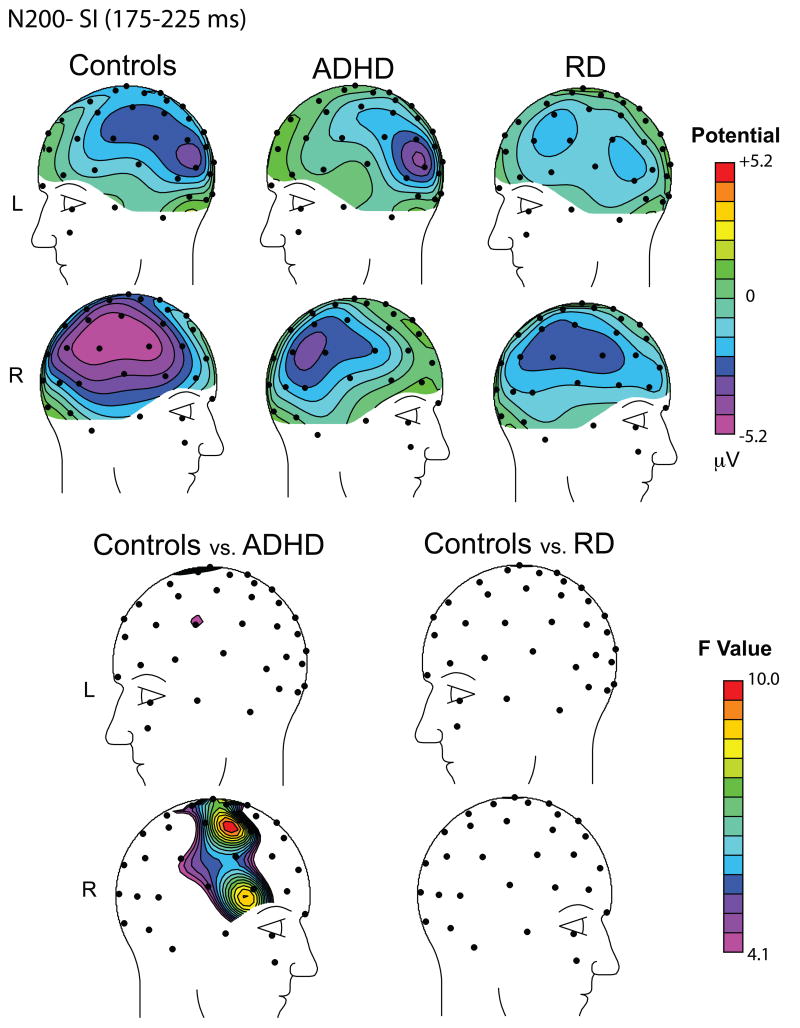
A global analysis including the three groups revealed a significant effect of diagnosis, F (2,48)=3.94, p=0.026. The N200 amplitude was significantly reduced in the ADHD compared to the Control group, t(37)=2.89, p=0.007, effect size (Cohen d′)= 0.72. In contrast, there were no significant N200 amplitude differences between the RD and the Controls, t(33)=1.46, p=0.15, or the RD and the ADHD group, t(27)= -.98, p=0.34. As expected, no overall N200 amplitude differences were evident among groups for the left frontal ROI, F(2,48)=0.78, p=0.47, or the left and right posterior ROIs [F(2,48)=0.95, p=0.39 and F(2.48)=2.23, p=0.12, respectively].
Failed Inhibitions
Unlike the Successful Inhibitions, no group differences in N200 amplitude were present for the right frontal ROI, F(2,48)=1.89, p=0.16, nor for any the other three ROIs (for all, p>0.14).
Frontal N200 wave to the Stop signal- Within-group effects
Healthy control Group
Over the frontal scalp, N200 amplitudes were greater for Successful than Failed Inhibitions, F(1,22)=7.35, p=0.013. However this effect was qualified by the significant trial type × hemisphere interaction, F(2,22)=5.50, p=0.029. Over the right frontal ROI, successful stops yielded greater N200s than failed stops, t(21)=-3.41, p=0.003, while the difference was not significant over the left frontal ROI, t(22)=-0.835, p=0.413. No N200 modulation as a function of trial type was evident over posterior scalp.
RD Group
Over frontal scalp, there was a main effect of trial type, with greater N200s for Successful than Failed Stops, F(1,12)=5.72, p=0.034. There was no hint of an interaction between trial and hemisphere, F(1,12)=0.007, p=0.94. Over posterior scalp, there were no main effects or interactions involving trial type (for all, F<.15).
ADHD Group
At variance with the Control and RD groups, over the frontal scalp there were no main effect or trial type [F(1,15)=0.32, p=0.58] nor interactions of trial type × hemisphere [F(1,15)=1.42, p=0.25]. No differences were evident over posterior scalp as well.
The effects on the N200 wave to the Stop signal for the Successful Inhibitions are illustrated in Figure 1 left (waveforms) and right (topographical maps).
Summary of N200 findings
The ADHD-C group exhibited a significant reduction of N200 amplitude to the Stop signal over right frontal scalp relative to the control group. In contrast, no such reduction was present in the RD group. A success-related enhancement of the N200, greater over frontal scalp was present in both control and RD children, while it was entirely absent in the ADHD-C group.
Nogo-P3 to the Stop signal (300-400 ms)
Within-Group effects
NoGo-P3 amplitude was significantly greater for the temporo-parietal than frontocentral ROIs in all groups. A significant success-related NoGo-P3 modulation (Successful greater than Failed Inhibitions) was present all three groups [RD: F(1,12)=8.63, p=.012; ADHD: F(1,16)=8.02, p=.013, Controls: F(1,21)=6.55, p=.018]. See an illustration of the findings in Figure 2.
Figure 2.
Topographic maps of the N200 wave (175-225 ms) to the Stop Signal for Successful Inhibitions (SI) illustrating the N200 group effects. Top: Scalp distribution of the N200 mean voltage amplitude in μV for the Control Group (Left), the ADHD Group (center) and the RD Group (right). Bottom: Scalp distribution of F-values in the contrast of Controls versus ADHD (left) and Controls versus RD (right). Maps are the result of omnibus F-tests at each scalp sensor, uncorrected for multiple comparisons. Note the presence of a significant right frontal N200 reduction in the ADHD group only.
Between Group effects
ADHD vs Controls
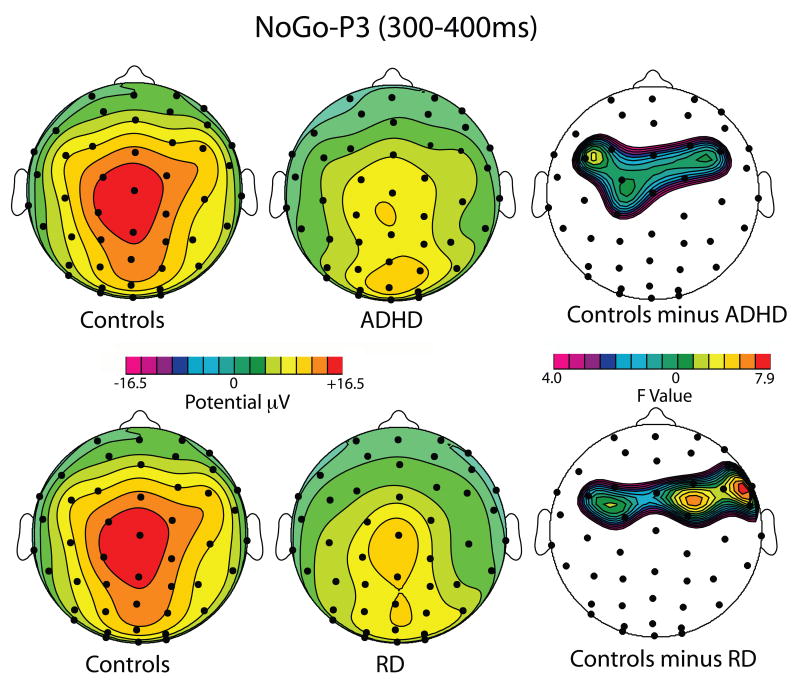
For Successful Inhibitions, mean noGo-P3 amplitude elicited by the Stop signal did not statistically differ between the two groups over any ROIs (for all, p>0.10). In contrast, for Failed Inhibitions, mean noGo-P3 voltage was significantly reduced for the ADHD group over frontocentral scalp (left ROI: F(1,36)=6.23, p=0.017; right ROI: F(1,36)=5.17, p=0.029), but not over temporoparietal scalp (for both hemispheres, p>0.10). See these effects in Figure 2.
RD vs. Controls
For Successful Inhibitions, mean NoGo-P3 amplitude was significantly reduced in the RD group over the right frontocentral ROI, F(1,33)= 4.30, p=0.046, but not the left frontocentral ROI (p>0.10). For Failed Inhibitions, mean NoGo-P3 amplitude was significantly reduced in the RD group over frontocentral scalp [left ROI: F(1,33)= 5.14, p=0.030; right ROI: F(1,33)=5.98, p=0.020], while it was not dissimilar over posterior scalp (for both hemispheres, p>0.16).
ADHD vs. RD
No mean noGo-P3 amplitude differences were present for either Successful or Failed Inhibitions over either frontocentral or posterior scalp (for all, p>0.47).
Summary of NoGo-P3 findings
NoGo-P3 amplitude in response to the Stop signal was modulated by success (Success Inhibitions greater than Fail Inhibitions) in all three groups. NoGo-P3 amplitude abnormalities were found in both ADHD-C and RD groups. Both ADHD children and RD children had smaller NoGo-P3 amplitudes for Failed Inhibition trials than control children. The effect was restricted to the frontocentral region (anterior ROI). In addition, the RD group appeared to have reduced NoGo-P3 also to Successful Inhibitions over the right frontal ROI.
Discussion
The main goal of this study was to ascertain whether previously reported behavioural and ERP abnormalities during the Stop Signal task in ADHD-C are restricted to this disorder, or shared by another common developmental condition, Reading Disorder. All three hypotheses were confirmed. Behaviourally, only the ADHD-C group had a significant slowing of the Stop Signal Reaction Time (SSRT) relative to the healthy controls. In the evoked response to the Stop Signal, the ADHD-C group had unique abnormalities of the right frontal N200. In contrast, the later fronto-central NoGo-P3 was abnormally reduced in both ADHD-C and RD children relative to the healthy controls. Such wave is associated to cognitive control operations which may be impaired in both developmental pathologies.
Behavioural findings
Mean SSRT was significantly longer for the ADHD-C group than for the healthy control group, consistent with previous behavioural findings in the literature (see meta-analysis of results in Lijffijt et al, 2005). More notably here, mean SSRT for the RD group was similar to the healthy control group, and significantly shorter than in the ADHD-C group.
Please note that the groups differed in mean RT to the GO signal, and the two clinical groups had more RT variability than the control group. Controls had the longer mean RT, in contrast to other studies reporting that children with ADHD more often have a longer mean RT than control groups. This suggests that in the present study, controls may have deliberately adopted a “slowing” strategy to enhance their ability to inhibit. Nonetheless, the differences in the inhibitory function remained significantly different even after controlling for differences in mean RT and variability on the GO trials, suggesting that the ADHD group truly had deficient inhibitory ability relative to the other groups. These methodological difficulties with strategic slowing can be avoided in the future by using the tracking version of the stop signal task, wherein the appearance of the stop signal is varied trial by trial based on whether or not the participant inhibited successfully on the previous stop trial (Logan, Schachar & Tannock, 1997).
Overall, the present behavioural results confirm our first prediction that RD without ADHD does not affect the primary response inhibition mechanism. To our knowledge, this is the first report of behavioural differences during the SST in a study directly comparing ADHD-C, RD and healthy children.
ERP findings: N200 effects
Consistent with our second hypothesis, ADHD-C children exhibited unique ERP abnormalities of the frontal N200 evoked by the Stop Signal, which were not shared by the RD group. Only the ADHD-C group showed reduced right frontal N200 amplitude to successful stops relative to the healthy controls (Pliszka et al, 2000; Dimoska et al, 2003; Albrecht et al, 2005; Liotti et al, 2007), while the same comparison between the RD group and the healthy control group did not show significant amplitude changes. Furthermore, the ADHD-C group failed to show a success-related increase of frontal N200 amplitude (Liotti et al, 2007), while a significant frontal N200 enhancement for successful relative to failed inhibitions was observed both in the healthy controls and in the RD group.
The present study provides novel evidence that previously reported abnormalities of the frontal N200 elicited by a Stop signal in ADHD-C children (see references above) are not found in a clinical sample with a different developmental disorder, i.e., RD children without ADHD. The present evidence for specificity of SST abnormalities further adds to a body of research supporting the inhibitory control model of ADHD (Barkley, 1997; Pliszka et al, 2000), and pointing to the notion that the dysfunction of a distributed right-lateralized striato-prefrontal network specialized for response inhibition may be a specific endophenotype of ADHD-C. In a recent ERP study in a larger cohort ADHD-C and control children, source-modeling of the right frontal N200 group difference revealed that a major source in right dorsolateral prefrontal cortex explained most of the variance (Liotti et al, 2007). This is in agreement with fMRI studies in healthy adolescents and adults which have consistently reported activation of the right middle-inferior frontal gyrus during inhibitory control tasks (such as the Go/NoGo task and the SST; e.g., Konishi et al., 1999; Rubia et al., 1999), and fMRI studies of the SST in ADHD adolescents, which have reported reduced activation of the same right frontal region (Rubia et al., 1999; Rubia et al, 2005).
Please note that a recent ERP study of the SST found similarly reduced N200 amplitudes for SI trials in children who met criteria for either ADHD-C only or oppositional defiant/conduct disorder (ODD/CD) only (Albrecht et al, 2005). Our ADHD-C sample could meet criteria for ODD (but not CD), therefore is possible that our results of a response inhibition deficit may be at least in part explained by a additive effect of ODD symptomatology. However, interestingly, in Albrecht et al's study a combined ADHD+ODD/CD group did not show a frontal N200 reduction, discrediting the notion that the two pathologies may simply have an additive effect on N200 abnormalities (Albrecht et al, 2005).
ERP findings: No-Go P3 effects
Unlike the Frontal N200 wave, which showed selective impairments in the ADHD-C group, the No-Go P3 in response to the Stop signal for the Failed Inhibitions was significantly reduced in both ADHD-C and RD relative to the healthy comparison group over fronto-central scalp. This deficit extended to Successful Inhibitions in the RD group, but only over right fronto-central scalp.
This pattern of results suggests an impairment of cognitive control mechanisms involved in error monitoring in both ADHD-C and RD. The finding of reduced frontocentral NoGo-P3s elicited by failed inhibitions in ADHD children has been reported before (Overtoom et al, 2002; Smith et al, 2004; Liotti et al, 2005; 2007). The functional significance of this abnormality has been recently emphasized (Schmajuk et al, 2006; Liotti et al, 2007), in that it appears that the NoGo-P3 to Failed Inhibitions is equivalent to the Error Positivity (Pe) associated to late conscious recognition of errors (Falkenstein et al, 1991). Consistent with this interpretation, Wiersema et al. (2005) used a Go-NoGo task and reported smaller Pe amplitude in ADHD children relative to healthy controls. Such ERP findings are consistent with the results of a companion event-related fMRI study performed in a subset of the same ADHD and control children as in the present study, showing failure-specific activation of the dorsal Anterior Cingulate Cortex (dACC) in healthy controls, and lack of such activity in ADHD children (Pliszka et al, 2006). Dipole modelling of the NoGo-P3 has suggested a main source in dACC (Fallgatter et al, 2004). Note, however, that a recent study using a flanker task did not find reduced Pe amplitude in ADHD-C relative to a control group, suggesting that there may be task-dependence in Pe findings (Albrecht et al, 2008). In relation to our results in the RD group, a recent study using a two-choice discrimination task reported that children with Learning Disorder, and particularly those with RD+Math disorder, displayed a reduction of the error positivity (Pe) when compared with children with RD alone (which only exhibited a trend), ADHD-C, ADHD-I and healthy controls, and interpreted their findings suggesting that children with RD+Math Disorder exhibit greater cognitive and executive function deficits than those with RD alone or ADHD (Burgio-Murphy et al, 2007). In summary, the above results suggest a shared deficit in error processing and conflict monitoring in ADHD-C and RD (reflected in the NoGo-P3 abnormalities), consistent with current interpretations of the role of dACC in cognitive control operations (e.g., Botvinick et al, 2001; Nieuwenhuius et al, 2003).
The NoGo-P3 results in the RD group in the present study replicate and extend recent findings of NoGo-P3 amplitude reductions in dyslexic adolescents during the OX version of the CPT (Taroyan et al, 2007). Importantly, in that study false alarms to noGo stimuli were not analyzed (equivalent to our Failed Inhibitions). Therefore, while both studies report abnormalities in the NoGo-P3 to Successful Inhibitions, only the present study additionally reports a reduction of the NoGo-P3 to Failed Inhibitions. Taroyan et al (2007) also reported that while right hemisphere's NoGo-P3s were greater in the control group, such asymmetry was absent in the RD group. In our study, noGo-P3 abnormalities in the RD group were stronger or only present over the right hemisphere, for both Successful and Failed Inhibitions. Taroyan et al (2007) interpreted their right lateralized NoGo-P3 reduction in RD as a deficit of predominantly right-sided posterior parietal lobe function, possibly affecting early attentional orienting to visual or auditory stimuli (see also Facoetti et al, 2005). It is worth noting, however, that in our study the NoGo-P3 deficit had a more anterior (frontocentral) and not a parietal scalp topography, suggesting a prefrontal abnormality likely affecting executive aspects of attention and not early attentional orienting. In support of this interpretation, the neuropsychological performance in the same three groups on test of attention and working memory, showed that children in the ADHD-C group scored more poorly than the RD and control groups on a standardized measure of selective attention and concentration (a standardized version of a cancellation test, the d2 Test of Attention (Brickenkamp & Zillmer, 1998). In contrast, both RD and ADHD-C had difficulties with measures of cognitive control and working memory compared to controls. On the Stroop color-word test which requires the child to inhibit a prepotent response, the ADHD-C and RD groups had the most difficulty. A similar deficit relative to controls was observed on measures of verbal working memory (see Semrud-Clikeman, Pliszka & Liotti, 2008, for a full report of such neuropsychological findings).
Convergent neuropsychological evidence for common and shared central executive deficits in ADHD and RD comes from a recent study in ADHD only, RD only, ADHD+RD and control children (Tiffin-Richards et al, 2008). On a measure of task-switching (an adaptation of the WCST) only the ADHD groups were significantly impaired relative to controls. In contrast, on a measure of working memory manipulating (digit span backword), a common deficit was reported for ADHD and RD children. These findings are in good agreement with our results of distinct and shared ERP abnormalities in ADHD-C and RD children
Conclusion
The present study provides further behavioural and electrophysiological evidence of a core-deficit in response inhibition in ADHD combined subtype. The right frontal N200 to the Stop Signal, indexing an early inhibitory control mechanism involved with triggering and modulating the efficiency of response inhibition (Pliszka et al, 2000; Liotti et al, 2007) was abnormally reduced and not modulated by success only in our ADHD-C group, while in a group of children with Reading Disorder (without ADHD) the primary inhibitory control mechanism as indexed by the right frontal N200 appeared to be spared. This dissociation was paralleled by our behavioural data in the SST task, with impaired SSRT only in the ADHD-C group. We propose that these abnormalities may provide an endophenotype for ADHD-C.
Our study suggested that only such early response inhibition mechanism (indexed by the right frontal N200) is specifically affected by ADHD. A later component of the evoked response to the Stop signal, the NoGo-P3, was equally reduced in amplitude in the ADHD-C and RD groups. It has been proposed that the NoGo-P3 reflects later monitoring of the outcome of the inhibitory process, both for successful and, in particular, for failed inhibitions by the dACC (Liotti et al, 2005; Pliszka et al, 2006). While no fMRI study of the SST has been conducted in RD individuals, we propose that reduced cognitive control and error processing by the dACC may be present in both disorders, and contribute to the executive control and working memory abnormalities reported both in ADHD-C and RD (Tiffin-Richards et al, 2008; Semrud-Clikeman et al, 2008). Further neuroimaging studies appear necessary to corroborate such conclusion.
Caveats
A first limitation of this study is that it did not include a group with combined ADHD+RD, allowing to better test specificity and shared abnormalities of both disorders (as in Tiffin-Richards et al, 2008). We can only presume that such group would display additive deficits of the frontal N200 and NoGo-P3. A related limitation of the present study is its emphasis on a single subtype of ADHD (the combined subtype). The ADHD cohort in the present study was part of a larger multi-methodology study focusing on neuroimaging and electrophysiology of inhibitory control and stimulant response in ADHD-combined type (Pliszka et al, 2006; Pliszka et al, 2007).
A third limitation of the present study is its reliance on the results of a single task, the SST. It appears to be critically important to study neural correlates of impaired executive function across more than one task, possibly within the same individuals, to evaluate how abnormalities of the N200 and P300 generalize across tasks, or are rather task-specific.
A final limitation of this study is the use of a version of the SST that does not allow online tracking and correction of performance (Logan et al, 1997). We rather adjusted the probability of errors in a given block based on the mean Go RT in the preceding block. This procedure may have been less than optimal in preventing control subjects from strategically slowing the RT to successfully inhibit. However, other measures of inhibitory control, such as slope of the inhibitory function and SSRT, yielded significant effects across groups. Furthermore, the group differences in slope remained significant after calculating the ZRFT, which corrects for differences in these primary task variables.
These limitations notwithstanding, this is to our knowledge the first ERP study to describe selective electrophysiological differences between ADHD-C and RD children during a response inhibition task.
Figure 3.
Topographic maps of the NoGo-P3 to Failed Inhibitions (300-400 ms), illustrating the main NoGo-P3 group effects. Top: Scalp distribution of the NoGo-P3 mean voltage amplitude in μV for the Control Group (Left), the ADHD Group (center) and the RD Group (right). Bottom: Scalp distribution of F-values in the contrast of Controls versus ADHD (left) and Controls versus RD (right). Maps are the result of omnibus F-tests at each scalp sensor, uncorrected for multiple comparisons. Note significant fronto-central NoGO-P3 reductions in both ADHD and RD groups.
Figure 4.
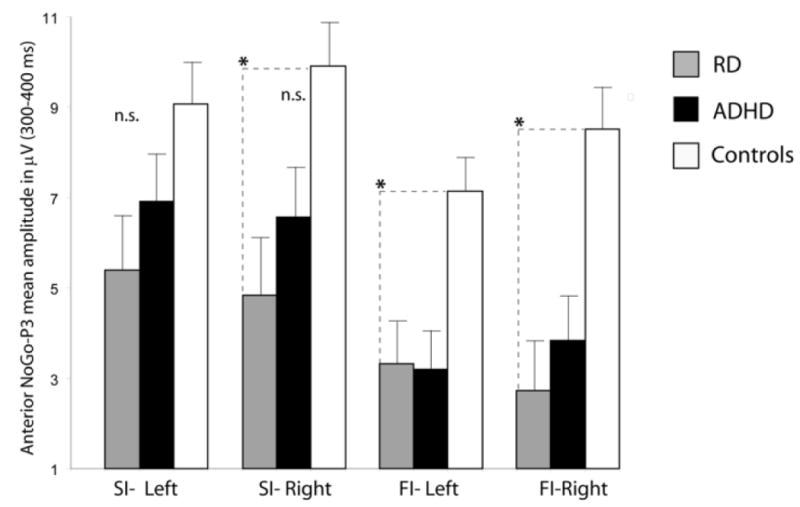
Top: NoGo-P3 Mean voltage amplitude of the NoGo-P3 (300-400 over frontocentral (anterior ROI) scalp for Successful Inhibitions (SI) and Failed Inhibitions (FI) over the left and right frontal ROI for the Control, ADHD and RD groups. *: p<0.05.
Acknowledgments
The authors wish to thank Kristen Vega, Crystal Mendoza and Charlotte Kay, LMSW-ACP for their assistance on this project, and dr. Theresa Newlove for valuable comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht B, Banaschewski T, Brandeis D, Heinrich H, Rothenberger A. Response inhibition deficits in externalizing child psychiatric disorders: An ERP-study with the Stop-task. Behavior and Brain Function. 2005;9:1–22. doi: 10.1186/1744-9081-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht B, Brandeis D, Uebel H, Heinrich H, Müller UC, Hasselhorn M, Steinhausen HC, Rothenberger A, Banaschewski T. Action Monitoring in boys with ADHD, their Nonaffected Siblings and Normal Controls: Evidence for Conflict Monitoring as an Endophenotype for ADHD. Biological Psychiatry. 2008;64:615–625. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Association of ADHD and conduct disorder - brain electrical evidence for the existence of a distinct subtype. Journal of Child Psychology and Psychiatry. 2003;44:356–376. doi: 10.1111/1469-7610.00127. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Questioning inhibitory control as the specific deficit of ADHD - evidence from brain electrical activity. Journal of Neural Transmission. 2004;111:841–864. doi: 10.1007/s00702-003-0040-8. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychologica. 2003;112(2):105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Barkley R. ADHD and the nature of self control. New York: Guildford Press; 1997. [Google Scholar]
- Bednarek DB, Saldaña D, Quintero-Gallego E, García I, Grabowska A, Gómez CM. Attentional deficit in dyslexia: a general or specific impairment? Neuroreport. 2004;15(11):1787–90. doi: 10.1097/01.wnr.0000134843.33260.bf. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver T, Barch D, Carter C, Cohen J. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brickenkamp R, Zillmer EA. D2 Test of Attention. Seattle: Hogrefe & Huber; 1998. [Google Scholar]
- Burgio-Murphy A, Klorman R, Shaywitz SE, et al. Error-related potentials in children with attention-deficit hyperactivity disorder, oppositional defiant disorder, reading disorder, and math disorder. Biological Psychology. 2007;75:75–86. doi: 10.1016/j.biopsycho.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting LE, Denckla MB. Attention: Relationships between Attention-Deficit Hyperactivity Disorder and learning disabilities. In: Swanson HL, Harris KR, Graham S, editors. Handbook of learning disabilities. New York: Guilford Press; 2003. pp. 125–139. [Google Scholar]
- Dimoska A, Johnstone S, Barry R, Clarke A. Inhibitory motor control in children with attention-deficit/hyperactivity disorder: event-related potentials in the stop-signal paradigm. Biological Psychiatry. 2003;54(12):1345–54. doi: 10.1016/s0006-3223(03)00703-0. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Lorusso ML, Cattaneo C, Galli R. Multi-modal attentional capture is sluggish in children with developmental dyslexia (2005) Acta Neurobiologica Experimentalis. 2005;65:61–72. doi: 10.55782/ane-2005-1540. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoorman J, Hohnsbein J. ERP components in Go/NoGo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–91. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoorman J, Hohnsbein J. Inhibition-related ERP components: variation with age and time-on-task. Journal of Psychophysiology. 2002;16:167–175. [Google Scholar]
- Fallgatter A, Ehlis A, Seifert J, Strik W, Scheuerpflug P, Zillessen K, et al. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clinical Neurophysiology. 2004;115(4):973–81. doi: 10.1016/j.clinph.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Greenhouse S, Geisser S. On methods in the analysis of profile data. Psychometrika. 1954;24:95–112. [Google Scholar]
- Helland T, Asbjørnsen A. Executive functions in dyslexia. Child Neuropsychology. 2000;6(1):37–48. doi: 10.1076/0929-7049(200003)6:1;1-B;FT037. [DOI] [PubMed] [Google Scholar]
- Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/Nogo task. Electroencephalography and Clinical Neurophysiology. 1992;82(6):477–82. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Kok A. Effects of degradation of visual stimulation on components of the event-related potential (ERP) in go/nogo reaction tasks. Biological Psychology. 1986;23(1):21–38. doi: 10.1016/0301-0511(86)90087-6. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajim K, Uchida I, Kiyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114(2):216–22. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Liotti M, Woldorff MG, Perez R, Mayberg HS. An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia. 2000;38(5):701–11. doi: 10.1016/s0028-3932(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka S, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41(3):377–88. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, III, Luus B, Glahn D, Semrud-Clikeman M. Electrophysiological correlates of response inhibition in children and adolescents with ADHD: Influence of gender, age and previous treatment history (2007) Psychophysiology. 2007;44(6):936–48. doi: 10.1111/j.1469-8986.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Logan G, Cowan W, Davis K. On the ability to inhibit thought and action: A theory of act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- Nichols SL, Waschbusch DA. A review of the validity of laboratory cognitive tasks used to assess symptoms of ADHD. Child Psychiatry and Human Development. 2004;34(4):297–315. doi: 10.1023/B:CHUD.0000020681.06865.97. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuius S, Yeung N, van den Wildenberg WPM, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/nogo task: Effects of response conflict and trial-type frequency. Cognitive, Affective and Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Overtoom C, Kenemans J, Verbaten M, Kemner C, van der Molen M, van Engeland H, et al. Inhibition in children with attention-deficit/hyperactivity disorder: a psychophysiological study of the stop task. Biological Psychiatry. 2002;51(8):668–76. doi: 10.1016/s0006-3223(01)01290-2. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Giard M, Echallier J. Mapping of scalp potentials by surface spline interpolation. Electroencephalography and Clinical Neurophysiology. 1987;66(1):75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- Petkov CI, O'Connor KN, Benmoshe G, Baynes K, Sutter ML. Auditory perceptual grouping and attention in dyslexia. Cognitive Brain Research. 2005;24:343–354. doi: 10.1016/j.cogbrainres.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Borcherding S, Spratley K, Irick S. Measuring inhibitory control in children. Journal of Developmental Behavioral Pediatrics. 1997;18:254–259. [PubMed] [Google Scholar]
- Pliszka SR, Glahn D, Xiong J, Semrud-Clikeman M, Franklin C, Perez R, Liotti M. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. American Journal of Psychiatry. 2006;163(6):1052–60. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory Control in Children with Attention-Deficit/Hyperactivity Disorder: Event-Related Potentials Identify the Processing Component and Timing of an Impaired Right-Frontal Response-Inhibition Mechanism. Biological Psychiatry. 2000;48:238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Bailey BY, Perez R, 3rd, Glahn D, Semrud-Clikeman M. Electrophysiological effects of stimulant treatment on inhibitory control in children with attention-deficit/hyperactivity disorder. Journal of Child Adolescent Psychopharmacology. 2007;17(3):356–66. doi: 10.1089/cap.2006.0081. [DOI] [PubMed] [Google Scholar]
- Purvis KL, Tannock R. Phonological processing, not inhibitory control, differentiate ADHD and Reading Disability. Journal of the American Academy of Child Adolescent Psychiatry. 2000;39(4):485–494. doi: 10.1097/00004583-200004000-00018. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, Bullmore E. Hypofrontality in Attention Deficit Hyperactivity Disorder During Higher-Order Motor Control: A Study with Functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Brammer M, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. American Journal of Psychiatry. 2005;162(6):1067–75. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Schmajuk M, Liotti M, Busse L, Woldorff MG. Electrophysiological Activity Underlying inhibitory Control Processes in Normal Adults. Neuropsychologia. 2006;44(3):384–95. doi: 10.1016/j.neuropsychologia.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Biederman J, Sprich-Buckminster S, Lehman BK, Faraone SV, Norman D. Comorbidity between ADDH and learning disability: a review and report in a clinically referred sample. Journal of the American Academy of Child Adolescent Psychiatry. 1992;31(3):439–48. doi: 10.1097/00004583-199205000-00009. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Pliszka SR, Liotti M. Executive Functioning in Children with ADHD:Combined Type with and without a Stimulant Medication History. Neuropsychology. 2008;22(3):329–40. doi: 10.1037/0894-4105.22.3.329. [DOI] [PubMed] [Google Scholar]
- Sergeant J. The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neuroscience Biobehavioral Reviews. 2000;24(1):7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE. Current concepts-dyslexia. New England Journal of Medicine. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Fletcher J, Shaywitz BA. Issues in the definition and classification of attention deficit disorder. Topics in Language Disorders. 1994;14:1–25. [Google Scholar]
- Smith J, Johnstone S, Barry R. Inhibitory processing during the Go/NoGo task: an ERP analysis of children with attention-deficit/hyperactivity disorder. Clinical Neurophysiology. 2004;115(6):1320–31. doi: 10.1016/j.clinph.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2002;57(11):1231–8. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Taroyan NA, Nicolson RI, Fawcett AJ. Behavioural and neurophysiological correlates of dyslexia in the continuous performance task. Clinical Neurophysiology. 2007;118:845–855. doi: 10.1016/j.clinph.2006.11.273. [DOI] [PubMed] [Google Scholar]
- Tiffin-Richards M, Hasselhorn M, Woerner W, Rothenberger A, Banaschewski T. Phonological Short-term Memory and Central Executive Processing in Attention-deficit/Hyperactivity Disorder with/without Dyslexia - Evidence of Cognitive Overlap. Journal of Neural Transmission. 2008;115:227–234. doi: 10.1007/s00702-007-0816-3. [DOI] [PubMed] [Google Scholar]