Abstract
Study Objectives
We used a sequence-learning task to assess whether: 1. The time interval between awakening and training equally affects the rate of acquisition of sequence order, a declarative component, and the kinematic optimization process, an implicit component; 2. Sleep enhances the retention of both these aspects of sequence learning.
Design
For aim 1, we compare the acquisition rate of a new motor sequence in a group trained in the morning and another in the evening. For aim 2, we tested retention of the same motor sequence twelve hours later, either without sleep (normal day activity or a night of sleep deprivation) or with interposed sleep (afternoon napping or regular full night sleep).
Setting
Training and Testing were performed in a controlled laboratory setting.
Participants
Thirty-six right-handed normal subjects (age range 18–24 years; 16 women).
Results
During the training, acquisition rate of the sequence order was significantly higher in the AM-trained than in the PM-trained group, without differences in the kinematic optimization processes. Both declarative and implicit learning indices were significantly higher in the subjects tested after sleep compared to those tested without interposed sleep.
Conclusion
The best time for fast and efficient acquisition of new declarative material is the morning, while the kinematic aspects of skill acquisition are not sensitive to the time of day. However, better retention of both declarative material and motor skills requires two conditions: a period of post-training sleep and the achievement of performance saturation during training.
Keywords: Learning, Reaching, Declarative learning, Motor skills, Consolidation
Introduction
Learning, the process of acquiring new knowledge or a new skill, is one of the most important functions of the brain at any age, as it provides the bases to do old and novel things, to cope with change and to promote change as well as progress. Many studies have been devoted to ascertain the factors that are central to learning and in particular, to memory retention.
An almost general consensus has been built around the fact that sleep improves memory retention (Diekelmann et al., 2008). On the other hand, the “proactive” effect of sleep has been investigated mostly in terms of attention and working memory. Attention and working memory fluctuate during the day, as a result of circadian rhythms and sleep pressure (Schmidt et al., 2007). However, whether the time of the day has an effect on the acquisition rate of new material, and in particular of new motor skills, remains matter of debate.
Most of the studies on sleep and consolidation of motor learning have used the finger-tapping task, a motor sequence learning paradigm, and showed unequivocally a positive effect of sleep on consolidation (Fischer et al., 2002; Walker et al., 2003; Rickard et al., 2008; Debas et al., 2010). Although this task has been conventionally defined “explicit”, subjects are asked to type repeatedly and fast a known, short sequence, and the outcome measures, accuracy and speed, all reflect kinematic or implicit aspects of skill formation. Indeed, the majority of sleep studies on motor learning and consolidation have largely focused on implicit aspects of learning, disregarding declarative components, which have been instead studied using verbal tasks (Barrett and Ekstrand, 1972; Gais et al., 2006; Tucker et al., 2006; Diekelmann et al., 2008). Motor sequence acquisition represents a particular case in the field of learning, as it involves at the same time declarative (the learning of the sequence order) and non-declarative components (the ability to perform the sequence flawlessly): to obtain winning actions in any type of sports, lectures and theory are always combined with extensive hands-on practice. In this study, we wished to assess whether the time interval between awakening and training equally affects the rate of acquisition of declarative sequence order and the kinematic optimization process. In addition, we determined whether sleep enhances the retention of both these aspects of sequence learning.
For this purpose, we used a reaching task in which the declarative and kinematic -or optimization- components can be measured simultaneously but with distinct variables (Ghilardi et al., 2000; Ghilardi et al., 2003a; Ghilardi et al., 2003b; Ghilardi et al., 2007; Ghilardi et al., 2008; Moisello et al., 2009). In this task, subjects are explicitly instructed to learn a complex sequence of targets while reaching for them. The acquisition of the elements’ order is reflected in the increase of correct anticipatory movements and in declarative scores (Ghilardi et al., 2009; Moisello et al., 2009). Interestingly, anticipatory movements show kinematic changes that cannot be perceived nor reported by subjects. These reflect the skill to perform the sequence efficiently, a form of optimization that parallels the explicit acquisition of the sequence order.
Subjects and methods
Subjects
Thirty-six right-handed neurologically normal subjects (16 women and 20 men) participated in the study. The age range of subjects was from 18 to 24 years (mean ± SE: 19.8 ± 0.34). The study was approved by the local IRB committee and all subjects signed an IRB-approved informed consent form.
Study design
Subjects were instructed to maintain a regular sleep schedule for at least one week preceding the study. In addition, they were asked to abstain from drinking caffeine or alcohol the day before and during the study. All the subjects were tested in two sessions separated by twelve hours. They performed a sequence learning task (SEQ) and an additional task (RAN) to assess general motor performance, as detailed below. Each complete session took approximately 25 minutes.
In the first session (Training session), after familiarizing with the apparatus and the tasks, the subjects performed a block of RAN and five blocks of SEQ. In the second session (Test session), that took place twelve hours later, subjects were tested with a block (Test Block) with the same sequence order they previously learned, followed by one block of RAN. Hours of sleep and wake time were recorded for each subject. Verbal scores (see below) were recorded after each SEQ block in the Train session and before the Test block.
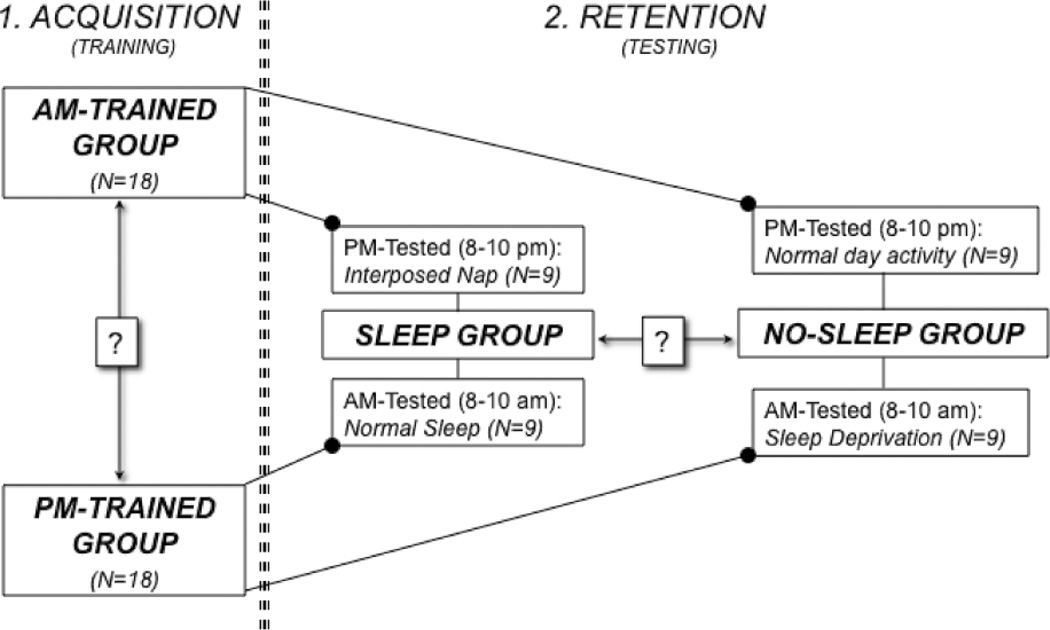
To ascertain whether the interval between waking and training impacted the rate of acquisition, the first training session of 18 subjects occurred between 7 am and 9 am (AM-trained group), and in the remaining 18 subjects it took place between 7 pm and 9 pm (PM-trained group). Then to determine whether retention was improved by intervening sleep, we further divided each of the two groups in two subgroups (9 subjects each, Fig. 1). Thus, nine subjects in one of the AM-trained subgroups were instructed to nap for at least one hour before the testing session (AM-nap-PM), while the remaining subjects were instructed not to sleep between training and testing sessions (AM-PM). One subgroup in the PM-trained was instructed to sleep their regular time before the testing session (PM-AM), while the other subjects were sleep deprived (PM-depr-AM). In the twelve hours between the two sessions, subjects in the PM-depr-AM subgroup were constantly observed by the experimenters. Mentally stimulating activities were conducted to maintain alertness. No caffeine consumption was allowed.
Fig. 1.
Experimental Design.
Methods
Details about the motor tasks are reported in previous publications (Ghilardi et al., 2003b; Ghilardi et al., 2008; Moisello et al., 2009). Briefly, in all the tasks, subjects moved a cursor on a digitizing tablet with their right hand. They made out and back movements from a central starting point to one of eight targets (distance: 4.8 cm) displayed as circles on a computer screen (see Fig. 1). The cursor and targets position were always visible on the screen. Instructions were to make the reaching movements as fast and accurately as possible, without correction and to reverse sharply within the target circle. Targets always appeared in synchrony with a tone at 1s intervals in separate blocks of 96 movements each. Subjects performed the following tasks:
-
–
SEQ: The eight targets were presented in repeating order in cycles of 16 elements. In each block, the sequence was repeated six times for a total of 96 movements. In each session, subjects performed five entire blocks. Subjects were informed of the sequence’s presence and were instructed to learn it, to anticipate the target appearance when known, thus reaching the target in synchrony with the tone. At the end of each block, subjects were instructed to report the sequence order and responses were scored from 0 (no sequence detected) to 16 (entire sequence order). At the beginning of the second session, declarative scores were also determined.
-
–
RAN: A reaction time task in which targets were presented in a pseudo-random, non-repeating, and unpredictable order. Subjects were required to move “as soon as possible”, minimizing reaction time but avoiding target anticipation. This task was used to assess the floor value of reaction time to detect anticipatory movements and to provide baseline values for movement time and peak acceleration changes in SEQ (see below).
Data analysis
As detailed in previous reports (Ghilardi et al., 2000; Ghilardi et al., 2003a; Ghilardi et al., 2003b; Ghilardi et al., 2007; Ghilardi et al., 2008), for each movement in both SEQ and RAN tasks, onset, peak velocity and reversal positions were identified and the following measures computed: (1) Onset time, the time from target and tone presentation to movement onset; (2) Movement time, the time from movement onset to the reversal point; (3) Path length, the length of path from the movement onset to the reversal point; (4) Amplitude of peak velocity.
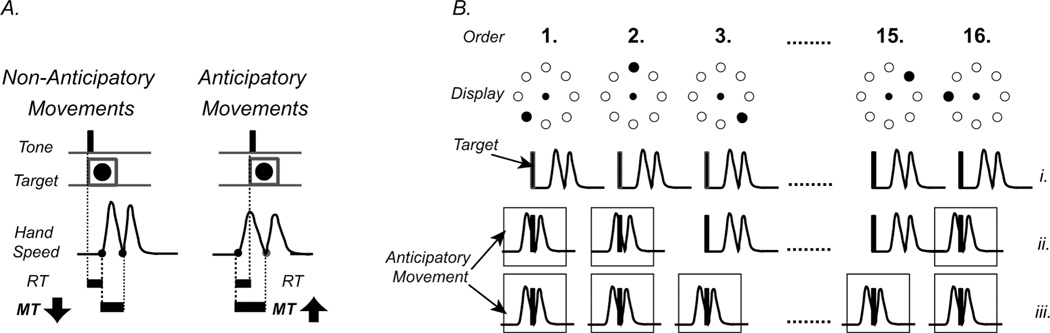
For SEQ, we also computed the number of correct anticipatory movements per cycle (i.e., every sixteen movements, see also Fig. 2). For each subject, the correct anticipatory movements were defined as those directed to the correct target and with onset times below the lowest reaction time achieved in RAN. These movements reflect explicit learning of sequence order, (Ghilardi et al., 2003a; Ghilardi et al., 2003b; Ghilardi et al., 2007; Ghilardi et al., 2008) as we have previously found a strong correlation (r2 > 0.7) between the number of these movements and the declarative report collected at the end of each block. In addition, for each block, we computed declarative scores, as described above. The quality of sleep before the training session was assessed by a subjective Sleep Rating Scale ranging from 1 (worst) to 10 (best).
Fig. 2.
A. Anticipatory and Non-Anticipatory Movements. Anticipatory Movements have RT below the lowest reaction time achieved in RAN as well as longer Movement Time (MT) and lower peak velocity compared to Non-Anticipatory Movements. B. Development of anticipatory movements during a 16-element sequence: In the beginning of the learning process, (i) Movements are initiated in response to target presentation. Soon, (ii) some movements become anticipatory (boxed hand-paths). Finally, (iii) when the whole sequence is acquired, all targets are anticipated.
To minimize inter-subject variability, the movement times and the peak velocities of the correct anticipatory movements in SEQ were normalized by the mean movement times and peak velocities in the corresponding blocks of RAN. In fact, as explained in the introduction, when target’s appearance is unpredictable as in RAN, movement duration are much shorter and peak velocities higher than when target’s appearance is totally predictable. During sequence learning, anticipatory movements display progressive duration increase and peak velocity decrease. Thus, in this scenario, RAN provides the starting point, baseline, for movement time and peak velocities.
Statistical analysis
For each block of SEQ and each experimental group, we computed the mean and SD of declarative scores as well as the number of correct anticipatory movements, of their movement time and peak velocity.
To ascertain whether declarative and optimization processes acquisition were more efficient in AM-trained group compared to the PM-trained group, we used mixed model ANOVAs: we compared the mean performance indices (that is, number of correct anticipatory movements, declarative scores, movement time, peak velocity, path length) across the five blocks and between the two groups.
To determine whether interposed sleep improved the retention of declarative and optimization processes, we used mixed model ANOVAs and compared the mean performance indices of the last Train block and the Test block (Block) of the Sleep group (PM-AM + AM-nap-PM subgroups) and the No Sleep group (AM-PM + PM-dep-AM subgroups). We also compared the difference between the Train and Test blocks normalized by the Train block performance (i.e., normalized delta) of the two groups with one-way ANOVAs.
Results
As reported in Table I, all the groups had similar sleep patterns in the weeks before the experiment, as they slept similar number of hours and did not differ in terms of the quality and the number of hours of sleep the night before the training session.
Table I.
Characteristics of AM- and PM-trained Groups.
| AM-trained | PM-trained | |
|---|---|---|
| Usual Hours of Sleep (h) | 6.9 ± 1.0 | 6.5 ± 1.0 |
| Hours of Sleep Before Training (h) | 6.8 ± 1.3 | 6.3 ± 1.5 |
| Sleep Rating Before Training* | 7.3 ± 1.7 | 8.1 ± 1.1 |
| Time Awake Before Training (h) | 2.0 ± 0.9 | 11.2 ± 1.7 |
Rating scale ranged from 1 (worst) to 10 (best) + p < 0.0001
1. Is the acquisition of declarative and optimization processes more efficient in the AM?
To determine whether learning occurs at a faster rate in the AM hours, we used the performance indices of the five blocks to compare the groups that trained in the AM to those trained in the PM. The mean time awake (± SE) at the time of the training session in the AM-trained group was 2.03 (± 0.21) hours and in the PM-trained group 11.21 (± 0.40) hours.
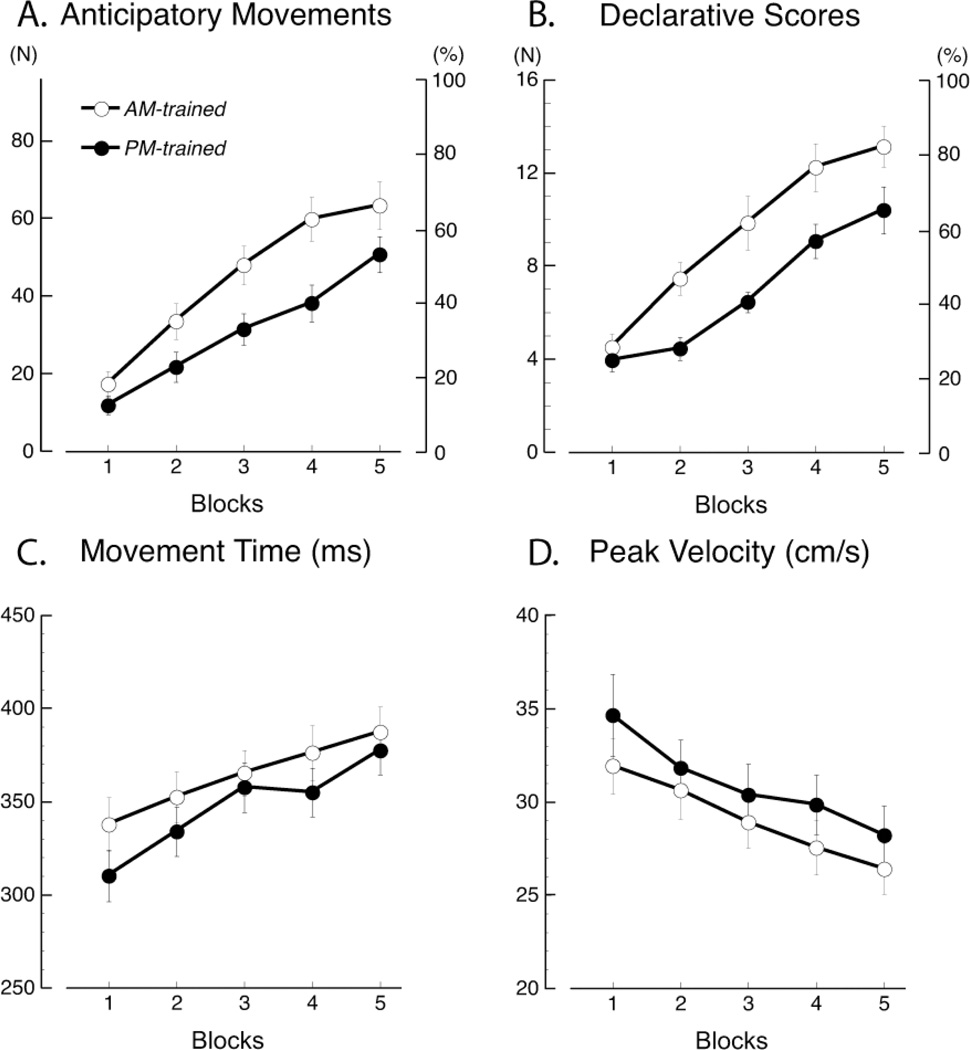
The number of anticipatory movements, representing declarative learning, significantly increased in the five training blocks in all the groups (F(4,136) = 75.19, p < 0.0001; see Fig. 3A). However, for the AM-trained group, the number of anticipatory movements grew at a significantly faster rate than the PM-trained group (group: F(1,136) = 5.835, p = 0.02; group × block: F(1, 4) = 2.395, p = 0.05, see Fig. 3). Similarly, the declarative scores recorded at the end of each block progressively increased in the two groups (F(4,136) = 52.97, p < 0.0001; see Fig. 3B), but they were always higher in the AM-trained group (group: F(1,136) = 10.203, p = 0.003; group × block: F(1, 4) = 1.77, p = 0.14, see Fig. 3B). As expected, there was a significant correlation between declarative scores and the number of anticipatory movements in all blocks (r = 0.67, p < 0.0001), confirming that the number of anticipatory movements is an index of declarative learning.
Fig. 3.
(A) Anticipatory Movements and (B) Declarative Scores significantly increased in the five training blocks of the two groups, although a faster rate of growth was observed in the AM-trained group. (C) Movement Time significantly increased in both groups during the course of the five blocks, while (D) Peak Velocity decreased. No significant difference in either Movement Time or Peak Velocity was observed between the AM- and PM- trained groups.
The movement time of the anticipatory movements, representing implicit learning, significantly increased in both groups in the course of the five blocks (F(4,136) = 18.41, p < 0.0001). However, there was no difference between the AM-trained and PM-trained groups (group: F(1,136) = 1.13, p = 0.30; group × block: F(1, 4) = 0.74, p = 0.57, see Fig. 3C). Moreover, no differences between groups were found for peak velocity (group: F(1,136) = 0.86, p = 0.36; block: F(4,136) = 20.39, p < 0.0001; group × block: F(1, 4) = 0.37, p = 0.83, see Fig. 3D). These results were not due to differences in the level of attention: in fact, the mean reaction times in the RAN task were similar in the AM- and PM-trained groups (AM: 278.18 ± 35.41 ms; PM: 270.20 ± 28.76 ms; p = 0.40).
Interestingly, the declarative scores and the number of anticipatory movements decreased with the time spent awake, as demonstrated by significant correlations between length of time awake and both the number of anticipatory movements (r = −0.35, p = 0.01), and the declarative scores (r = −0.43, p = 0.001), computed on the combined cohort of subjects trained in AM and PM. However, we did not find any correlation with either movement time (r = 0.19, p > 0.1) or peak velocity (r = 0.02, p > 0.1).
Altogether, these results suggest that rate of declarative learning depends on the time interval between the waking and the training time. On the other hand, the rate of acquisition of implicit skills involved in motor optimization does not seem to depend on such an interval.
2. Does interposed sleep improve retention of declarative and optimization processes?
To ascertain whether sleep improves retention, we used the performance indices of the last Train block and compared to the first Test block in the subjects that slept between the sessions (Sleep group: PM-AM + AM-nap-PM) and those that did not (No Sleep group: AM-PM + PM-dep-AM). There were no statistically significant differences in the performance indices between the two subgroups of the Sleep group (all performance indices differences: p > 0.4) and between the two subgroups in the No Sleep group (all performance indices differences: p > 0.2).
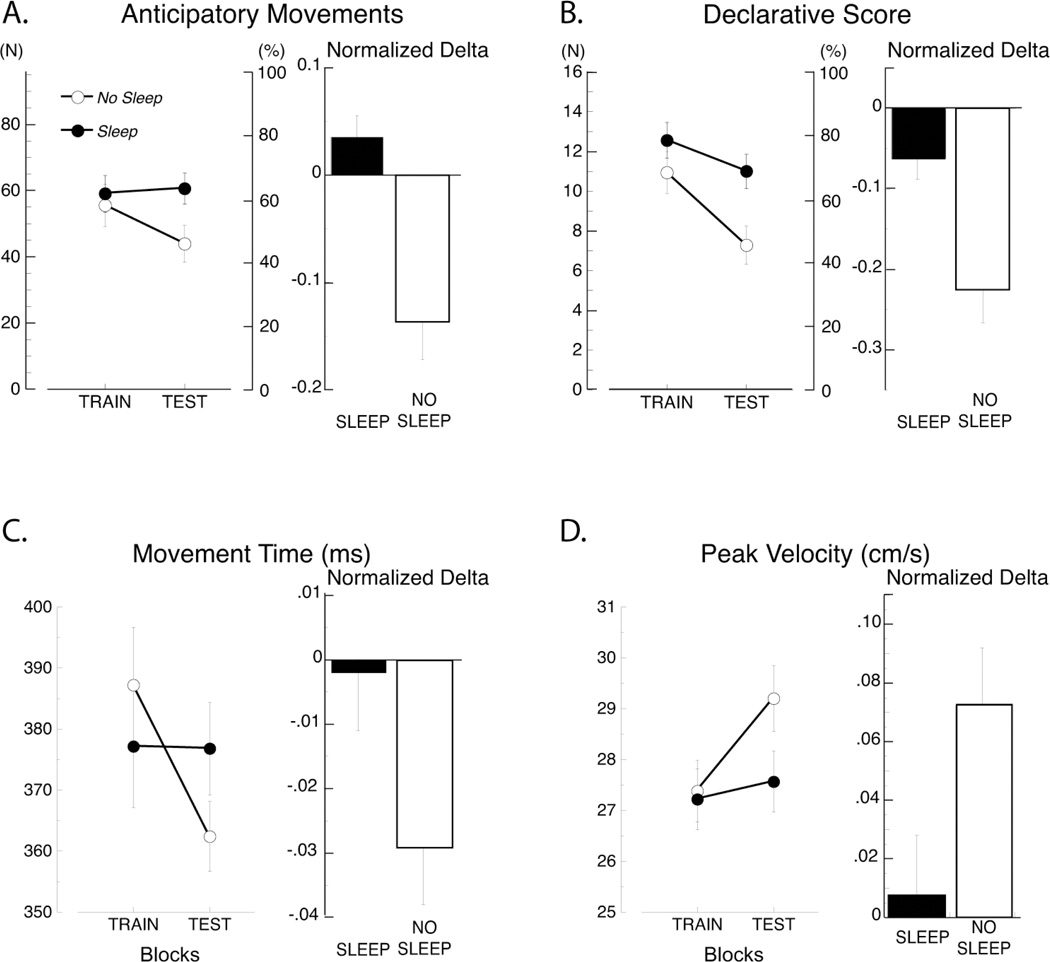
In the Test block, compared to the last Train block, the number of anticipatory movements did not noticeably changed in the Sleep group, but it decreased in the No Sleep group (group: F(1,34) = 1.73, p = 0.20; block: F(1,34) = 6.08, p = 0.02; Fig. 4A). The different gain at test was reflected in the significant group × time of testing interaction (group × block: F(1, 1) = 10.43, p = 0.003; Fig. 4A). A post hoc test revealed that the differences between the two groups were significant only for the Test block (p = 0.02) and not for the last Train Block (p = 0.68), suggesting that sleep improves retention of declarative learning. To obtain further support to this conclusion, we normalized the difference between the Train and Test blocks by the Train block performance (i.e., normalized delta) and compared the normalized deltas of the two groups. This was done in order to remove possible differences in the Train block that might have been underestimated by the post-hoc test. Indeed, there was a significant difference between the Sleep and No Sleep groups (F(1,34) = 13.72, p = 0.0007, Fig. 4A). Similar results were obtained when comparing the declarative scores collected at the end of the last Train block and those collected just before the Test block (Fig. 4B; group: F(1,34) = 4.49, p = 0.04; block: F(1,34) = 32.54, p < 0.0001; group × block: F(1, 1) = 5.32, p = 0.03; normalized delta: F(1,34) = 10.83, p = 0.002).
Fig. 4.
Sleep affected the retention of both declarative (A–B) and kinematic (C–D) learning. For each variable, the left panel shows the average for the last block of Training and the block of Test in the SLEEP and NO-SLEEP groups, while the right panel illustrates the difference between the Train and Test blocks normalized by the Train block performance (i.e., normalized delta) in the two groups.
Interestingly, analyses of movement time and peak velocity, whose changes represent implicit aspects of learning, showed similar results. In the Sleep group, movement time was the same in both the Train and Test block, while in the no-sleep group, it decreased in the Test block (Fig. 4C; group: F(1,34) = 0.001, p = 0.99; block: F(1,34) = 5.128, p = 0.03; group × block: F(1, 1) = 4.195, p = 0.048; normalized delta: F(1,34) = 4.64, p = 0.032). This difference was also apparent in the analysis of peak velocity (Fig. 4D; group: F(1,34) = 0.08, p = 0.78; block: F(1,34) = 4.26, p = 0.04; group × block: F(1, 1) = 0.89, p = 0.34; normalized delta: F(1,34) = 4.40, p = 0.04). The changes in the kinematic parameters were not due to changes in the length of the movement: in fact, path length had similar values at all testing points in all the groups (group: F(1,34) = 0.25, p = 0.64; block: F(1,34) = 0.55, p = 0.46; group × block: F(1, 1) = 0.60, p = 0.44).
Finally, we found that reaction times in RAN, a choice-reaction time task, were similar in the training and testing sessions (Train: 274.19 ± 32.05 ms; Test: 272.95 ± 41.56 ms; p = 0.77), suggesting that the declarative and kinematic changes at test were not related to a difference in attention levels between the two sessions.
Altogether, these results suggest that preservation of both declarative knowledge and the skills involved in motor optimization processes benefit from sleep.
Discussion
In the present study, we employed a single task that captures simultaneously, but separately, the learning of declarative and kinematic, optimization-related components of a motor skill (Ghilardi et al., 2008; Ghilardi et al., 2009; Moisello et al., 2009). We found that, first, the acquisition rate of the sequence order, the declarative component, was greater in the AM-trained than in the PM-trained group, while the kinematic changes were similar in the two groups. Secondly, the retention of both the declarative and the skill-related components was always better after sleep, independently of the time of training. However, rather than a sleep-related performance enhancement, we observed a preservation of learning achieved at the end of the training.
Declarative but not skill-related acquisition is better in the AM
Declarative scores were higher and the anticipatory movements grew at a faster rate during the morning than during the evening training. However, movement duration and peak velocity of the anticipatory movements changed similarly both during the morning and the evening training. Altogether, the present results suggest that, while the declarative learning of a motor task is better in the morning, the time of the day is not an important factor in kinematic optimization.
The finding that the acquisition of the declarative component of a motor skill is better in the morning is in agreement with most studies on the acquisition of declarative and explicit material in general (see Schmidt et al., 2007 for a review). The results showing that the time of training does not affect the acquisition of kinematic-related skills are in general agreement with studies on the kinematic optimization of either motor sequence learning (Cajochen et al., 1999; Wyatt et al., 1999; Walker et al., 2002; Wright et al., 2002; Doyon et al., 2003) or visuomotor adaptation (Huber et al., 2004; Maatta et al., 2010).
The decreased declarative learning in the evening hours could be related to decreased attentional resources in this time of day. Indeed, both circadian rhythms and homeostatic pressure influence attentional and working memory resources. However, when we used a choice-reaction time task (RAN) to measure psychomotor vigilance and attention, we found no differences in the groups tested in morning and in the afternoon. Another possible explanation is that general mental effort or fatigue in the hours preceding the training, i.e., homeostatic pressure, could have interfered proactively with the acquisition of the declarative material. The differential effects of time of the day on the acquisition of kinematic-related components might be due to the fact that it requires less attentional resources and less involvement of a fronto-parietal network, so that proactive interference does not occur for the acquisition of skill-related components. Indeed, all the subjects were students and during the day hours preceding the evening training, they were engaged in the acquisition of declarative material through lectures and other types of mental activities, but not into sports or motor-related training. Thus, the pre-training activity mostly engaged the neural systems for declarative learning and not those for motor skills.
Retention of both declarative and skillrelated components is always better after sleep
One of the most important findings is that retention of both declarative and skill was always better in the groups that were tested after sleep. This was true independently from the time of the initial training, as there were no differences between the AM- and PM-trained subjects, when retention indices were normalized by the learning achieved in the training session. Altogether, these results suggest that first, interposed sleep is the single, most important factor for the retention of any type of learned material, in agreement with the bulk of literature on many types of motor and non-motor learning (Diekelmann et al., 2008). Secondly, one-hour nap during the day had beneficial effects on performance similarly to an entire night of sleep; thirdly, absence of sleep either in the course of a normal working day or during a 24-hour sleep deprivation session had similar degrading effects on both performance measures.
These last results are in agreement with the reports that performance in other types of tasks was equally improved by either a nap or a full night sleep (Mednick et al., 2003; Lahl et al., 2008; Tucker and Fishbein, 2009). Nevertheless, the lack of difference in retention within the two sleep groups and within the two non-sleep groups might be due to the small sample of the subgroups (N = 9 each) and thus these effects need to be confirmed by studies with larger samples.
Lack of sleep-related performance enhancement
Finally, we did not find any sleep-related performance enhancement for either the declarative or the kinematic components. In fact, at testing, the level of learning achieved in the last training block was, at best, maintained after sleep and was visibly degraded without interposed sleep. These results are in apparent conflict with our previous work (Ghilardi et al., 2009) that showed a significant improvement in both indices after sleep using this type of motor sequence learning task. However, in the previous study, we used motor sequences with a reduced learning load (eight-target sequences): subjects easily achieved a complete declarative learning (100%) with several correct reiterations of the sequence during the training session. In the present work, instead, we used longer sequences (sixteen elements) and, by the end of training, verbal scores and anticipatory movements were on average less than 80%, with performance levels far away from saturation (see Fig. 3). Therefore, it is possible that to prevent decay or forgetting and to obtain sleep-related performance enhancement, saturation in learning must be achieved during the training session. This conclusion is in agreement with reports showing that the level of proficiency reached at the end of a training session for declarative or other types of learning predicts the performance improvement following sleep (Tucker and Fishbein, 2008). In addition, this interpretation might help understanding the lack of sleep-related enhancement in some motor and nonmotor tasks (Gais et al., 2006; Rickard et al., 2008).
In summary, we conclude that the best time to acquire declarative material fast and efficiently is the morning, while kinematic skill acquisition is not sensitive to the time of day. However, better retention of both declarative material and motor skills requires two conditions: a period of sleep at any time after training and the achievement of performance saturation during training.
Acknowledgments
This work was supported by NIH grant NS054864 (MFG).
References
- Barrett TR, Ekstrand BR. Effect of sleep on memory. 3. Controlling for time-of-day effects. J. Exp. Psychol. 1972;2:321–327. doi: 10.1037/h0033625. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am. J. Physiol. 1999;3(Pt 2):R640–R649. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Hadj Tahar A, Bellec P, Karni A, Ungerleider LG, Benali H, Doyon J. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc. Natl. Acad. Sci. USA. 2010;41:17839–17844. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Landolt HP, Lahl O, Born J, Wagner U. Sleep loss produces false memories. PLoS One. 2008;10:e3512. doi: 10.1371/journal.pone.0003512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;3:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc. Natl. Acad. Sci. USA. 2002;18:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learn. Mem. 2006;3:259–262. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi MF, Alberoni M, Rossi M, Franceschi M, Mariani C, Fazio F. Visual feedback has differential effects on reaching movements in Parkinson’s and Alzheimer’s disease. Brain Res. 2000;1–2:112–123. doi: 10.1016/s0006-8993(00)02635-4. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, Ghez C, Eidelberg D. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann. Neurol. 2003a;1:102–109. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Eidelberg D, Silvestri G, Ghez C. The differential effect of PD and normal aging on early explicit sequence learning. Neurology. 2003b;8:1313–1319. doi: 10.1212/01.wnl.0000059545.69089.ee. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Feigin AS, Battaglia F, Silvestri G, Mattis P, Eidelberg D, Di Rocco A. L-Dopa infusion does not improve explicit sequence learning in Parkinson’s disease. Parkinsonism Relat. Disord. 2007;3:146–151. doi: 10.1016/j.parkreldis.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Silvestri G, Feigin A, Mattis P, Zgaljardic D, Moisello C, Crupi D, Marinelli L, Dirocco A, Eidelberg D. Implicit and explicit aspects of sequence learning in pre-symptomatic Huntington’s disease. Parkinsonism Relat. Disord. 2008;6:457–464. doi: 10.1016/j.parkreldis.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi MF, Moisello C, Silvestri G, Ghez C, Krakauer JW. Learning of a sequential motor skill comprises explicit and implicit components that consolidate differently. J. Neurophysiol. 2009;5:2218–2229. doi: 10.1152/jn.01138.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;6995:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J. Sleep Res. 2008;1:3–10. doi: 10.1111/j.1365-2869.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- Maatta S, Landsness E, Sarasso S, Ferrarelli F, Ferreri F, Ghilardi MF, Tononi G. The effects of morning training on night sleep: a behavioral and EEG study. Brain Res. Bull. 2010;1–2:118–123. doi: 10.1016/j.brainresbull.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat. Neurosci. 2003;7:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Moisello C, Crupi D, Tunik E, Quartarone A, Bove M, Tononi G, Ghilardi MF. The serial reaction time task revisited: a study on motor sequence learning with an arm-reaching task. Exp. Brain Res. 2009;1:143–155. doi: 10.1007/s00221-008-1681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard TC, Cai DJ, Rieth CA, Jones J, Ard MC. Sleep does not enhance motor sequence learning. J. Exp. Psychol. Learn. Mem. Cogn. 2008;4:834–842. doi: 10.1037/0278-7393.34.4.834. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: circadian rhythms in human cognition. Cogn. Neuropsychol. 2007;7:755–789. doi: 10.1080/02643290701754158. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol. Learn. Mem. 2006;2:241–247. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Fishbein W. Enhancement of declarative memory performance following a day-time nap is contingent on strength of initial task acquisition. Sleep. 2008;2:197–203. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MA, Fishbein W. The impact of sleep duration and subject intelligence on declarative and motor memory performance: how much is enough? J. Sleep Res. 2009;3:304–312. doi: 10.1111/j.1365-2869.2009.00740.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;1:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn. Mem. 2003;4:275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;6:R1370–R1377. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am. J. Physiol. 1999;4(Pt 2):R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]