Abstract
Background
Pseudomonas aeruginosa is a gram-negative bacterium that is ubiquitously present in the aerobic biosphere. As an antibiotic-resistant facultative pathogen, it is a major cause of hospital-acquired infections. Its rapid and accurate identification is crucial in clinical and therapeutic environments.
Methods
In a large-scale MALDI-TOF mass spectrometry-based screen of the Harvard transposon insertion mutant library of P. aeruginosa strain PA14, intact-cell proteome profile spectra of 5547 PA14 transposon mutants exhibiting a plethora of different phenotypes were acquired and analyzed.
Results
Of all P. aeruginosa PA14 mutant profiles 99.7% were correctly identified as P. aeruginosa with the Biotyper software on the species level. On the strain level, 99.99% of the profiles were mapped to five different individual P. aeruginosa Biotyper database entries. A principal component analysis-based approach was used to determine the most important discriminatory mass features between these Biotyper groups. Although technical replicas were consistently categorized to specific Biotyper groups in 94.2% of the mutant profiles, biological replicas were not, indicating that the distinct proteotypes are affected by growth conditions.
Conclusions
The PA14 mutant profile collection presented here constitutes the largest coherent P. aeruginosa MALDI-TOF spectral dataset publicly available today. Transposon insertions in thousands of different P. aeruginosa genes did not affect species identification from MALDI-TOF mass spectra, clearly demonstrating the robustness of the approach. However, the assignment of the individual spectra to sub-groups proved to be non-consistent in biological replicas, indicating that the differentiation between biotyper groups in this nosocomial pathogen is unassured.
Introduction
Although a broad range of bacterial identification systems are commercially available, microbial identification based on matrix-assisted laser desorption/ionization time-of-fight mass spectrometry (MALDI-TOF MS) is currently making its way into routine bacteriological practice at a rapid pace [1–3]. While the idea of differentiating bacteria through mass spectrometric techniques dates back to 1981 [4], biotyping by intact-cell MALDI-TOF MS was initially described for bacterial species in 1996 [5]. Today, MALDI-TOF MS biotyping provides an easy-to-use, rapid and reliable proteomic alternative to established phenotypic techniques such as the Vitek2 system [6]. Combining low sample preparation costs with high-throughput capabilities, MALDI-TOF MS biotyping is exceptionally well-suited for routine use and has huge implications for clinical applications [7–9]. Sufficiently sensitive MALDI-TOF instruments are commonly available at moderate costs, the underlying database and algorithm structures used for assigning unknown bacterial profiles to know strains however, have developed at a much slower rate [10–11]. Correct strain identification seems to be strongly influenced by the size and quality of the database spectra collection as well as by the investigated proteomic fingerprint range [12–13]. Provided a well-organized database, MALDI-TOF MS biotyping results were shown to be very robust in terms of colony-to-colony differences even if the culture conditions were not identical [14–17]. The high reproducibility could be owed to the fact that the proteomic MALDI-TOF MS fingerprint is largely based on ribosomal proteins that are constitutively expressed in the cell in very high abundance and thus not subject to the expression variability seen in phenotypic methods [18–20]. In a systematic large-scale approach, we recorded the mass spectra of the comprehensive Harvard transposon insertion mutant library of the Pseudomonas aeruginosa strain PA14 (http://ausubellab.mgh.harvard.edu/cgi-bin/pa14/home.cgi, accessed 04–2013) [21]. The extensive MS dataset acquired in this study provides information on the robustness of MALDI-TOF MS biotyping identification and sheds light on whether knock-out mutations in thousands of different genes impact MALDI-TOF intact-cell profiles and subsequent MALDI-TOF MS biotyping.
Methods
Cultivation and preparation of PA14 mutants
All 5833 transposon insertion mutants of the Harvard Medical School P. aeruginosa PA14 library were transferred onto LB agar plates in batches of 96 mutants. In total, 5547 mutants exhibited sufficient growth for further analyses. PA14 mutants were prepared on target plates (MSP 96 polished steel target, Bruker Daltonics, Bremen, Germany) by standardized smear procedure and immediately overlaid with 1μL of saturated alphacyano-4-hydroxycinnamic acid matrix solution.
MALDI-TOF MS
Intact-cell bacterial profile spectra were acquired in duplicates using a MicroflexLT MALDI-TOF device (Bruker Daltonics). Escherichia coli DH5α bacterial test standard (Bruker Daltonics) was used for external calibration. Spectra exceeding 1000 ppm maximal peak shift were excluded from further analysis as non-recalibratable and remeasured. The full raw spectra collection as universal csv data and Bruker fid spectra including detailed acquisition parameters can be downloaded from https://www3.mh-hannover.de/proteomix/pa14/spectra (user: plos1, password: dataset).
Data Analysis
A total number of 11094 mass spectra, consisting of two replicas of 5547 PA14 mutants, were analyzed in the range of 3000–15000 m/z. The mutant spectra were grouped into subclasses according to their unambiguous Biotyper 3.1 (Bruker Daltonics, database build 66) classification scores. The resulting P. aeruginosa subclasses were PA 19955–1 CHB, PA 8147–2 CHB, PA ATCC 27853 CHB, PA DSM 1117 DSM, and PA DSM 50071 T HAM, respectively. All 11094 raw spectra were normalized to 100 arbitrary intensity units and baseline corrected by regressing the varying baselines to a set of fixed window points (250) using a spline approximation (MATLAB 7.7.0). Class specific mean spectra were calculated for each sub-class. For peak detection, a wavelet-based MATLAB 7.7.0 peak picking algorithm (mspeaks) with a base of 2 and 10 levels as well as median-absolute-deviation for noise threshold estimation was applied on each individual spectrum [22]. Rigorous peak picking was achieved by a minimum height filter of 0.3 normalized intensity units and a minimum distance of 5 m/z between peaks [23]. Only peaks with a minimum full-width-at-half-height (FWHH) of 4 were considered valid [24].
The individual peak lists were used to generate pseudo-spectra of equal length by indexing the m/z-range for each of the spectra. In the next step spectral information was decomposed by using principal component analysis (PCA) in order to reduce and harmonize the variance between the analyzed spectra. Internal validation of the model was achieved by using all scores of the resulting PCA in linear discriminant analysis (LDA), thereby securing that the covariance matrix is positive definite and the loss of variance information is minimized [25]. In comparison to the PCA model, an approach using only the pseudo-spectra as input was also followed, allowing both model validation and the classification of external biological replicas. All classification runs were stratified by 10-fold cross-validation. The PCA approach also allowed the calculation of distances between individual spectra and clusters. The cluster centroids were used to calculate Ward’s distance between the five classes.
Results
MALDI Biotyper classification of the PA14 mutant library
The main objective of this study was to elucidate to what extent the inactivation of single genes influences MALDI-TOF MS based biotyper identification. Overall, of the 11094 mass spectra generated from the entire set of 5547 PA14 mutants, 11060 (99.7%) were correctly classified as P. aeruginosa by Biotyper 3.1 analysis (using default parameters according to standard biotyper rules (the highest scoring hit with identification score > 2.0)) as shown in Table 1. Species-misidentification and ambiguous results for replicas was exclusively due to poor spectrum quality (or contamination). A detailed table with the ten highest-scoring Biotyper database (build 66) matches for each spectrum can be downloaded from https://www3.mh-hannover.de/proteomix/pa14/bt (user: plos1, password: dataset).
Table 1. MALDI Biotyper 3.1 classification of the PA14 mutant library.
| TaxId | Species | Spectra | Proportion (%) | Mean Score |
|---|---|---|---|---|
| 287 | Pseudomonas aeruginosa DSM 1117 DSM | 5887 | 53.06 | 2.28 |
| 287 | Pseudomonas aeruginosa ATCC 27853 CHB | 2077 | 18.72 | 2.13 |
| 287 | Pseudomonas aeruginosa 8147_2 CHB | 1424 | 12.84 | 2.17 |
| 287 | Pseudomonas aeruginosa DSM 50071T HAM | 1291 | 11.64 | 2.08 |
| 287 | Pseudomonas aeruginosa 19955_1 CHB | 381 | 3.43 | 2.13 |
| 287 | Pseudomonas aeruginosa A07_08_Pudu FLR | 1 | 0.01 | 1.79 |
| - | Other Pseudomonas | 14 | 0.13 | 1.39 |
| 562 | Escherichia coli DH5alpha BRL | 3 | 0.03 | 2.12 |
| 470 | Acinetobacter baumannii LMG 994 HAM | 2 | 0.02 | 1.31 |
| 29378 | Staphylococcus arlettae DSM 20673 DSM | 2 | 0.02 | 1.40 |
| 32001 | Alcaligenes faecalis ssp faecalis DSM 30030T HAM | 1 | 0.01 | 1.42 |
| - | Other species | 11 | 0.10 | 1.44 |
| Sum | 11094 | 100.00 |
Revealing discriminatory proteomic traits among Biotyper groups
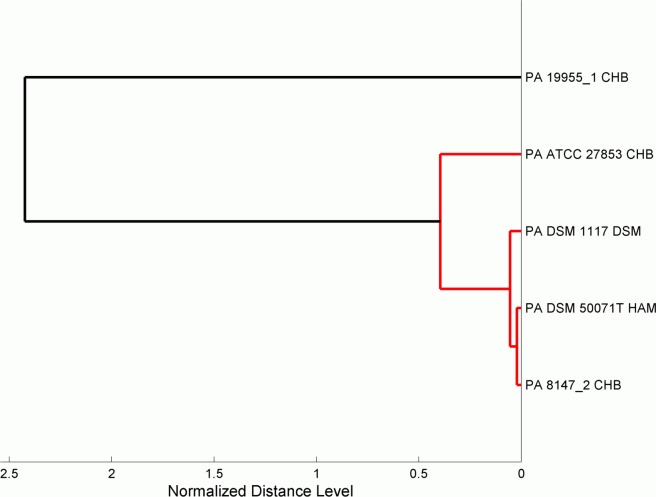
All MALDI-TOF MS PA14 mutant profiles classified as P. aeruginosa were mapped to five individual Biotyper database entries (PA 19955–1 CHB, PA 8147–2 CHB, PA ATCC 27853 CHB, PA DSM 1117 DSM, and PA DSM 50071 T HAM). Fig. 1 shows a dendrogram of the five Biotyper groups based on the normalized distance level between Biotyper group-specific mean spectra. The dendrogram demonstrates that the spectra of all Biotyper groups (except PA 19955–1 CHB) are highly similar. With the aim to evaluate whether the spectral differences are caused by differences in the protein composition of the various P. aeruginosa transposon mutants we applied automatic peak detection for each Biotyper group and harmonized the variance of the spectral information by using principal component analysis (PCA) on the pooled co-variance matrix. An optimal set of discriminatory masses was selected by optimizing peak numbers and peak indices of each Biotyper group and calculating the mean correct classification rate. All classification runs were stratified by 10-fold cross-validation. Using a panel of 187 mass peaks per Biotyper group, all mutant spectra could be matched to the Biotyper groups with a mean accuracy of 99.13% (Table 2).
Fig 1. Dendrogram of the five main P. aeruginosa Biotyper database strains the PA14 mutants were mapped to.
The dendrogram demonstrates that four classes are highly similar in terms of generalized spectral distances. PA 19955_1 CHB shows the largest deviation from the other four P. aeruginosa database entries.
Table 2. Confusion matrix of the LDA model for classifying PA biotyper groups.
| PA 19955_1 CHB | PA 8147_2 CHB | PA ATCC 27852 CHB | PA DSM 1117 DSM | PA 50071T HAM | |
|---|---|---|---|---|---|
| PA 19955_1 CHB | 368 | 0 | 10 | 2 | 1 |
| PA 8147_2 CHB | 0 | 1403 | 0 | 6 | 15 |
| PA ATCC 27852 CHB | 0 | 2 | 2064 | 7 | 4 |
| PA DSM 1117 DSM | 0 | 11 | 0 | 5872 | 4 |
| PA 50071T HAM | 0 | 19 | 1 | 14 | 1257 |
Robustness of the assignment of MALDI-TOF MS spectra to individual P. aeruginosa Biotyper database entries
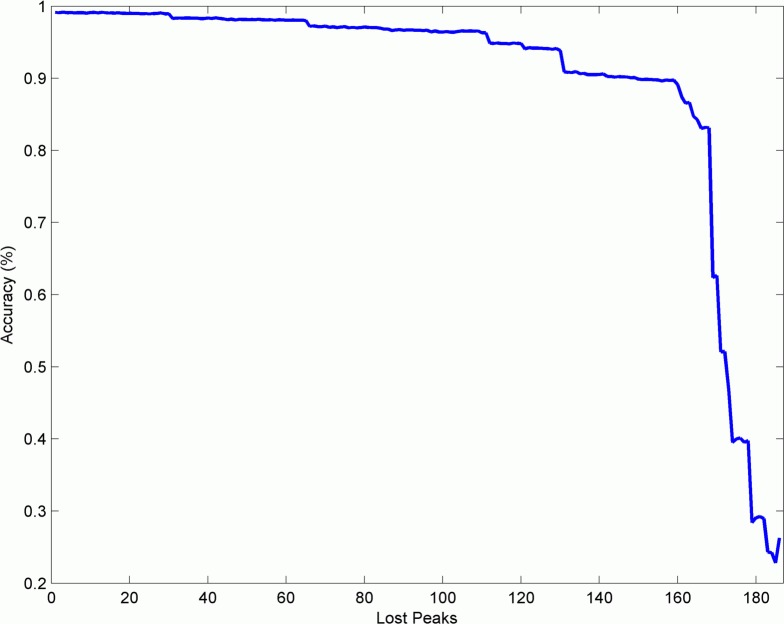
To further test the potential of well-characterized proteomic panels for strain sub-classification, our MALDI-TOF MS peak-panel-based approach was evaluated in respect to its robustness. The optimized panel of 187 peaks for PA14 mutant sub-classification was highly robust in terms of Biotyper group prediction (99.13% accuracy). However, failure to detect single mass peaks for example due to inter-laboratory variability may induce a significant loss in accuracy. Therefore, we successively deleted masses from the peak list while monitoring the behavior of the accuracy using 10-fold cross-validation and discriminant analysis. The elimination of peaks continually reduced the general accuracy of the model. However, eliminating the first 111 peaks still yielded an accuracy of 95% which is an indicator for the robustness of the found model. Further peak elimination led to a more dramatic drop in accuracy. When 149 peaks were removed, the model quickly fell below 90% accuracy. Fig. 2 shows a graphic representation of this behavior which demonstrates that the combination of 187 masses forms a highly stable model for the differentiation between five of the currently 14 Biotyper groups which is also robust against the loss of multiple peaks in MALDI-TOF MS spectra. A full peaklist can be found in the supplementary material (S1 Table).
Fig 2. The plot shows the drop in accuracy when relevant peaks are removed.
Although the classification result experiences continuous loss in accuracy when fewer peaks are included, the result is rather stable and stays above 95% until 111 peaks are removed. This indicates a highly stable peak list which can be used for mathematical subclass prediction.
MALDI-TOF MS spectra do not support stable classification of biological replicas
From the entire set of 5547 PA14 mutants we generated mass spectra of technical replicas of which 11060 (99.7%) were classified as P. aeruginosa. Thereby, 99.99% of the technical replicas of individual mutant profiles mapped to one of five P. aeruginosa Biotyper database entries. Table 3 shows the distribution of the technical replicas after Biotyper classification. 5.83% of the technical replicas were assigned ambiguously. We next tested whether biological replicas could be stably assigned to sub-groups. We therefore re-streaked individual P. aeruginosa transposon mutants and generated 132 MALDI-TOF-MS spectra. Unfortunately, as depicted in Table 4, 21.97% of the biological replicas were assigned unambiguously. These results clearly demonstrate that the identification of distinct clonal groups of this nosocomial pathogen is unassured.
Table 3. Analysis of technical replicas (MALDI Biotyper 3.1 classification).
| Subgroup | No. of techn. rep. 1 | No. of techn. rep. 2 | Sum | Ratio 1 (%) | Ratio 2 (%) |
|---|---|---|---|---|---|
| Pseudomonas aeruginosa 19955_1 CHB | 173 | 208 | 381 | 45.41 | 54.59 |
| Pseudomonas aeruginosa 8147_2 CHB | 715 | 709 | 1424 | 50.21 | 49.79 |
| Pseudomonas aeruginosa ATCC 27853 CHB | 1044 | 1033 | 2077 | 50.26 | 49.74 |
| Pseudomonas aeruginosa DSM 1117 DSM | 2963 | 2924 | 5887 | 50.33 | 49.67 |
| Pseudomonas aeruginosa DSM 50071T HAM | 640 | 651 | 1291 | 49.57 | 50.43 |
| Sum | 5535 | 5525 | 11060 |
Table 4. Distribution of classified biological replicas (use of the LDA model for validating PA sub-groups).
| PA 19955_1 CHB | PA 8147_2 CHB | PA ATCC 27852 CHB | PA DSM 1117 DSM | PA 50071T HAM | |
|---|---|---|---|---|---|
| PA 19955_1 CHB | 103 | 0 | 2 | 0 | 1 |
| PA 8147_2 CHB | 10 | 0 | 0 | 0 | 1 |
| PA ATCC 27852 CHB | 8 | 0 | 0 | 0 | 0 |
| PA DSM 1117 DSM | 3 | 0 | 0 | 0 | 0 |
| PA 50071T HAM | 4 | 0 | 0 | 0 | 0 |
Discussion
Using a fast and reproducible profiling procedure, the extraordinary robustness of the MALDI-TOF Biotyper procedure [26] was demonstrated in this study by analyzing the whole Harvard P. aeruginosa PA14 mutant library. This library comprises a total of 5833 single gene transposon insertion mutants exhibiting a plethora of different phenotypes in the genetic background of the P. aeruginosa type strain PA14. Here, this library served to generate the largest coherent microbial intact-cell MALDI-TOF MS profile spectra collection, which is now publicly available to the community (https://www.mh-hannover.de/proteomix/pa14/spectra). The simple, fast and cost effective MALDI-TOF Biotyper procedure compared very well with the Vitek2 standard routine species identification method. The obtained mass spectra correctly identified 99.7% of the studied mutants at the species level and proved to be an even more robust procedure as compared to standard biochemical testing [27].
Interestingly, all obtained MALDI-TOF MS PA14 mutant profiles that were correctly classified as P. aeruginosa were mapped to five individual Biotyper database entries (PA 19955–1 CHB, PA 8147–2 CHB, PA ATCC 27853 CHB, PA DSM 1117 DSM, and PA DSM 50071 T HAM). Identification of not only the bacterial species but also the affiliation to a distinct clonal group of nosocomial pathogens is expected to facilitate population dynamics studies in chronically infected patients and to improve the detection of inter-patient transmission of epidemic clones [28–29]. Only recently it was demonstrated that characteristic mass fingerprints as recorded by MALDI-TOF MS were sufficiently discriminatory to identify Escherichia coli subgroups as a promising tool for the optimization of infection control [30].
In this study, we evaluated whether the MALDI-TOF Biotyper could be further used for the robust identification of distinct clonal groups of the nosocomial pathogen P. aeruginosa. We found that the combination of 187 masses forms a highly stable model for the differentiation between five of the 14 currently Biotyper groups which is also robust against the loss of multiple peaks in MALDI-TOF MS spectra. However, differences between discriminatory peak positions of the clusters were only marginal and the assignment of Biotyper groups based entirely to these small shifts seemed unassured. Indeed, we found only a restricted accuracy of the classification of technical replicas and an insufficient accuracy for biological replicas.
Clearly the impact of bacterial growth conditions and/or sample preparation procedures on P. aeruginosa subtyping has to be evaluated in more detail. Thus, in conclusion although this study demonstrates that MALDI-TOF MS-based identification can routinely identify P. aeruginosa irrespective of the location of a transposon insertion to the species level with very high diagnostic accuracy, it remains to be shown that P. aeruginosa intact-cell profile spectra contain masses that prove to be suitable to accurately characterize subgroups found within the P. aeruginosa strains on the proteomic level.
Supporting Information
(XLSX)
References
- 1. Bessède E, Angla-Gre M, Delagarde Y, Sep Hieng S, Ménard A, et al. (2011) Matrix-assisted laser-desorption/ionization biotyper: experience in the routine of a University hospital. Clin Microbiol Infect 17: 533–8. 10.1111/j.1469-0691.2010.03274.x [DOI] [PubMed] [Google Scholar]
- 2. Ford BA, Burnham CA (2013) Optimization of routine identification of clinically relevant gram-negative bacteria by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry and the bruker biotyper. J Clin Microbiol. 51: 1412–20. 10.1128/JCM.01803-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deak E, Charlton CL, Bobenchik AM, Miller SA, Pollett S, et al. (2015) Comparison of the Vitek MS and Bruker Microflex LT MALDI-TOF MS platforms for routine identification of commonly isolated bacteria and yeast in the clinical microbiology laboratory. Diagn Microbiol Infect Dis. 81:27–33. 10.1016/j.diagmicrobio.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 4. Wieten G, Haverkamp J, Meuzelaar HL, Engel HW, Berwald LG (1981) Pyrolysis mass spectrometry: a new method to differentiate between the mycobacteria of the ‘tuberculosis complex’ and other mycobacteria. J Gen Microbiol 122: 109–118. [DOI] [PubMed] [Google Scholar]
- 5. Holland RD, Wilkes JG, Rafii F, Sutherland JB, Persons CC, et al. (1996) Rapid identification of intact whole bacteria based on spectral patterns using matrix-assisted laser desorption/ionization with time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 10: 1227–32. [DOI] [PubMed] [Google Scholar]
- 6. Davies AP, Reid M, Hadfield SJ, Johnston S, Mikhail J, et al. (2012) Identification of clinical isolates of α-hemolytic streptococci by 16S rRNA gene sequencing, matrix-assisted laser desorption ionization-time of flight mass spectrometry using MALDI Biotyper, and conventional phenotypic methods: a comparison. J Clin Microbiol 50: 4087–90. 10.1128/JCM.02387-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, et al. (2009) Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis 49: 543–51. 10.1086/600885 [DOI] [PubMed] [Google Scholar]
- 8. Tani A, Sahin N, Matsuyama Y, Enomoto T, Nishimura N, et al. (2012) High-throughput identification and screening of novel Methylobacterium species using whole-cell MALDI-TOF/MS analysis. PLoS One. 7(7):e40784 10.1371/journal.pone.0040784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tong DL, Boocock DJ, Coveney C, Saif J, Gomez SG, et al. (2011) A simpler method of preprocessing MALDI-TOF MS data for differential biomarker analysis: stem cell and melanoma cancer studies. Clin Proteomics. 9;8:14 10.1186/1559-0275-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Z, Dunlop K, Long SR, Li L (2002) Mass spectrometric methods for generation of protein mass database used for bacterial identification. Anal Chem 74: 3174–3182. [DOI] [PubMed] [Google Scholar]
- 11. Sogawa K, Watanabe M, Sato K, Segawa S, Miyabe A, et al. (2012) Rapid identification of microorganisms by mass spectrometry: improved performance by incorporation of in-house spectral data into a commercial database. Anal Bioanal Chem. 403: 1811–22. 10.1007/s00216-011-5656-1 [DOI] [PubMed] [Google Scholar]
- 12. Cunningham SA, Patel R (2013) Importance of Using Bruker's Security-Relevant Library for Biotyper Identification of Burkholderia pseudomallei, Brucella Species , and Francisella tularensis . J Clin Microbiol. 51: 1639–40 10.1128/JCM.00267-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szabados F, Woloszyn J, Richter C, Kaase M, Gatermann S (2010) Identification of molecularly defined Staphylococcus aureus strains using matrix-assisted laser desorption/ionization time of flight mass spectrometry and the Biotyper 2.0 database. J Med Microbiol. 59: 787–90. 10.1099/jmm.0.016733-0 [DOI] [PubMed] [Google Scholar]
- 14. Sogawa K, Watanabe M, Sato K, Segawa S, Ishii C, et al. (2011) Use of the MALDI BioTyper system with MALDI-TOF mass spectrometry for rapid identification of microorganism. Anal Bioanal Chem. 400: 1905–1911. 10.1007/s00216-011-4877-7 [DOI] [PubMed] [Google Scholar]
- 15. McElvania Tekippe E, Shuey S, Winkler DW, Butler MA, Burnham CAD, et al. (2013) Optimizing identification of clinically relevant gram-positive organisms by use of the bruker biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry system. J Clin Microbiol. 51: 1421–7. 10.1128/JCM.02680-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alatoom AA, Cunningham SA, Ihde SM, Mandrekar J, Patel R (2011) Comparison of direct colony method versus extraction method for identification of gram-positive cocci by use of Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 49: 2868–73. 10.1128/JCM.00506-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson NW, Buchan BW, Riebe KM, Parsons LN, Gnacinski S, et al. (2012) Effects of solid-medium type on routine identification of bacterial isolates by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 50: 1008–13. 10.1128/JCM.05209-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teramoto K, Sato H, Sun L, Torimura M, Tao H, et al. (2007) Phylogenetic classification of Pseudomonas putida strains by MALDI-MS using ribosomal subunit proteins as biomarkers. Anal Chem. 79: 8712–8719. [DOI] [PubMed] [Google Scholar]
- 19. Khot PD, Couturier MR, Wilson A, Croft A, Fisher MA (2012) Optimization of matrix-assisted laser desorption ionization-time of flight mass spectrometry analysis for bacterial identification. J Clin Microbiol. 50: 3845–52. 10.1128/JCM.00626-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He Y, Li H, Lu X, Stratton CW, Tang YW (2010) Mass spectrometry biotyper system identifies enteric bacterial pathogens directly from colonies grown on selective stool culture media. J Clin Microbiol. 48: 3888–92. 10.1128/JCM.01290-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, et al. (2006) An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A. 103: 2833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coombes KR, Tsavachidis S, Morris JS, Baggerly KA, Hung MC, et al. (2005) Improved peak detection and quantification of mass spectrometry data acquired from surface-enhanced laser desorption and ionization by denoising spectra with the undecimated discrete wavelet transform. Proteomics. 5(16), 4107–4117. [DOI] [PubMed] [Google Scholar]
- 23. Yang C, He Z, Yu W (2009) Comparison of public peak detection algorithms for MALDI mass spectrometry data analysis. BMC Bioinformatics. 10:4 10.1186/1471-2105-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Q, Sung AH, Qiao M, Chen Z, Yang JY, et al. (2009) Comparison of feature selection and classification for MALDI-MS Data. BMC Genomics. 10 Suppl 1:S3 10.1186/1471-2164-10-S1-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dossat N, Mangé A, Solassol J, Jacot W, Lhermitte L, et al. (2007) Comparison of supervised classification methods for protein profiling in cancer diagnosis. Cancer Inform. 3:295–305. [PMC free article] [PubMed] [Google Scholar]
- 26. Clark AE, Kaleta EJ, Arora A, Wolk DM (2013) Matrix-assisted laser desorption ionization-time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin Microbiol Rev. 26(3):547–603. 10.1128/CMR.00072-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pommerenke C, Müsken M, Becker T, Dötsch A, Klawonn F, et al. (2010) Global genotype-phenotype correlations in Pseudomonas aeruginosa . PLoS Pathog. 6(8):e1001074 10.1371/journal.ppat.1001074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berrazeg M, Diene SM, Drissi M, Kempf M, Richet H, et al. (2013) Biotyping of multidrug-resistant Klebsiella pneumoniae clinical isolates from France and Algeria using MALDI-TOF MS. PLoS One. 8(4):e61428 10.1371/journal.pone.0061428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolters M, Rohde H, Maier T, Belmar-Campos C, Franke G, et al. (2011) MALDI-TOF MS fingerprinting allows for discrimination of major methicillin-resistant Staphylococcus aureus lineages. Int J Med Microbiol. 301(1):64–8. 10.1016/j.ijmm.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 30. Novais A, Sousa C, de Dios Caballero J, Fernandez-Olmos A, Lopes J, et al. (2014) MALDI-TOF mass spectrometry as a tool for the discrimination of high-risk Escherichia coli clones from phylogenetic groups B2 (ST131) and D (ST69, ST405, ST393). Eur J Clin Microbiol Infect Dis. Epub ahead of print. 10.1007/s10096-014-2071-5 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)