Abstract
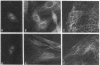
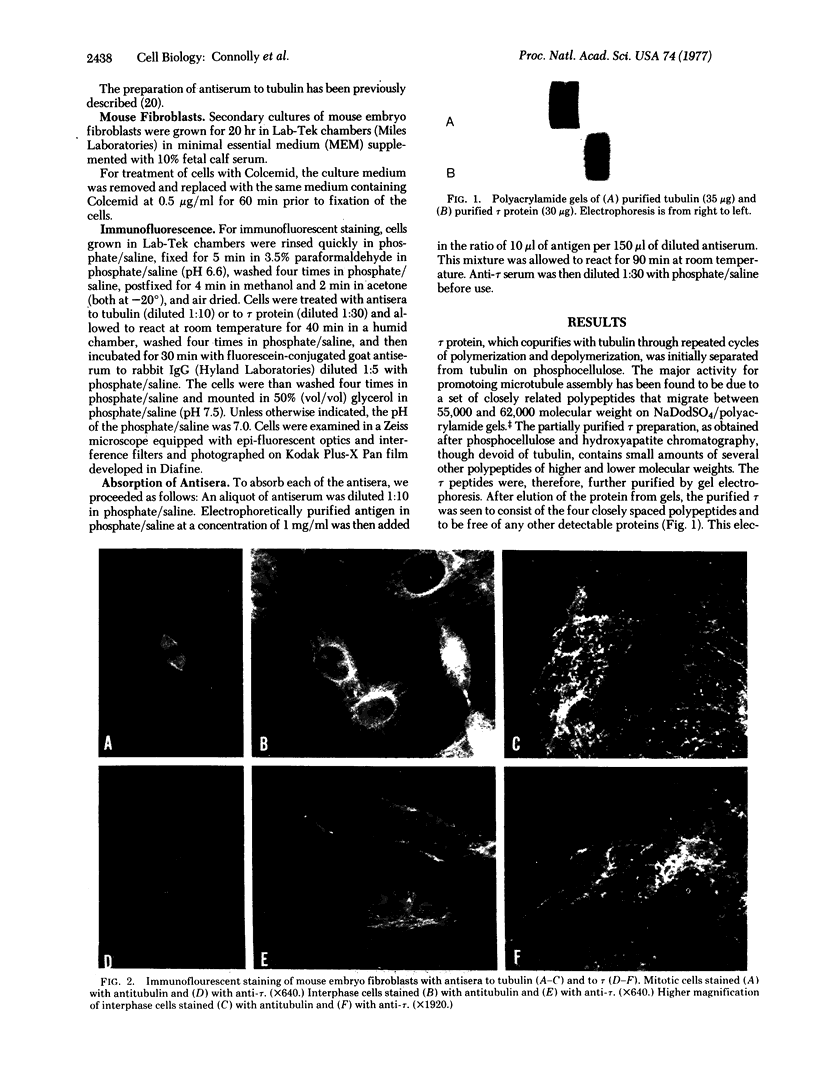
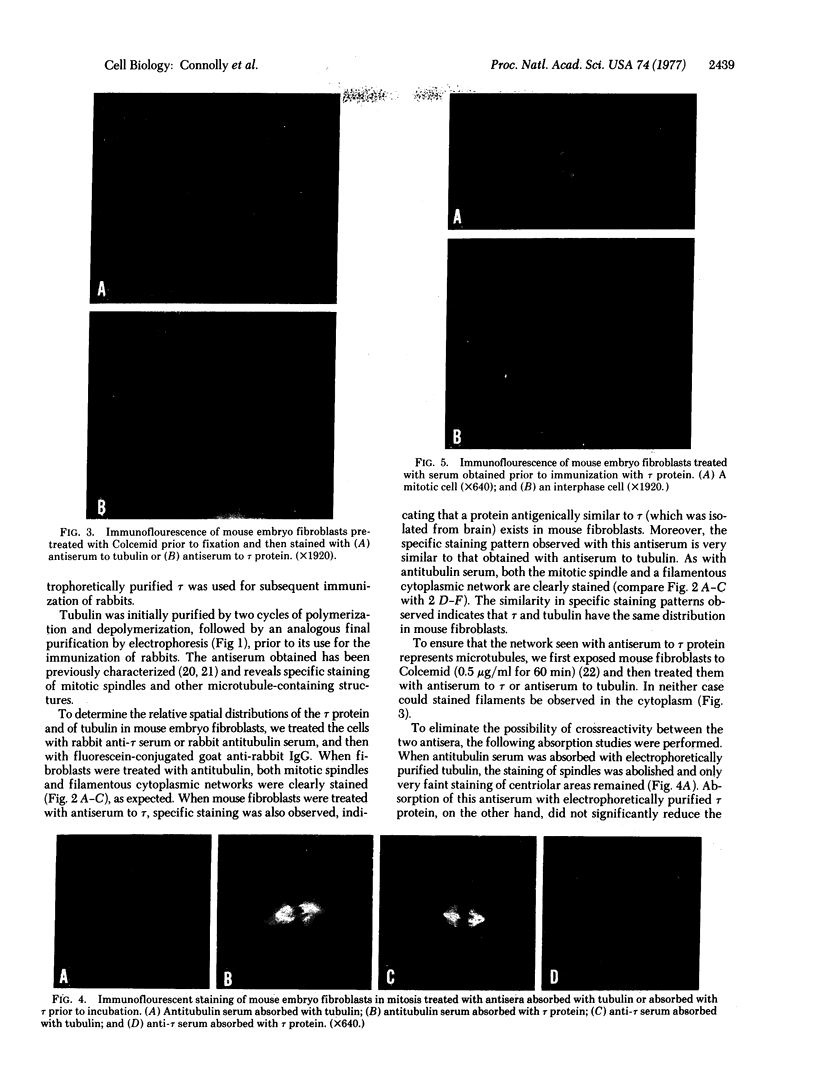
tau protein isolated from porcine brain microtubules was further purified by electrophoretic elution from polyacrylamide gels and used to prepare antisera in rabbits. The antiserum to tau specifically stains mitotic spindles and a filamentous network within mouse fibroblasts when the indirect immunofluorescence technique is used. The staining of the filamentous network and mitotic spindles is identical to that observed when cells are treated with antiserum prepared against electrophoretically purified tubulin. The filamentous network observed with either serum is sensitive to Colcemid. Absorption of anti-tau serum with electrophoretically purified tubulin does not remove the immunofluorescent staining of the mitotic spindle, whereas absorption with electrophoretically purified tau protein does. Conversely, absorption of antitubulin serum with tubulin eliminates its ability to stain the mitotic spindle, whereas absorption with tau has no effect. We conclude that tau protein and tubulin are antigenically distinct proteins and that tau is an integral part of microtubules in vivo. These results also provide evidence that tau protein, or an antigenically related protein, is associated with microtubules not only in brain but also in other cell types.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubin J. E., Subrahmanyan L., Kalnins V. I., Ling V. Antisera against electrophoretically purified tubulin stimulate colchicine-binding activity. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1246–1249. doi: 10.1073/pnas.73.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B. Nucleated assembly of microtubules in porcine brain extracts. Science. 1972 Sep 29;177(4055):1196–1197. doi: 10.1126/science.177.4055.1196. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- De Mey J., Hoebeke J., De Brabander M., Geuens G., Joniau M. Immunoperoxidase visualisation of microtubules and microtubular proteins. Nature. 1976 Nov 18;264(5583):273–275. doi: 10.1038/264273a0. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Voter W. A. Polycation-induced assembly of purified tubulin. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2813–2817. doi: 10.1073/pnas.73.8.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forer A., Kalnins V. I., Zimmerman A. M. Spindle birefringence of isolated mitotic apparatus: further evidence for two birefringent spindle components. J Cell Sci. 1976 Oct;22(1):115–131. doi: 10.1242/jcs.22.1.115. [DOI] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- Himes R. H., Burton P. R., Kersey R. N., Pierson G. B. Brain tubulin polymerization in the absence of "microtubule-associated proteins". Proc Natl Acad Sci U S A. 1976 Dec;73(12):4397–4399. doi: 10.1073/pnas.73.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keates R. A., Hall R. H. Tubulin requires an accessory protein for self assembly in microtubules. Nature. 1975 Oct 2;257(5525):418–421. doi: 10.1038/257418a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The reconstitution of microtubules from purified calf brain tubulin. Biochemistry. 1975 Nov 18;14(23):5183–5187. doi: 10.1021/bi00694a025. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Cytoplasmic microtubules in tissue culture cells appear to grow from an organizing structure towards the plasma membrane. Proc Natl Acad Sci U S A. 1976 Mar;73(3):867–871. doi: 10.1073/pnas.73.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda R. D., Dentler W. L., Rosenbaum J. L. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry. 1976 Oct 5;15(20):4497–4505. doi: 10.1021/bi00665a026. [DOI] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Witman G. B., Cleveland D. W., Weingarten M. D., Kirschner M. W. Tubulin requires tau for growth onto microtubule initiating sites. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4070–4074. doi: 10.1073/pnas.73.11.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]