Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1 RA) are attractive options for the treatment of type 2 diabetes (T2D) because they effectively lower A1C and weight while having a low risk of hypoglycemia. The GLP-1 RA class has grown in the last decade with several agents available for use in the US and Europe and several more in development. Since the efficacy and tolerability, dosing frequency, administration requirements, and cost may vary between agents within the class, each agent may offer unique advantages and disadvantages. Through a review of phase III clinical programs for exenatide twice daily, exenatide once weekly, liraglutide, albiglutide, lixisenatide, and dulaglutide, eight head-to-head trials have evaluated the safety and efficacy of GLP-1 RA active comparators. The purpose of this review is to provide an analysis of these trials. The GLP-1 RA head-to-head clinical studies have demonstrated that all GLP-1 RA agents are effective therapeutic options at reducing A1C. However, differences exist in terms of magnitude of effect on A1C and weight as well as frequency and severity of adverse effects.
Keywords: GLP-1 receptor agonist, type 2 diabetes
Introduction
Globally, it is estimated that 382 million people have diabetes, correlating with a prevalence of 8.3% [International Diabetes Federation, 2013]. Type 2 diabetes (T2D) accounts for approximately 95% of all cases. Coinciding with the growing rates of obesity, the global prevalence of T2D continues to rise. If inadequately treated, T2D increases the risk of heart attacks and strokes, kidney failure, blindness, and amputations. Although many treatment options exist, achieving adequate glycemic control remains challenging. Beta-cell function progressively declines, often necessitating treatment intensification over time to maintain glycemic control. Current diabetes treatment guidelines recommend a patient-specific approach to treatment with a goal of achieving glycemic control while minimizing adverse effects, particularly weight gain and hypoglycemia [Inzucchi et al. 2012; Garber et al. 2013]. While lifestyle modifications, weight loss, and metformin are typically considered first-line options, several medication classes are available for use as add-on therapy. When selecting diabetes medications, clinicians should consider specific patient goals and needs, complementary mechanisms of action, efficacy, adverse effects, cost, treatment burden, and long term safety. The glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are attractive options for the treatment of T2D because they effectively lower A1C and weight while having a low risk of hypoglycemia. The GLP-1 RA class has grown in the last decade with several agents available for use in the US and Europe and several more in development. Since the efficacy and tolerability, dosing frequency, administration requirements, and cost may vary between agents within the class, each agent may offer unique advantages and disadvantages. The purpose of this review is to provide an analysis of current head-to-head comparative data of GLP-1 RAs.
GLP-1 RAs: general effects and comparisons
GLP-1 is a peptide hormone that increases insulin secretion and decreases glucagon secretion from the pancreas in a glucose-dependent manner. GLP-1 RAs provide pharmacologic levels of GLP-1 which reduces glucose and weight by increasing glucose-dependent insulin secretion and decreasing glucagon secretion, delaying gastric emptying and increasing satiety. All of the GLP-1 RA agents are administered as subcutaneous (SC) injections. Although rates of adverse effects differ between specific agents, the most common adverse effects with the GLP-1 RA class are gastrointestinal (GI) related (nausea, vomiting, and diarrhea) and injection-site reactions. There are currently four GLP-1 receptor agonists approved for use in United States and five approved for use in the European Union (Table 1): exenatide twice daily, liraglutide once daily, exenatide once weekly, albiglutide once weekly, and lixisenatide once daily (available in Europe). Dulaglutide once weekly has been submitted for approval in both the US and Europe. Several key differences exist between the products in terms of molecular structure, pharmacokinetics, dose, administration, storage as well as efficacy, tolerability, and patient satisfaction. With the considerable heterogeneity and complexity across the class of GLP-1 RAs, each agent should be evaluated independently, as opposed to assuming a class effect, and head-to-head clinical trials can lend important information regarding differences within the GLP-1 RA class.
Table 1.
The glucagon-like peptide 1 receptor agonists (GLP-1 RAs) currently available and in development.
| Drug | Brand name | Dosing frequency | US FDA approval status | EMA approval status | Phase III clinical trial program |
|---|---|---|---|---|---|
| Exenatide | Byetta® | Twice daily | Approved 28 April 2005 | Approved 20 November 2006 | AMIGO |
| Liraglutide | Victoza® | Daily | Approved 25 January 2010 | Approved 30 June 2009 | LEAD |
| Exenatide | Bydureon® | Weekly | Approved 26 January 2012 | Approved 17 June 2011 | DURATION |
| Lixisenatide | Lyxumia® (Europe) | Daily | Submitted Withdrawn 12 September 2013 | Approved 1 February 2013 | GetGoal |
| Albiglutide | Tanzeum® (US) Eperzam® (Europe) | Weekly | Approved 15 April 2014 | Approved 23 January 2014 | HARMONY |
| Dulaglutide | Weekly | Submitted | Submitted | AWARD |
Abbreviations: US FDA, United States Food and Drug Administration; EMA, European Medicines Agency.
Head-to-head clinical studies
The GLP-1 RA agents have all been extensively evaluated in phase III clinical programs (Table 1). Through a review of phase III clinical programs for exenatide twice daily, exenatide once weekly, liraglutide, albiglutide, lixisenatide, and dulaglutide, we identified eight head-to-head trials that evaluated the safety and efficacy of GLP-1 RA active comparators. A summary of the design of the head-to-head studies is provided in Table 2. All eight trials were designed as noninferiority trials. The primary efficacy endpoint in all of the trials was change in A1C from baseline with a noninferiority margin of 0.4%. Figures 1 and 2 display the differences in A1C and weight observed within these studies.
Table 2.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs): summary of head-to-head clinical trials.
| Study | Design | Baseline characteristics | Background therapy | Active comparators |
|---|---|---|---|---|
| DURATION-1 [Drucker et al. 2008] | R, OL, AC, NI N=295, 30 weeks | Mean age 55 years, A1C 8.3%, weight 102 kg, BMI 35 kg/m2, duration of diabetes 6.7 years | Drug naïve or metformin, SU, TZD or a combination of two of those agents | Exenatide 10 µg BID |
| Exenatide 2 mg QW | ||||
| LEAD-6 [Buse et al. 2009] | R, OL, AC, NI N=464, 26 weeks | Mean age 57 years, A1C 8.1%, weight 93 kg, BMI 32.9 kg/m2, duration of diabetes 8.2 years | metformin, SU, or both | Exenatide 10 µg BID |
| Liraglutide 1.8 mg QD | ||||
| DURATION-5 [Blevins et al. 2011] | R, OL, AC, NI N=252, 24 weeks | Mean age 56 years, A1C 8.4%, weight 96 kg, BMI 33.3 kg/m2, duration of diabetes 7 years | Drug naïve or metformin, SU, TZD or any combination | Exenatide 10 µg BID |
| Exenatide 2 mg QW | ||||
| DURATION-6 [Buse et al. 2013] | R, OL, AC, NI N=911, 26 weeks | Mean age 57 years, A1C 8.5%, weight 91 kg, BMI 32.3 kg/m2, duration of diabetes 8.5 years | Metformin, SU, both, or metformin + pioglitazone | Exenatide 2 mg QW |
| Liraglutide 1.8 mg QD | ||||
| GetGoal-X [Rosenstock et al. 2013] | R, OL, AC, NI N=634, 24 weeks | Mean age 57 years, A1C 8.0%, weight 95 kg, BMI 33.6 kg/m2, duration of diabetes 6.8 years | Metformin | Lixisenatide 20 µg QD |
| Exenatide 10 µg BID | ||||
| HARMONY-7 [Pratley et al. 2014] | R, OL, AC, NI N=841, 32 weeks | Mean age 56 years, A1C 8.2%, weight 92 kg, BMI 32.8 kg/m2, duration of diabetes 8.4 years | metformin, pioglitazone, SU, or any combination | Albiglutide 50 mg QW |
| Liraglutide 1.8 mg QD | ||||
| AWARD-1 [Wysham et al. 2014] | R, OL, PC, AC, S*, NI N=978, 26 weeks | Mean age 56 years, A1C 8.1%, weight 96 kg, BMI 33 kg/m2, duration of diabetes 9 years | Metformin + pioglitazone | Dulaglutide 1.5 mg QW |
| Dulaglutide 0.75 mg QW | ||||
| Exenatide 10 µg BID | ||||
| Placebo | ||||
| AWARD-6 [Dungan et al. 2014] | R, OL, AC, NI N=599, 26 weeks | Mean age 57 years, A1C 8.1%, weight 94 kg, BMI 33.5 kg/m2, duration of diabetes 7.2 years | Metformin | Dulaglutide 1.5 mg QW |
| Liraglutide 1.8 mg QD |
Abbreviations: R, randomized; OL, open label; AC, active comparator; PC, placebo controlled; S, superiority; NI, noninferiority; PC, placebo controlled; BID, twice daily; QD, once daily; QW, once weekly; SU, sulfonylurea; TZD, thiazolidinedione; BMI, body mass index.
Superiority testing versus placebo, noninferiority testing versus exenatide.
Figure 1.
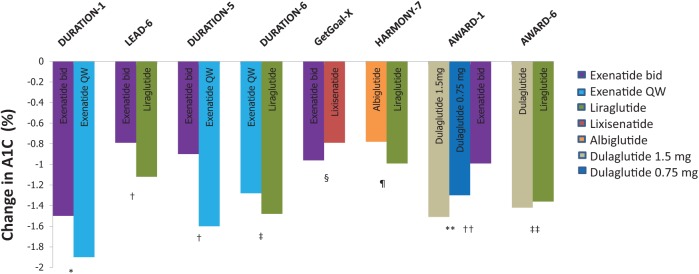
Changes in A1C values with glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in head-to-head clinical studies.
p-values are for statistical superiority unless otherwise noted as noninferiority; *p < 0.0025, †p < 0.0001, ‡p = 0.02, §p = not significant, noninferiority p-value not reported (95% confidence interval 0.033–0.297, meeting predefined noninferiority margin), ¶ noninferiority p-value = 0.846 (not meeting predefined noninferiority margin), **p < 0.001 for both doses of dulaglutide versus exenatide bid, ††p = not significant, noninferiority p-value < 0.0001 (meeting predefined noninferiority margin).
Figure 2.
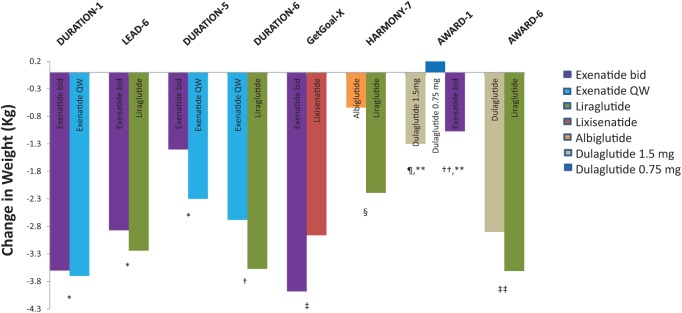
Changes in weight with glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in head-to-head clinical studies.
p-values are for statistical superiority (unless noted for noninferiority); *p = not significant, †p = 0.0005, ‡p-value not reported for weight difference of 1.02 kg (95% confidence interval 0.456–1.581), §p < 0.0001, ¶ p < 0.001 versus dulaglutide 0.75 mg, **p = not significant between dulaglutide 1.5 mg versus exenatide bid, ‡‡p = 0.011.
Efficacy
The DURATION-1 study compared exenatide once weekly with exenatide twice daily in patients with uncontrolled T2D being treated with either diet, one or two oral therapies [Drucker et al. 2008]. Baseline characteristics for this study are reported in Table 2. After 30 weeks, exenatide once weekly reduced A1C significantly more compared with the twice daily formulation (−1.9% versus −1.5%; 95% confidence interval [CI] −0.54% to −0.12%, p = 0.0023). The percentage of patients achieving a goal A1C of ≤7% was also greater with exenatide once weekly compared with exenatide twice daily (77% versus 61%, p = 0.0039). Body weight decreased similarly between the two groups throughout the 30-week study with a −3.7 and −3.6 kg reduction from baseline in the exenatide weekly and twice daily groups, respectively (p = 0.89, Figure 2). An extension study of DURATION-1 to 52 weeks was conducted by Buse and colleagues [Buse et al. 2010]. The extension study converted the exenatide twice daily patients to the weekly formulation for an additional 22 weeks, while those originally randomized to exenatide once weekly continued this during the follow up period. After 52 weeks patients continued on the once weekly exenatide maintained an A1C improvement (−2.0%) while those switching from twice daily to once weekly further reduced A1C to achieve a similar reduction in A1C as those originally on exenatide once weekly.
Patients on maximally tolerated doses of metformin, sulfonylurea or both were randomized to liraglutide or exenatide twice daily in the LEAD-6 trial [Buse et al.2009]. Liraglutide reduced A1C significantly more than exenatide twice daily (−1.12% versus −0.79%; 95% CI −0.47 to −0.18, p < 0.0001), while improving the proportion of patients achieving an A1C of <7% (54% versus 43%, respectively, p = 0.0015). The percentage of subjects achieving weight loss (liraglutide 78% versus exenatide 76%) and overall weight loss (liraglutide −3.24 kg versus exenatide 2.87 kg, p = 0.22) was similar between groups.
The DURATION-5 study also compared exenatide once weekly to exenatide twice daily [Blevins et al. 2011]. After 24 weeks, a significant reduction in A1C was observed with once weekly compared with twice daily exenatide (−1.6 versus −0.9%, p < 0.0001). As with the DURATION-1 trial, exenatide once weekly significantly lowered fasting glucose when compared with the twice daily formulation (−1.9 versus −0.7 mmol/l, p = 0.0008). The proportion of patients achieving an A1C <7% was 58% and 30% in the weekly and twice daily exenatide groups, respectively (p < 0.0001). A similar reduction in body weight was observed between groups (Figure 2).
Exenatide once weekly was compared to liraglutide in the DURATION-6 trial [Buse et al. 2013]. Reductions in A1C from baseline were significantly greater in patients taking liraglutide compared with exenatide once weekly (−1.48 versus −1.28%, p = 0.02). This difference of 0.21% did not meet predefined noninferiority criteria (95% CI 0.08–0.33). The proportion of patients achieving an A1C <7% was 60% and 53% in the liraglutide and exenatide once weekly groups, respectively (p = 0.0011). Patients in the liraglutide group lost 0.9 kg more body weight compared with exenatide once weekly (−3.57 versus −2.68, p = 0.0005). Both liraglutide and exenatide significantly reduced fasting blood glucose from baseline (−2.12 versus −1.76 mmol/l, p = 0.02).
The GetGoal-X trial compared the efficacy and safety of lixisenatide to exenatide twice daily in patients with uncontrolled T2D on metformin [Rosenstock et al. 2013]. The mean change in A1C was −0.79% in the lixisenatide group compared with −0.96% in the exenatide twice daily group. This difference of 0.17% between groups met predefined criteria for noninferiority (95% CI 0.033–0.297). A similar proportion of patients in each group achieved a goal A1C of <7% (48.5% lixisenatide and 49.8% exenatide, p = not significant). Body weight was significantly reduced in both groups, although a greater reduction was seen with exenatide (lixisenatide −2.96 kg versus exenatide −3.98 kg; 95% CI 0.45–1.58).
The HARMONY-7 study compared albiglutide once weekly to liraglutide once daily [Pratley et al. 2014]. A greater reduction in A1C of 0.21% favored liraglutide (0.99 versus 0.78%, 95% CI 0.08–0.34; noninferiority p-value = 0.0846), thus not meeting predefined noninferiority criteria. Fasting blood glucose was significantly more reduced with liraglutide (–1.68 versus –1.22 mmol/l, p = 0.0048). Weight loss was also significantly better with liraglutide. The mean change in weight in the liraglutide and albiglutide groups was −2.16 and −0.64 kg, respectively, with a mean difference of 1.55 kg (95% CI 1.05–2.06, p < 0.0001).
Two different doses of dulaglutide (1.5 and 0.75 mg) given weekly were compared with exenatide twice daily and placebo in the AWARD-1 study [Wysham et al. 2014]. Changes in A1C at 26 weeks were −1.51%, −1.30%, −0.99% and −0.46% for the dulaglutide 1.5 mg, dulaglutide 0.75 mg, exenatide and placebo groups, respectively (both doses of dulaglutide were superior to exenatide; p < 0.001). A greater percentage of patients achieving an A1C of <7% was observed with dulaglutide 1.5 and 0.75 mg groups compared with exenatide (78% and 66% versus 52%, p < 0.001 for both comparisons). Change in weight over 26 weeks was −1.30, +0.2, −1.07 and +1.24 for dulaglutide 1.5mg, dulaglutide 0.75mg, exenatide and placebo, respectively. The difference in weight loss between exenatide twice daily and dulaglutide 1.5mg was not significant (−0.24 kg, p = 0.474), though there was a statistical difference between exenatide twice daily and dulaglutide 0.75 mg (−1.27 kg, p < 0.001).
The AWARD-6 trial compared once weekly dulaglutide 1.5 mg versus daily liraglutide [Dungan et al. 2014]. Mean change in A1C was −1.42 and −1.36 in the dulaglutide and liraglutide groups, respectively (95% CI −0.19 to 0.07, noninferiority p-value < 0.0001) thus meeting predefined noninferiority criteria. Both groups resulted in 68% patients achieving an A1C of <7%. Weight reduction was significantly greater in the liraglutide groups compared with dulaglutide (−3.61 kg versus −2.90 kg; 95% CI 0.17–1.26, p = 0.011).
Safety/tolerability
The major adverse events seen with the head-to-head GLP-1 RA trials are summarized in Tables 3 and Table 4. Predictably, most of the adverse events experienced were GI in nature. Hypoglycemia rates were similar across GLP-1 RA treatment groups, as shown in Table 4, and were primarily seen in patients treated with concomitant sulfonylurea (SU) therapy. Across trials, however, there were some differences highlighted between comparators with regards to reported adverse events (AEs).
Table 3.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs): a comparison of common adverse effects in head-to-head trials.
| Study | Active comparators | Nausea | Vomiting | Diarrhea | Injection-site reactions | Withdrawal due to AEs (N) |
|---|---|---|---|---|---|---|
| DURATION-1 [Drucker et al. 2008] | Exenatide 10 µg BID | 50/145 (34.5%) | 27/145 (18.6%) | 19/145 (13.1%) | 17/145 (11.7%) | 7 |
| Exenatide 2 mg QW | 39/148 (26.4%) | 16/148 (10.8%) | 20/148 (13.5%) | 33/148 (22.3%) | 9 | |
| LEAD-6 [Buse et al. 2009] | Exenatide 10 µg BID | 65/232 (28.0%) | 23/232 (9.9%) | 28/232 (12.1%) | 21/232 (9.1%) | 31 |
| Liraglutide 1.8 mg QD | 60/235 (25.5%) | 14/235 (6.0%) | 29/235 (12.3%) | 21/235 (8.9%) | 23 | |
| DURATION-5 [Blevins et al. 2011] | Exenatide 10 µg BID | 43/123 (35.0%) | 11/123 (8.9%) | 5/123 (4.1%) | 16/123 (13%) | 6 |
| Exenatide 2 mg QW | 18/129 (14.0%) | 6/129 (4.7%) | 12/129 (9.3%) | 13/129 (10%) | 6 | |
| DURATION-6 [Buse et al. 2013] | Exenatide 2 mg QW | 43/461 (9.3%) | 17/461 (3.7%) | 28/461 (6.1%) | 73/461 (15.8%) | 12 |
| Liraglutide 1.8 mg QD | 93/450 (20.7%) | 48/450 (10.7%) | 59/450 (13.1%) | 9/450 (2.0%) | 25 | |
| GetGoal-X [Rosenstock et al. 2013] | Lixisenatide 20 µg QD | 78/318 (24.5%) | 32/318 (10.1%) | 33/318 (10.4%) | 27/318 (8.5%) | 33 |
| Exenatide 10 µg BID | 111/316 (35.1%) | 42/316 (13.3%) | 42/316 (13.3%) | 5/316 (1.6%) | 41 | |
| HARMONY-7 [Pratley et al. 2014] | Albiglutide 50 mg QW | 40/404 (9.9%) | 20/404 (5.0%) | 60/404 (14.9%) | 52/404 (12.9%) | 31 |
| Liraglutide 1.8 mg QD | 119/408 (29.2%) | 38/408 (9.3%) | 55/408 (13.5%) | 22/408 (5.4%) | 41 | |
| AWARD-1 [Wysham et al. 2014] | Dulaglutide 1.5 mg QW | 78/279 (28.0%) | 47/279 (16.8%) | 31/279 (11.1%) | 1/279 (0.4%) | 8 |
| Dulaglutide 0.75 mg QW | 45/280 (16.1%) | 17/280 (6.1%) | 22/280 (7.9%) | 0/280 (0%) | 4 | |
| Exenatide 10 µg BID | 71/276 (25.7%) | 30/276 (10.9%) | 16/276 (5.8%) | 1/276 (0.4%) | 9 | |
| Placebo | ||||||
| AWARD-6 [Dungan et al. 2014] | Dulaglutide 1.5 mg QW | 61/299 (20.4%) | 21/299 (7.0%) | 36/299 (12.0%) | 1/299 (0.3%) | 18 |
| Liraglutide 1.8 mg QD | 54/300 (18.0%) | 25/300 (8.3%) | 36/300 (12.0%) | 2/300 (0.7%) | 18 |
Abbreviations: BID, twice daily; QD, once daily; QW, once weekly; AE, adverse event.
Table 4.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs): a comparison of hypoglycemia rates in head-to-head clinical trials.
| Study | Active comparators | Major hypoglycemia | Minor hypoglycemia |
|---|---|---|---|
| DURATION-1 [Drucker et al. 2008] | Exenatide 10 µg BID | No SU = 0/93 (0%) | No SU = 1/93 (1.1%) |
| SU use = 0/54 (0%) | SU use = 8/54 (14.8%) | ||
| Exenatide 2 mg QW | No SU = 0/93 (0%) | No SU = 0/93 (0%) | |
| SU use = 0/55 (0%) | SU use = 8/55 (14.5%) | ||
| LEAD-6 [Buse et al. 2009] | Exenatide 10 µg BID | No SU = 0/63 (0%) | No SU = 7/63 (11.1%) |
| SU use = 2/169 (1.2%) | SU use = 71/169 (42.0%) | ||
| Liraglutide 1.8 mg QD | No SU = 0/64 (0%) | No SU = 4/64 (6.2%) | |
| SU use = 0/171 (0%) | SU use = 56/171 (32.7%) | ||
| DURATION-5 [Blevins et al. 2011] | Exenatide 10 µg BID | No SU = 0/89 (0%) | No SU = 0/89 (0%) |
| SU use = 0/34 (0%) | SU use = 4/34 (11.8%) | ||
| Exenatide 2 mg QW | No SU = 0/91 (0%) | No SU = 0/91 (0%) | |
| SU use = 0/40 (0%) | SU use = 5/40 (12.5%) | ||
| DURATION-6 [Buse et al. 2013] | Exenatide 2 mg QW | No SU = 0/167 (0%) | No SU = 6/167 (3.6%) |
| SU use = 0/294 (0%) | SU use = 45/294 (15.3%) | ||
| Liraglutide 1.8 mg QD | No SU = 0/154 (0%) | No SU = 4/154 (2.6%) | |
| SU use = 0/296 (0%) | SU use = 36/296 (12.2%) | ||
| GetGoal-X [Rosenstock et al. 2013] | Lixisenatide 20 µg QD | No SU = 0/318 (0%) | No SU = 8/318 (2.5%) |
| Exenatide 10 µg BID | No SU = 0/316 (0%) | No SU = 25/316 (7.9%) | |
| HARMONY-7 [Pratley et al. 2014] | Albiglutide 50 mg QW | No SU = 0/165 (0%) | No SU = 4/165 (2.4%) |
| SU use = 0/239 (0%) | SU use = 38/239 (15.9%) | ||
| Liraglutide 1.8 mg QD | No SU = 0/176 (0%) | No SU = 8/176 (4.5%) | |
| SU use = 0/232 (0%) | SU use = 45/232 (19.3%) | ||
| AWARD-1 [Wysham et al. 2014] | Dulaglutide 1.5 mg QW | No SU = 0/279 (0%) | No SU = 29/279 (10.4%) |
| Dulaglutide 0.75 mg QW | No SU = 0/280 (0%) | No SU = 30/280 (10.7%) | |
| Exenatide 10 µg BID | No SU = 2/276 (0.7%) | No SU = 44/276 (15.9%) | |
| Placebo | No SU = 0/141 (0%) | No SU = 5/141 (3.5%) | |
| AWARD-6 [Dungan et al. 2014] | Dulaglutide 1.5 mg QW | No SU = 0/299 (0%) | No SU = 26/299 (8.7%) |
| Liraglutide 1.8 mg QD | No SU = 0/300 (0%) | No SU = 17/300 (5.7%) |
Abbreviations: SU, sulfonylurea; BID, twice daily; QD, once daily; QW, once weekly.
In the DURATION-1 trial, exenatide twice daily showed a higher incidence of both nausea and vomiting compared to the exenatide once weekly formulation, with similar rates of diarrhea [Drucker et al. 2008]. Injection-site reactions were more common with the once weekly formulation, which is expected, as symptoms, particularly itching, have been shown to have a higher incidence with injectable sustained-release products that degrade over time in the body [Garbutt et al. 2005].
In the LEAD-6 trial, overall adverse events were lower with the liraglutide group, compared with exenatide twice daily (74.9% versus 78.9%, respectively) but the severity of these effects where higher with liraglutide (serious AEs 5.1%, severe AEs 7.2%) than exenatide twice daily (serious AEs 2.6%, severe AEs 4.7%) [Buse et al. 2009]. There was no clear trend in the type of serious or severe AE experienced in either group. In general, GI side effects were similar across both treatment groups. It was observed that while initial nausea rates were similar between groups, it was less persistent in liraglutide compared with exenatide twice daily (reported at week 26 in 3% of liraglutide patients versus 9% in the exenatide twice daily group).
DURATION-5 highlighted higher rates of nausea (and subsequent vomiting) with use of exenatide twice daily compared with the once-weekly formulation, with two patients in the twice daily arm reporting severe nausea, compared to none in the once weekly arm [Blevins et al. 2011]. Injection-site reactions were again higher with the exenatide once weekly group, although the differences were smaller (13% versus 10%).
DURATION-6 showed higher rates of nausea, vomiting, and diarrhea with the liraglutide-treated group, compared with exenatide once weekly [Buse et al. 2013]. Both groups noted an attenuation of these symptoms over time. Exenatide once weekly had higher reporting of injection site reactions, including nodule formation after injection.
Lixisenatide demonstrated slightly lower rates of reported GI side effects compared with exenatide twice daily in the GetGoal-X trial, with statistically lower rates of nausea (24.5% versus 35.1%, p < 0.05) [Rosenstock et al. 2013]. These symptoms appeared to improve over time in both groups, although the exenatide twice daily group had a slightly longer attenuation time (5 weeks) compared to lixisenatide (3 weeks). Interestingly, this was one trial where there was an observed difference in the incidence of hypoglycemia; lixisenatide had statistically fewer episodes of symptomatic hypoglycemia compared with exenatide twice daily (2.5% versus 7.9%, p < 0.05). Neither group included patients taking concomitant SU therapy.
The HARMONY-7 trial showed similar rates of GI side effects across both the liraglutide and albiglutide groups; the liraglutide arm had slightly higher rates of nausea and vomiting, which were most pronounced early in treatment [Pratley et al. 2014]. Injection site reactions were statistically higher in the albiglutide group (12.9% versus 5.4%, p = 0.0002), possibly due to its once weekly formulation.
For the AWARD-1 trial, GI AEs were similar between the 1.5 mg dulaglutide and exenatide groups, with nausea and vomiting being statistically higher than placebo at 26 weeks (p < 0.05) [Wysham et al. 2014]. Slightly lower rates were seen with the lower dose 0.75 mg dulaglutide arm. All groups reported the highest incidence of GI events early (within the first 2 weeks) in treatment.
The AWARD-6 showed no difference in reported GI AEs between dulaglutide and liraglutide [Dungan et al. 2014]. The frequency of nausea in both groups peaked at week one and gradually declined thereafter.
Upper respiratory tract infections (URIs) continue to be reported as adverse events in patients receiving GLP-1 RA therapy. This was reported in the DURATION-1 (8.1% of the exenatide twice daily group, 17.2% of the exenatide once weekly group), LEAD-6 (6.4% with liraglutide versus 6.0% with exenatide twice daily), DURATION-5 (4.1% with exenatide twice daily, 7.0% with exenatide once weekly), DURATION-6 (3% in each group), and HARMONY-7 (10.4% with albiglutide compared with 11.0% with liraglutide). In the AWARD-1 trial, rates were consistent across groups, including placebo (4% dulaglutide 1.5 mg, 5% dulaglutide 0.75 mg, 4% exenatide and 4% placebo), and URIs were not reported in the GetGoal-X or the AWARD-6 trials. The mechanism for the GLP-1 RAs increasing URIs has not been elucidated, but the consistency across trials suggests that this is still an important consideration with this class.
Patient satisfaction
When considering potential GLP-1 RA options for therapy, evaluating patient satisfaction with treatment becomes important. DURATION-1 utilized a Diabetes Treatment Satisfaction Questionnaire (DTSQ) to examine satisfaction with therapy [Drucker et al. 2008]. They found that patients using exenatide once weekly reported a significant increase in treatment satisfaction from baseline compared with exenatide twice daily, despite similar adherence rates (98%) between the two groups. The authors theorized this may be due to reduced frequency of injections. A publication on the patient-reported outcomes in the LEAD-6 trial, reported superior patient-reported outcomes with liraglutide treatment compared with exenatide twice daily [Schmidt et al. 2011]. They, too, utilized the DTSQ, and showed a greater improvement from baseline with liraglutide, with a between-group difference of 3.04 (95% CI 1.73–4.35, p < 0.0001). Items from this scale that showed significant differences between the two groups included convenience, flexibility, recommend the therapy, and continue therapy. A similar 30-week open-label trial comparing exenatide twice daily with exenatide once weekly also demonstrated significant DTSQ treatment satisfaction changes at 30 weeks with willingness to continue current treatment and perceived hypoglycemia frequency, both favoring the once weekly formulation [Best et al. 2009].
The HARMONY-7 trial utilized a Diabetes Medication Satisfaction questionnaire, and demonstrated that treatment satisfaction scores improved from baseline for both the liraglutide and albiglutide groups (4.38 for liraglutide and 3.29 for albiglutide), with no significant differences between groups (p = 0.207) [Pratley et al. 2014]. The AWARD-6 utilized a European quality of life five dimensions visual analog scale, which did not demonstrate any statistical differences between the dulaglutide and liraglutide groups [Dungan et al. 2014]. Taken as a whole, it appears that changes in overall patient satisfaction may be limited to transitioning away from twice daily GLP-1 RA treatment to a longer dosing-interval GLP-1 RA therapy.
Discussion
The GLP-1 RA class offers important advantages in the treatment of T2D. All agents within the class have demonstrated significant reductions in A1C and the class generally has a favorable effect on weight with minimal risk of hypoglycemia. The use of GLP-1 RAs may be limited by the adverse effects (mostly GI and injection-site related), need for subcutaneous administration, and cost. For those patients that would benefit from therapy with GLP1-RA, clinicians should consider the available literature regarding comparative effects on A1C and weight, rates of adverse effects, administration requirements and cost when selecting a specific agent within the class.
Most clinical experience, to date, is with exenatide twice daily, liraglutide once daily, and exenatide once weekly. Data regarding these three agents from published head-to-head studies suggest that liraglutide may have the largest A1C lowering capability of the three, followed by exenatide once weekly and then exenatide twice daily [Drucker et al. 2008; Buse et al. 2009, 2010, 2013; Blevins et al. 2011]. Most of the evidence showed similar effects on weight between these agents, however one study showed more weight loss with liraglutide compared with exenatide once weekly [Buse et al. 2013]. The data also suggests that GI AEs appear similar between liraglutide and exenatide twice daily, while the occurrence of GI AEs appears to be less in patients taking exenatide once weekly compared with exenatide twice daily and compared to liraglutide once weekly [Drucker et al. 2008; Buse et al. 2009, 2010, 2013; Blevins et al. 2011].
Incorporating information about the newer GLP-1 RAs, additional head-to-head studies suggest similar A1C reductions between lixisenatide and exenatide twice daily while showing more weight loss with exenatide twice daily and less GI AEs with lixisenatide [Rosenstock et al. 2013]. Albiglutide once weekly was less efficacious at both lowering A1C and lowering weight compared with liraglutide, but did have less GI AEs [Prately et al. 2014]. Dulaglutide once weekly was more efficacious at lowering A1C compared with exenatide twice daily and was noninferior to liraglutide at lowering A1C. Weight loss was similar between dulaglutide and exenatide twice daily but was greater with liraglutide compared to dulaglutide. In the dulaglutide head-to-head comparisons with exenatide twice daily and liraglutide, GI AEs were similar between groups [Wysham et al. 2014; Dungan et al. 2014].
Injection-site reactions may be more common with the longer acting agents, particularly exenatide once weekly which can cause transient small nodules at the injection site. However, patient satisfaction data indicate that once weekly injections result in higher patient satisfaction compared with twice daily injections. No difference in patient satisfaction was found between once daily and once weekly injections. Discontinuation rates due to adverse events vary between agents and studies, but are low overall with less than 10% of patients in the studies discontinuing GLP-1 RA therapy due to adverse events. In clinical practice, discontinuation rates are likely to be higher; possibly due to less time and resources dedicated to patient education, support, and follow-up.
The risk of hypoglycemia is low with GLP-1 RAs and rates were similar across all GLP-1 RA treatment groups in the head-to-head clinical studies; although the risk was increased with concomitant SU therapy.
Although the purpose of this review was to provide an analysis of current head-to-head comparative data of GLP-1 RAs, there are several other important factors that may influence the choice of GLP-1 RA. Clinicians should use a patient-centered approach when selecting a specific GLP-1 RA agent, incorporating evidence on efficacy as well as other practical considerations such as self-administration requirements and other psychosocial factors [Kalra, 2014]. In addition, each GLP-1 RA requires specific medication counseling and self-administration education specific to that product.
Conclusion
The phase III studies that have compared GLP-1 RA agents head-to-head have demonstrated that all GLP-1 RA agents are effective therapeutic options at reducing A1C. However, differences clearly exist in terms of magnitude of effect on A1C and weight as well as frequency and severity of adverse effects. When selecting the most appropriate agent, a comprehensive review of all head-to-head data indicates that liraglutide appears to still offer the best A1C and weight reduction, while the once weekly agents may cause less GI AEs compared with the once daily or twice daily options. Additional studies with different active comparators will further expand our understanding of the differences in efficacy and tolerability within this class of agents. In addition, more data from large-scale studies are required to evaluate and compare the long-term safety of the GLP-1 RA agents including cardiovascular safety and the risk of pancreatitis.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Contributor Information
Jennifer M. Trujillo, Associate Professor, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Mail Stop C238, 12850 East Montview Boulevard, V20-1222; Aurora, CO 80045, USA
Wesley Nuffer, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO, USA.
Samuel L. Ellis, University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO, USA
References
- Best J., Boye K., Rubin R., Cao D., Kim T., Peyrot M. (2009) Improved treatment satisfaction and weight-related quality of life with exenatide once weekly or twice daily. Diabetic Med 26: 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins T., Pullman J., Malloy J., Yan P., Taylor K., Schulteis C., et al. (2011) DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 96: 1301–1310. [DOI] [PubMed] [Google Scholar]
- Buse J., Drucker D., Taylor K., Kim T., Walsh B., Hu H., et al. (2010) DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 33: 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse J., Nauck M., Forst T., Sheu W., Shenouda S., Heilmann C., et al. (2013) Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 381: 117–124. [DOI] [PubMed] [Google Scholar]
- Buse J., Rosenstock J., Sesti G., Schmidt W., Montanya E., Brett J., et al. (2009) Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374: 39–47. [DOI] [PubMed] [Google Scholar]
- Drucker D., Buse J., Taylor K., Kendall D., Trautmann M., Zhuang D., et al. (2008) Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372: 1240–1250. [DOI] [PubMed] [Google Scholar]
- Dungan K., Povedano S., Forst T., Gonzalez J., Atisso C., Sealls W., et al. (2014) Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- Garber A., Abrahamson M., Barzilay J., Blonde L., Bloomgarden Z., Bush M., et al. (2013) AACE comprehensive diabetes management algorithm 2013. Endocrine Pract 19: 327–336. [DOI] [PubMed] [Google Scholar]
- Garbutt J., Kranzler H., O’Malley S., Gastfriend D., Pettinati H., Silverman B., et al. (2005) Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. J Am Med Assoc 293: 1617–1625. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation (2013) IDF Diabetes Atlas, 6th edn. Brussels, Belgium: International Diabetes Federation; Available at: http://www.idf.org/diabetesaltas (accessed 6 July 2014). [Google Scholar]
- Inzucchi S., Bergenstal R., Buse J., Diamant M., Ferrannini E., Nauck M., et al. (2012) Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 55: 1577–1596. [DOI] [PubMed] [Google Scholar]
- Kalra S. (2014) Choosing appropriate glucagon-like peptide 1 receptor agonists: a patient-centered approach. Diabetes Ther 5: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratley R., Nauck M., Barnett A., Feinglos M., Ovalle F., Harman-Boehm I., et al. (2014) Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol 2: 289–297. [DOI] [PubMed] [Google Scholar]
- Rosenstock J., Raccah D., Koranyi L., Maffei L., Boka G., Miossec P., et al. (2013) Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care 36: 2945–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W., Christiansen J., Hammer M., Zychma M., Buse J. (2011) Patient-reported outcomes are superior in patients with Type 2 diabetes treated with liraglutide as compared with exenatide, when added to metformin, sulphonylurea or both: results from a randomized, open-label study. Diabetic Med 28: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysham C., Blevins T., Arakaki R., Colon G., Garcia P., Atisso C., et al. (2014) Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 37: 2159–2167. [DOI] [PubMed] [Google Scholar]


