Abstract
Background
Previous studies have shown that the injection of dehydrated alcohol has been successful for the treatment of Morton's neuroma in the foot. In this study, we determined the cellular effect of injection of alcohol into and around the sciatic nerve of rats, and measured the extent of cell necrosis and/or any associated histologic or inflammatory changes.
Methods
Twenty-two male (~375g) Wistar rats were randomized into two groups each receiving alcohol injections into or around the sciatic nerve after nerve exposure under sterile technique. Group 1 rats were injected with a 0.5ml solution of 0.5% Marcaine in the left sciatic nerve as a control group. In the right sciatic nerve a 0.5ml solution of 4% ethanol with 0.5% Marcaine was injected. Group 2 rats received 0.5ml of 20%ethanol with 0.5% Marcaine injected into the left sciatic nerve and 0.5 ml of 30% ethanol with 0.5% Marcaine injected into the right sciatic nerve. In each group, the rats were placed in 3 subgroups: intraneural, perineural, perimuscular injections. All rats were sacrificed and tissue harvested for histologic evaluation at day 10 post injection.
Results
No evidence of alcohol-associated cell necrosis, apoptosis or apparent inflammation was observed in histologic specimens of any injected nerves, perineural tissue, or muscles in controls or experimental groups regardless of concentration of ethanol injected on day 10.
Conclusion
We concluded that alcohol injection (≤30% ethanol) into and/or around the sciatic nerve or the adjacent muscle of rats has no histologic evidence of necrosis or inflammation to the nerve or surrounding tissue. There was no observable histological change in apoptosis, or cell number, in response to the alcohol injection.
Keywords: Interdigital Neuroma, Morton’s Neuroma, Alcohol, Ethanol, Sclerosing Therapy, Foot
INTRODUCTION
Morton’s metatarsalgia or “Morton’s digital neuralgia” of the foot was first described in 1876 by Thomas G. Morton of Philadelphia.14,24 The condition is believed to be due to an entrapment neuropathy secondary to compression of the common interdigital nerve under the overlying transverse metatarsal ligament. Documented cases have shown irregular swelling of the plantar nerve.8,24 Histologically, the condition is not a true neuroma, but is characterized by fibrosis, endoneurial edema, axonal degeneration and local vascular proliferation.8,14 Common clinical signs include reproducible pain and paresthesias with plantar pressure directed between the metatarsal heads; Mulder’s sign (reproducible painful click upon simultaneously squeezing the forefoot while pushing upward with the thumb in the involved interspace); and symptomatic relief following injection approximately 2 cm proximal to the metatarsal head beneath the intermetatarsal ligament with lidocaine.8,16,23
In recent years, the injection of dehydrated alcohol has been reported to successfully treat Morton’s neuroma in the foot.11 The use of alcohol as a sclerosing agent has been reported for different medical conditions.1,3,10-12,15 However, the mechanism of action and efficacy of this particular therapy in Morton’s neuroma remains highly controversial.5,11,18,21 High concentrations of alcohol (up to 98%) have been reported to induce damage to the endothelium in cystic disease and cause resultant fibrosis.1,3,15 Similarly, in portal hypertension sclerosing therapy has been used to damage the vascular endothelium and smooth muscles by inducing fibrosis.17,19 Several clinical studies have also reported success with concentrations of alcohol as low as 4%.11,18
Currently, our approach has been to treat clinically assessed, symptomatic Morton’s neuroma non-surgically at first with modifications to shoe wear, plantar pad placement, stretching and NSAIDs. When these are unsuccessful a cortisone injection (lml of dexamethasone and 1ml of local anesthetic) is administered at the site. If the injection is unsuccessful surgical excision of the neuroma through a dorsal incision and nerve transection is performed.23 After reviewing the literature no studies where alcohol was injected into or around the peripheral nerves and that determined histologic changes in the tissue that could help explain a clinical response were identified. Since successful treatment relies on the accurate identification of the mechanically induced degenerative neuropathy and cessation of the mechanical or noxious stimuli over the nerve end, alcohol sclerosis treatment of the neuroma would seem counterintuitive. However, if alcohol injection induced histological changes such as apoptosis of the nerve and surrounding cells, altered cell proliferation or changes in local vessel patency, with some local moderating effects on the pain, then some alcohol efficacy on the causal neuroma would be conceivable.
Therefore, our objective was to evaluate the effect of alcohol injection in animals to compare histological effects with the clinical effects reported by others.4,6,11,12 We chose the rat sciatic nerve as a model since it is a peripheral nerve and its size is comparable to the human plantar digital nerve.20 The purpose of this study was to directly determine the cellular effects of alcohol injection on the intraneural or perineural tissues of the sciatic nerve of rats.
MATERIALS AND METHODS
All animals were used in this study according to the NIH Guide for the Care and Use of Laboratory Animals under an IACUC-approved protocol consistent with local institutional guidelines for the humane use of animals in research. All animals were housed in individual cages in temperature (22°C) and humidity (50%) controlled rooms having a 12 h light/12 h dark cycle with food and water ad libitum. All animals were handled by animal care personnel for 5-7 days prior to surgery.
Following Institutional animal use review board approval, a small pilot study was performed to ascertain efficacy and safety. Throughout the study, all rats were anesthetized with an injection of 80mg/kg ketamine and 12mg/kg xylazine. Adequacy of the anesthesia was assessed by toe pinch and monitoring of respiration. The rat’s limbs were aseptically prepared with betadine scrub and a 2-3 cm incision was made to expose the sciatic nerve of both hind limbs. The sciatic nerve distal to the sciatic notch was exposed aseptically using a dissecting microscope or magnifying loupes. In the pilot study, one rat was directly injected with 0.5 ml of 0.5% Marcaine in the left sciatic nerve and 0.5ml of 4% alcohol in a 0.5% Marcaine solution into the right sciatic nerve with no observable ill-effects and no evidence of histological change. A second rat was injection with 0.5 ml of 20% ethanol in a 0.5% Marcaine solution on the left sciatic nerve and 0.5ml of 30% ethanol on the right sciatic nerves with no ill effects or adverse histologic changes. Since injections caused no adverse effects to the rats, the study was expanded to an additional 20 rats divided into two groups.
The twenty additional male Winstar rats (weighing ~ 375g) were divided into two groups of ten and randomized to receive alcohol injections of different concentrations. In Group 1, the rats were injected with a 0.5ml of 0.5% Marcaine in the left sciatic nerve to serve as a control and 4% ethanol with 0.5% Marcaine in the right sciatic nerve. Group 2 rats were injected with 0.5ml of 20%ethanol with 0.5% Marcaine in the left sciatic nerve and 0.5ml of 30% ethanol with 0.5% Marcaine in the right sciatic nerve.
Animals were divided into 3 injection subgroups: intra-neural injection, perineural injection and peri-muscular injection. The injection of alcohol was performed using surgical loupes to directly visualize which tissues were being injected. The intraneural injection was placed in the mid-substance of the nerve ensuring there was slight expansion of the nerve fibers to confirm appropriate location. The perineural injections were placed in the perineural connective tissues while observing that the actual nerve fibers remained the same size under magnification and that the perineural tissues expanded. The intramuscular injection was done in the biceps femoris muscle tissue directly adjacent to the nerve. The three locations were chosen to mimic the possible injection combinations that could occur with either an ultrasound guided or by using anatomic landmarks to inject the intermetatarsal neuroma.
A total of 44 injections (including the 2 pilot study rats) were performed with 27-gauge needles and the area examined histologically. There were different concentrations of ethanol used in 0.5% Marcaine solution: 0% (control), 4%, 20%, and 30%. Each concentration was injected into 11 sites. Each injection was performed intra-neurally in 4 nerves, peri-neurally in 4 nerves, and intramuscularly in 3 nerves( Table 1). All animals survived surgery and were observed for changes in limb usage, self-mutilation and pain. All were euthanized 10 days after the procedure. The nerves and adjacent muscles were harvested for gross evaluation and histologic examination.
Table 1.
Table 1 shows the different injection groups and locations and the number of injections performed in each area
| Allocation of Subjects | ||||
|---|---|---|---|---|
| Marcaine Control |
4% EtOH in Marcaine |
20% EtOH in Marcaine |
30% EtOH in Marcaine |
|
| Intraneural | 4 | 4 | 4 | 4 |
| Perineural | 4 | 4 | 4 | 4 |
| Intramuscular | 3 | 3 | 3 | 3 |
Histology and Apoptosis
All harvested muscle and nerve were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. Five to seven micron sections (20 per specimen) were cut on a microtome (Leitz 1512, Wetzlar, Germany) for hematoxylin and eosin staining (H&E). Unstained sections were also used for apoptosis staining. To detect apoptotic cells in the sciatic nerve, the TUNEL assay was performed using a commercial kit (In Situ Cell Death Detection Kit; Roche, Mannheim, Germany). Samples from all animals were assessed for extent of apoptosis and underwent the same immunohistochemical staining. In brief, sections were fixed in 10% neutral buffered formaldehyde for 72 h and then pretreated with 100 mg/ml proteinase K for 30 min at 37 °C. Subsequently, sections of 5 μm thickness were mounted on a gelatinized glass slide, incubated in TUNEL reaction mixture for 1 h at 37 °C. For staining, DAB solution was used, and apoptotic cell nuclei stain brown. Mayer’s hematoxylin (DAKO, Glostrup, Denmark) was used for counterstaining before dehydration and mounting onto gelatinized glass slide. For the assessment of apoptotic cells, the stained sections were viewed under a bright field microscope and TUNEL-positive cells were detected based on their brownish color
RESULTS
There was no evidence of complications, including pain or functional deficiencies postoperatively. No numbness or irritation of sciatic nerve injection site was noted. Mild bilateral muscle weakness in the lower limbs was noted during the immediate postoperative period but resolved within 24 hours. No self-mutilating behaviors were observed, which would have been justification to stop intervention and exclude the subjects from the study. On pathological investigation, no alcohol-injected samples showed any evidence of enhanced inflammatory cell infiltration or other infiltrating cells.
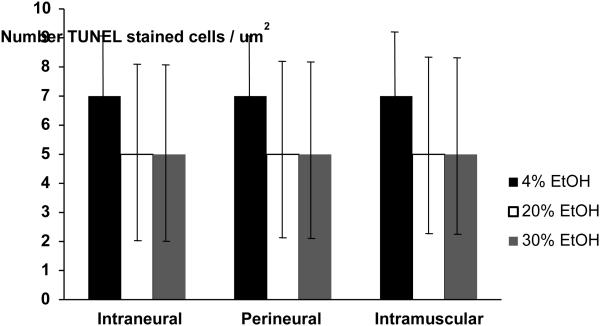
In addition, no observable differences in apoptosis of any cells were identified between alcohol and control groups regardless of the concentration or location of the injection (Figure 1). These data demonstrate that no alcohol injection at any site was able to elicit cellular changes in apoptosis in or around the nerve. The apoptotic rates per high–power-field were 7 ± 2 in the intraneural injections, regardless of concentration and 5 ± 3 in both the perinerual and perimuscular injections. No significant differences between either concentration of alcohol solution injected or site of injection were observed. No differences in TUNEL-positive cells were detected in the sciatic nerve, perineural tissue or surrounding muscle of alcohol injected nerves or controls regardless of concentration. As such we conclude that alcohol sclerosis treatment is not able to elicit robust changes in cell apoptosis in vivo.
Figure 1.
The number of TUNEL-stained apoptotic cells per high-power field (um2) in controls and various concentrations of ethanol (EtOH) for each injection location. (Mean ± SD). No significant differences were found between groups.
DISCUSSION
Better understanding of Morton’s neuroma, once a totally obscure and poorly understood entity, marked a great advance in differential diagnosis and logical treatment of painful disorders of the forefoot. However, few randomized or controlled trials documenting treatment efficacy have been performed. Historically treatment involved surgical excision of the neuroma and surrounding perineural fibrosis. Some clinicians treat Morton’s neuroma conservatively with shoe wear modification, steroid injections and if unsuccessful then resort to surgical intervention.2 Shoe wear modification might include open types of shoes, metatarsal pads, orthotic inserts and limitation of weight-bearing activities. Corticosteroid injection typically follows failure of shoe wear modification. After failure of corticosteroid injection, surgical excision is recommended and has good short term results.22 Most recently, a technique using the sclerosing effects of alcohol delivered by multiple ultra-sound guided injections over a period of time has been the topic of study. Improvement of symptoms with no long-term adverse events was reported in several cases, although some patients complained of localized pain at the injection site. An average of 84% of patients in these studies reported symptom resolution or improvement after receiving 3-5 4% alcohol injections over the course of several months.21 Ethanol injected around a nerve was documented to produce chemical neurolysis through dehydration, necrosis, and precipitation of protoplasm.19 The maximum effect was said to be in larger myelinated fibers. In one study, a series of 100 patients described an 82% complete response rate and 89% complete or partial response rate after series of 4% ethyl alcohol injections.4 Fanucci et al. reported a 90% partial or complete response at l0 month follow-up in a cohort of 40 patients after a series of four injections of 30% alcohol.6 Hyer et al. in 2005 also reported positive results in their small study in six out of eight observed neuromas.12 However, in a recent study, the 5 year outcomes of sclerosing alcohol treatment for Morton’s neuroma had only 29% of patients symptom free, 36% had subsequently undergone surgery and 29% still reported symptoms.9
In our study, solutions of 4%, 20% and 30% alcohol mixed with local anesthetic were used. These concentrations were chosen based on the earlier studies documenting short term clinical improvement and nervous signal inhibition at these concentrations.2,4,6,11,12 We used a 30% alcohol concentration in an attempt to elicit observable cell-based changes in the nerve or perineural tissues greater than those expected with lower concentrations of alcohol. In addition, three different injection site locations were used to simulate the different scenarios that occur with ultrasound or non-ultrasound guided injections of a neuroma. We observed no significant alteration in the pathology of the tissue harvested from the various groups, regardless of the alcohol concentration or location of injection compared with the control injection group.
Clearly, the rat sciatic nerve may not be the ideal model to study human intermetatarsal neuromas, although others have demonstrated similar effects in the rat model.13 We also recognize that the results of animal studies do not always translate to human subjects, however our findings do question the effectiveness of single alcohol injections for the treatment neural-based pathologies such as Morton’s neuroma. Since we examined only single injections of alchohol, it is interesting to speculate that despite the lack of effect at the highest dose (30%), repeated injection into or around the nerve may produce cellular changes. Although unlikely, we are currently attempting to determine if this is indeed the case.
In conclusion, despite the fact there have been several studies suggesting that sclerosing nerves with dehydrated alcohol is a viable alternative to surgical excision of Morton’s neuroma,2,4,6,11,12,21 the results of our study suggests that injection of alcohol into or around the sciatic nerve of a rat has no demonstrable effect on cellular histology, apoptosis or cell survival. No evidence of necrosis or inflammation was observed in any of the treatment groups. Our study has shown that injection of multiple concentrations of alcohol and local anesthetic into or surrounding the rat sciatic nerve did not produce any observable clinical or histologic effect. In our increasingly financially driven healthcare environment, each practitioner should carefully weigh the risks and benefits of using interventions with a low potential long-term benefit in order to avoid prolonging pain and suffering in patients and to reduce unnecessary healthcare expenditures.
Clinical Relevance.
The lack of any measureable changes in nerve or adjacent muscle histology with ethanol injection into the rat sciatic nerve (and surrounding tissues) raises questions about the efficacy of using ethanol injections in the treatment of Morton’s neuroma in human clinical practice.
ACKOWLEDGEMENT
These studies were supported by the Carl L. Nelson Chair in Orthopaedic Creativity and the UAMS Translational Research Institute (TRI) (CTSA grant Award #1 UL1TR000039).
Contributor Information
Mathew J. Mazoch, Department of Orthopaedic Surgery, University of Arkansas for Medical Sciences – Suite # 531, 4301 W. Markham, Little Rock, AR 72205
Gulraiz A. Cheema, Department of Orthopaedic Surgery, University of Arkansas for Medical Sciences – Suite # 531, 4301 W. Markham, Little Rock, AR 72205
Larry J. Suva, Center for Orthopaedic Research, University of Arkansas for Medical Sciences – Suite # 531, 4301 W. Markham, Little Rock, AR 72205.
Ruth L. Thomas, Department of Orthopaedic Surgery, University of Arkansas for Medical Sciences – Suite # 531, 4301 W. Markham, Little Rock, AR 72205
REFERENCES
- 1.Andersson R, et al. Alcohol sclerotherapy of non-parasitic cysts of the liver. British journal of surgery. 1989;76(3):254–255. doi: 10.1002/bjs.1800760313. [DOI] [PubMed] [Google Scholar]
- 2.Bennett GL, Graham CE, Mauldin DM. Morton's interdigital neuroma: A Comprehensive treatment protocol. Foot Ankle Int. 1995 doi: 10.1177/107110079501601204. [DOI] [PubMed] [Google Scholar]
- 3.Cobellis PL, et al. Alcohol sclerotherapy: a new method for Bartholin gland cyst treatment. Minerva ginecologica. 2006;58(3):245. [PubMed] [Google Scholar]
- 4.Dockery GL. The treatment of intermetatarsal neuromas with 4% alcohol sclerosing injections. J Foot and Ankle Surg. 1999;38:403–408. doi: 10.1016/s1067-2516(99)80040-4. [DOI] [PubMed] [Google Scholar]
- 5.Espinosa Norman, et al. Alcohol sclerosing therapy is not an effective treatment for interdigital neuroma. Foot & ankle international/American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society. 2011;32(6):576. doi: 10.3113/FAI.2011.0576. [DOI] [PubMed] [Google Scholar]
- 6.Fanucci E, et al. Treatment of intermetatarsal Morton's neuroma with alcohol injection under US guide: 10-month follow-up. Eur Radiol. 2004;14:514–518. doi: 10.1007/s00330-003-2057-7. [DOI] [PubMed] [Google Scholar]
- 7.Giannini S, Bacchini P, Ceccarelli F, Vannini F. Interdigital neuroma:Clinical examination and histopathologic results in 63 cases treated with excision. Foot Ankle Int. 2004;25:79–84. doi: 10.1177/107110070402500208. [DOI] [PubMed] [Google Scholar]
- 8.Gould John S. The Foot Book. Williams and Wilkins; Baltimore, US: 1988. pp. 269–273. MD. [Google Scholar]
- 9.Gurdezi S, White T, Ramesh P. Alcohol injection for Morton's neuroma: a five-year follow-up. Foot Ankle Int. 2013 Aug;34(8):1064–7. doi: 10.1177/1071100713489555. [DOI] [PubMed] [Google Scholar]
- 10.Hanna RM, Dahniya MH. Aspiration and sclerotherapy of symptomatic simple renal cysts: value of two injections of a sclerosing agent. American Journal of Roentgenology. 1996;167(3):781–783. doi: 10.2214/ajr.167.3.8751700. [DOI] [PubMed] [Google Scholar]
- 11.Hughes Richard Jet, et al. Treatment of Morton's neuroma with Alcohol Injection under Sonographic Guidance Follow-up of 101 cases. AJA. 2007;188:1535–1539. doi: 10.2214/AJR.06.1463. 16:760-763. [DOI] [PubMed] [Google Scholar]
- 12.Hyer Christopher F., et al. Treatment of recalcitrant intermetatarsal neuroma with 4% sclerosing alcohol injection: a pilot study. J Foot and Ankle Surg. 2005;44:287–291. doi: 10.1053/j.jfas.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Islam MS. Animal models of diabetic neuropathy: progress since 1960s. J Diabetes Res. 2013;2013:149452. doi: 10.1155/2013/149452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahass Melvin H., MD . Disorders of the Foot. W.B. Saunders Co; Philadelphia, US: 1982. pp. 702–704. [Google Scholar]
- 15.Junor Brian, JR, et al. Sclerosing peritonitis-the contribution of chlorhexidine in alcohol. Peritoneal Dialysis International. 1985;5(2):101–104. [Google Scholar]
- 16.Klenerman Leslie. The Foot and its Disorders. second Blackwell Scientific Publications; Oxford, UK: 1976. pp. 143–144. [Google Scholar]
- 17.Kochhar Rakesh, Goenka Mahesh K., Mehta Satish K. Outcome of injection sclerotherapy using absolute alcohol in patients with cirrhosis, non-cirrhotic portal fibrosis, and extrahepatic portal venous obstruction. Gastrointestinal endoscopy. 1991;37(4):460–464. doi: 10.1016/s0016-5107(91)70780-3. [DOI] [PubMed] [Google Scholar]
- 18.Mozena John D., Clifford Jared T. Efficacy of Chemical Neurolysis for the Treatment of lnterdigital Nerve Compression of the Foot. Journal of American Podiatric Medical Association. 2007 May-Jun;97(3) doi: 10.7547/0970203. DPM. DPM. [DOI] [PubMed] [Google Scholar]
- 19.Rengachary SS, Wantanbe IS, Singer P, Bopp WJ. Effects of glycerol on peripheral nerve: an experimental study. Neurosurgery. 1983;13:681–688. doi: 10.1227/00006123-198312000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Rigaud M, Gemez G, Barabas M, et al. Species and strain differences in rodent sciatic nerve anatomy: Implications for studies of neuropathic pain. Pain. 2008 May;136(1-2):188–201. doi: 10.1016/j.pain.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber Kent, et al. What is the best way to treat Morton's Neuroma. Journal of Family practice. 2011 Mar;60(30):157–158. [PubMed] [Google Scholar]
- 22.Thomson CE, Beggs I, Martin DJ, McMillan D, Edwards RT, Russell D, Yeo ST, Russell IT, Gibson JN. Methylprednisolone injections for the treatment of Morton neuroma: a patient-blinded randomized trial. J Bone Joint Surg Am. 2013 May 1;95(9):790–8. doi: 10.2106/JBJS.I.01780. [DOI] [PubMed] [Google Scholar]
- 23.Title C, Schon L. Morton Neuroma: Primary and Secondary Neurectomy. J Am Acad Orthop Surg September. 2008;16(9):550–557. [PubMed] [Google Scholar]
- 24.Wilson D, Helal B. The Foot: Volume I. Churchill Livingstone; London, UK: 1988. pp. 492–493. [Google Scholar]