Abstract
Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are cardiac hormones that regulate blood pressure and volume, and exert their biological actions via the natriuretic peptide receptor-A gene (Npr1). Mice lacking Npr1 (Npr–/–) have marked cardiac hypertrophy and fibrosis disproportionate to their increased blood pressure. This study examined the relationships between ANP and BNP gene expression, immunoreactivity and fibrosis in cardiac tissue, circulating ANP levels, and ANP and BNP mRNA during embryogenesis in Npr1–/– mice. Disruption of the Npr1 signaling pathway resulted in augmented ANP and BNP gene and ANP protein expression in the cardiac ventricles, most pronounced for ANP mRNA in females [414 ± 57 in Npr1–/– ng/mg and 124 ± 25 ng/mg in wild-type (WT) by Taqman assay, P < 0.001]. This increased expression was highly correlated to the degree of cardiac hypertrophy and was localized to the left ventricle (LV) inner free wall and to areas of ventricular fibrosis. In contrast, plasma ANP was significantly greater than WT in male but not female Npr1–/– mice. Increased ANP and BNP gene expression was observed in Npr1–/– embryos from 16 days of gestation. Our study suggests that cardiac ventricular expression of ANP and BNP is more closely associated with local hypertrophy and fibrosis than either systemic blood pressure or circulating ANP levels.
Keywords: atrial natriuretic peptide, brain natriuretic peptide
natriuretic peptides are a family of hormones that regulate blood pressure and body fluid homeostasis through their combined actions on vasculature, kidneys, and adrenal glands. Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are predominantly produced by cardiac atria and ventricles, respectively, in response to increased cardiac stretch. ANP and BNP exert their biological actions by binding to the natriuretic peptide receptor-A (NPR-A), resulting in the generation of the second messenger cGMP. These two natriuretic peptides have pronounced hypotensive, diuretic, and natriuretic effects (8).
Plasma levels of ANP and BNP are markedly elevated in heart failure (17) and after myocardial infarction (MI) (9) and are powerful predictors of ventricular dysfunction and mortality (14). Moreover, within heart tissue, gene expression of both ANP and BNP is reportedly upregulated in animal models of MI and heart failure (10, 16, 20, 23) and in human heart disease (12, 21). Whereas ANP is expressed primarily in the atria in adults, the ventricle is the major site of both ANP and BNP expression in embryos (3). The appearance of increased ANP expression in adult ventricles is a marker for the induction of the embryonic gene program during the development of hypertrophy (6). It has been reported that ANP inhibits cardiac hypertrophy in cultured cardiac myocytes (1, 11, 24) and that ANP effects apoptosis in cardiac myocytes in culture (28). In addition to inhibiting cardiac hypertrophy, the three natriuretic peptides ANP, BNP, and C-type natriuretic peptide (CNP) suppress cardiac fibroblast growth (5). This raises the possibility that these peptides may function in a paracrine manner to modulate the development of cardiac hypertrophy and fibrosis during remodeling of the cardiac ventricle.
Mice lacking natriuretic peptide receptor NPR-A (Npr1–/–) have marked cardiac hypertrophy and fibrosis disproportionate to their increased blood pressure (13, 19). The cardiac hypertrophy observed in these Npr1–/– mice is greater than that seen in other mouse models of hypertension, suggesting the NPR-A pathway directly modulates the hypertrophic response independent of blood pressure. Additional support for this hypothesis was provided by a recent study in which the blood pressure of Npr1–/– mice was maintained within the normal range by chronic treatment with antihypertensive agents without resulting in significantly diminished cardiac hypertrophy. Furthermore, Npr1–/– mice had a greater hypertrophic response than control mice to pressure overload induced by transverse aortic constriction (13). Therefore, it appears that the NPR-A pathway directly regulates cardiac hypertrophy. Furthermore, we hypothesize that local factors involved in the hypertrophic response may regulate expression of the natriuretic peptides within cardiac tissue.
To further characterize the effects of the deletion of the NPR-A gene on expression of the natriuretic peptide system and the hypertrophic response it elicits, ANP and BNP gene expression in adult hearts and embryonic tissues of Npr1–/– mice were examined using the technique of in situ hybridization and compared with those of wild-type (WT) control mice. The expression of ANP and BNP in the ventricles of adult Npr1–/– mice was quantified by real-time polymerase chain reaction (PCR) by using the Taqman assay system. The distribution of ANP immunoreactivity (IR) in adult Npr1–/– and WT hearts was compared with the sites of cardiac fibrosis. Associated levels of circulating ANP in Npr1–/– and WT mice was determined by radioimmunoassay.
MATERIALS AND METHODS
Generation of Npr1–/– mice
Mouse experiments were carried out under protocols approved by the Institutional Animal Care and Use Committees of the University of North Carolina. Most of the studies, unless otherwise stated, were performed on Npr1–/– and WT control mice backcrossed at least six generations to C57BL/6 mice derived from the original mutants, as previously reported (19).
Adult male and female Npr1–/– and WT mice (n = 4 per group) ranging from 4 to 12 mo of age were euthanized with an anesthetic overdose, and the hearts were rapidly dissected and then immersion fixed in 4% paraformaldehyde in 0.1 M borate buffer (pH 9.5). Embryos from mice of a mixed 129/C57BL6 genetic background were obtained from timed pregnant mice euthanized at 12 and 16 days post coitum. The embryos were dissected out of the uterine horns and separated from the placenta and were immersion fixed as above. Tissues were stored at 4°C. One day before being sectioned, tissues were transferred to a paraformaldehyde solution containing 10% sucrose, which was used as a cryoprotectant, and then embedded in OTC medium (Miles; Elkhart, IN).
Generation of ANP and BNP probe sequences
Riboprobes for in situ hybridization were generated by in vitro transcription from ANP and BNP DNA templates that had been extended by the PCR so that the 5′ ends of each strand encoded the T3 or T7 RNA polymerase promoter sequences, as described below. Oligonucleotide primers were designed from the published murine ANP (22) and BNP (18) DNA sequences, and encompassed exon 2 of each of these genes coding regions. A DNA fragment of 350 bp was generated by PCR of mouse genomic DNA using primers for ANP (ANP forward primer, 5′-GAACCTGCTAGACCACCT; reverse primer, 5′-CCTAGTCCACTCTGGGCT). A 240-bp mouse BNP product was PCR amplified using specific BNP primers (BNP forward primer, 5′-AAGCTGCTGGAGCTGATAAGA; reverse primer, 5′-GTTACAGCCCAAACGACTGAC). PCR amplicon sequences were confirmed by sequencing.
Riboprobe synthesis by in vitro transcription using T3 and T7 RNA polymerase was performed on PCR-generated templates, as described by Logel et al. (15). A second round of PCR amplification was performed on the ANP and BNP PCR templates generated above with primers with 5′ extensions encoding the T3 and T7 RNA polymerase promoter sequences on the sense and antisense strands, respectively, as illustrated by the following ANP primer set. The RNA polymerase promoter sequence is underlined and the ANP-specific sequence is in bold: ANP forward (T3) primer, 5′-CAGAGATGCAATTAACCCTCACTAAAGGGAGA-GAACCTGCTAGACCACCT and ANP reverse (T7) primer, 5′-CCAAGCTTCTAATACGACTCACTATAGGGA-CCTAGTCCACTCTGGGCT.
Generation of T3/T7 extensions to the murine ANP and BNP DNA fragments was performed by PCR using parameters identical to those described by Logel et al. (15). After amplification of each natriuretic peptide, a single PCR product that was ~70 bp larger than the original fragment was visualized on a 0.75% agarose gel. ANP and BNP riboprobes were generated by the procedure of in vitro transcription incorporating [35S]CTP, as previously described (3, 4).
In situ hybridization
The method of in situ hybridization was used to study ANP and BNP gene expression in cardiac and embryonic tissues from Npr1–/– and WT animals. The hybridization protocol was performed on 20-μm-thick cryostat sections by following the methods of Simmons et al. (25). Briefly, the slides were washed twice in 0.05 M KPO4-buffered saline to remove the embedding compound and postfixed in 10% neutral buffered formalin. Prehybridization treatment included 0.25% acetic anhydride in 0.1 M triethanolamine to block positive charges on the tissue, dehydration through increasing ethanol concentrations, and vacuum drying the tissue. Hybridization was performed at 55°C overnight with 1 × 107/ml probe in 100 μl of hybridization solution (25). A probe was applied to each slide, coverslipped, and sealed with DPX mountant (BDH; Poole, UK). After the coverslip was removed, the slides were rinsed four times in standard saline citrate (SSC) and incubated in RNAse A (20 μg/ml) at 37°C for 30 min. Sections were washed in decreasing concentrations of SSC, finishing with a high-stringency wash of 0.1× SSC at 68°C, dehydrated through ascending concentrations of ethanol, and vacuum dried. The slides were exposed to autoradiographic film (Hyperfilm-MP, Amersham; Little Chalfont, UK) for 1–2 days and then dipped in NTB-2 nuclear track emulsion (Eastman Kodak; Rochester, NY). Slides were exposed for 14 days and then developed and counterstained with hematoxylin and eosin. Adjacent sections were hybridized with ANP and BNP and their respective sense probes.
Measurement of ANP and BNP expression using Taqman assay
At death, hearts from adult male and female Npr1–/– and WT mice (n = 7 per group) were snap-frozen in liquid nitrogen and stored at –80°C in RNAlater solution (Ambion; Austin, TX) until RNA extraction. RNA samples were prepared from homogenized tissue with the use of an automated machine (model 7700, ABI; Foster City, CA). mRNA expression of ANP and BNP were characterized by real-time quantitative reverse transcription-PCR with a ABI 6700 machine. Primers for ANP were 5′-GAGAAGATGCCGGTAGAAGA-3′ and 5′-AAGCACTGCCGTCTCTCAGA-3′ (forward and reverse, respectively), and the probe for ANP detection was 5′-FAM-ATGCCCCCGCAGGCCCGG-Tamra-3′. Primers for BNP were 5′-CTGCTGGAGCTGATAAGAGA-3′ and 5′-TGCCCAAAGCAGCTTGAGAT-3′, and the probe for BNP detection was 5′-FAM-CTCAAGGCAGCACCCTCCGGG -Tamra-3′. All reactions included a β-actin internal standard. The primers used for β-actin amplification were 5′-CTGCCTGACGGCCAAGTC-3′ and 5′-CAAGAAGGAAGGCTGGAAAAGA-3′. The probe for β-actin detection was 5′-TET-CACTATTGGCAACGAGCGGTTCCG-Tamra-3′. The reactions were performed with 0.5 μg total RNA with minor differences from ABI 6700 manufacturer's instructions.
ANP plasma levels in Npr1–/– and WT mice
Whole blood samples from Npr1–/– and WT mice (n = 8 each for WT males and females, n = 5 Npr1–/– males, and n = 6 Npr1–/– females) were collected in EDTA tubes. Plasma was separated by centrifugation and stored at –80°C before analysis. Plasma (200 μl) was extracted through Sep-Pak C18 columns (Waters; Milford, MA) and used for ANP radioimmunoassay. ANP radioimmunoassay was performed by the method described by Yandle et al. (29). Cross reactivity in this ANP assay with mouse ANP-28 was 100%, and with mouse BNP-45 it was <0.02%.
ANP immunohistochemistry and histology
Immunohistochemistry for the detection of ANP-IR (ANP–) was performed on 7-μm-thick sections of paraffin-embedded hearts from Npr1–/– and WT mice with the use of a peroxidase-labeled streptavidin-biotin kit (DAKO; Carpinteria, CA). The antiserum against rat ANP (29) was used at a final dilution of 1:500. Adjacent heart sections were also stained with Masson trichrome for the presence of collagen, thus indicating cardiac fibrosis.
Statistical analyses
Two-way analysis of variance was used to analyze the genotype and gender effects on the heart weight-to-body weight ratio (HW/BW), left ventricular (LV) ANP, and BNP mRNA and circulating ANP plasma levels. Associations between age, HW/BW, LV ANP, and BNP mRNA were tested for significance using Pearson's correlation coefficients.
RESULTS
Npr1–/– mice are hypertensive and have cardiac hypertrophy versus Npr1 mice at baseline
A representative sample of Npr1–/– mice had significantly higher blood pressure levels than WT control mice (Npr1–/– = 126 ± 3 mmHg, n = 8 vs. WT = 108 ± 2 mmHg, n = 26). However, there was no significant difference in the blood pressures between male and female Npr1–/– mice, which is consistent with previous reports (13). Hearts of the Npr1–/– mice were also significantly larger than those of WT mice as presented as HW/BW in Table 1.
Table 1.
HW/BW ratios, levels of ANP and BNP mRNA in LV, and plasma concentrations of ANP in male and female backcrossed Npr1–/– mice compared with WT control mice
| HW/BW | LV ANP mRNA, ng/mg | LV BNP mRNA, ng/mg | Plasma ANP, pmol/l | |
|---|---|---|---|---|
| Male Npr1–/– | 6.7 ± 0.2* | 159 ± 16 | 54 ± 6 | 22.5 ± 3.9* |
| Male WT | 5.4 ± 0.2 | 111 ± 14 | 69 ± 11 | 11.6 ± 0.5 |
| Female Npr1–/– | 7.0 ± 0.2* | 414 ± 57* | 146 ± 38 | 13.2 ± 1.3 |
| Female WT | 5.1 ± 0.2 | 124 ± 25 | 90 ± 22 | 15.6 ± 1.6 |
Values are means ± SE. ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; Npr1–/–, mice lacking the gene that encodes natriuretic peptide receptor-A; WT, wild type; HW/BW, heart weight-to-body weight ratio; LV, left ventricular. HW/BW is expressed as a ratio ×10–3. LV ANP mRNA refers to the expression of ANP and BNP mRNA quantified by Taqman assay.
P < 0.001, statistically significant difference between Npr1–/– and WT control.
In situ hybridization reveals that Npr1–/– mice have increased ventricular expression of ANP and BNP
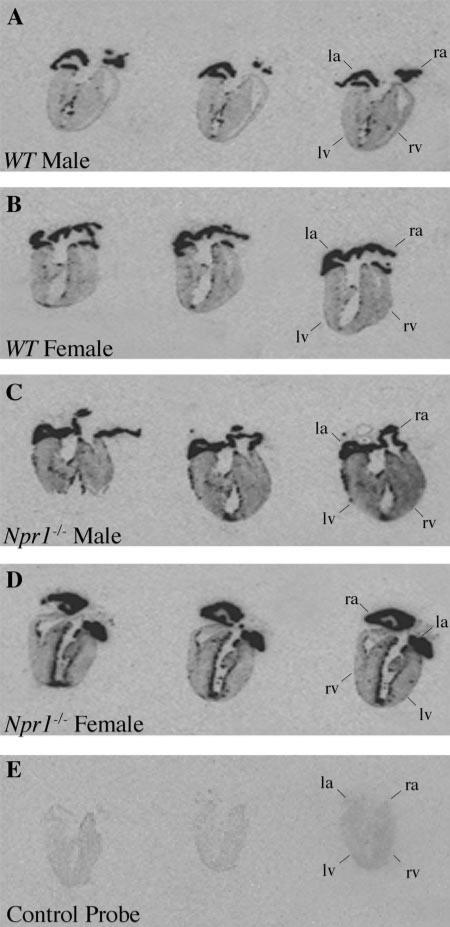
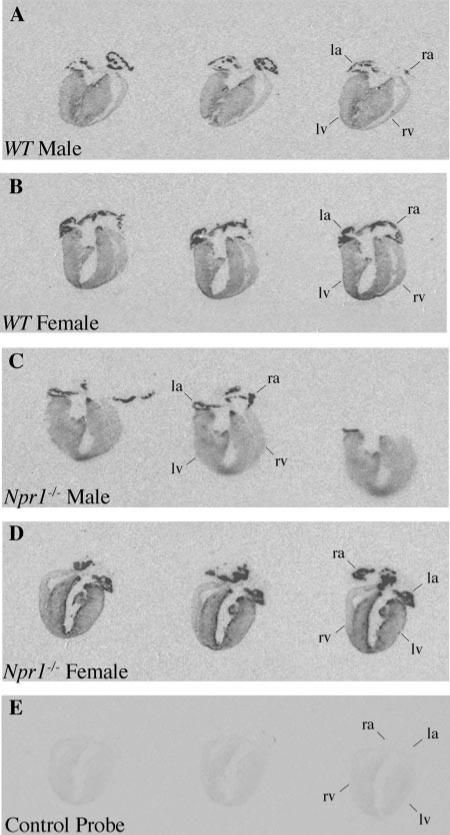
Expression of ANP in the whole hearts of Npr1–/– and WT mice are shown in Fig. 1. In the atria, ANP expression was very intense in both Npr1–/– and WT mice. However, in the ventricles, ANP expression was markedly increased in Npr1–/– mice compared with WT controls. This was particularly pronounced in female hearts, with intense expression along the endocardium lining the left ventricle (LV) and in patches within the walls of both the LV and right ventricles. Increased thickness of the LV free wall was observed in both male and female Npr1–/– mice compared with the LV of WT mice. Expression of BNP in the whole hearts of Npr1–/– and WT mice are shown in Fig. 2. Similar to ANP, BNP expression was greatly increased in the LV of Npr1–/– mice compared with WT controls. This was most marked in female Npr1–/– mice.
Fig. 1.
Representative autoradiographs of longitudinal sections through entire mouse hearts from wild-type (WT) male (A) and female (B) mice and natiuretic peptide receptor-A knockout (Npr1–/–) male (C) and female (D) mice hybridized with atrial natriuretic peptide (ANP) and ANP control probe (E). Positive ANP expression is observed as darkened regions in the left atria (la), left ventricle (lv), right atria (ra), and right ventricle (rv).
Fig. 2.
Representative autoradiographs of longitudinal sections through entire male and female WT (A and B) and male and female Npr1–/– (C and D) adult mouse hearts hybridized with brain natriuretic peptide (BNP) and BNP control probe (E). Positive BNP expression is observed as darkened regions (see Fig. 1 for abbreviations).
Expression quantitated by Taqman system
To quantify the ventricular ANP and BNP expression in Npr1–/– and WT mice, RNA was extracted from LV tissue from the hearts, and levels of ANP and BNP mRNA were assessed using Taqman real-time PCR (Table 1). These data confirmed the results of the in situ hybridization, with LV ANP mRNA being significantly greater in Npr1–/– mice than in WT mice. There was a highly significant effect of both genotype (P < 0.001) and gender (P < 0.001) on ANP mRNA. The increase in ANP mRNA was 3.3 times greater in female Npr1–/– mice compared with WT mice, whereas the increase in ANP mRNA was 1.4 times greater in male Npr1–/– mice compared with WT mice. The difference between the genders was also significant (P < 0.001). LV BNP mRNA was increased by 60% in female Npr1–/– mice compared with female WT mice. However, neither the effect of genotype nor gender was statistically significant for BNP expression.
There was a highly significant correlation between HW/BW and both LV ANP mRNA (r = 0.699, P < 0.001) and LV BNP mRNA (r = 0.374, P < 0.05). There was also a highly significant correlation between LV ANP and LV BNP mRNA (P < 0.001). There was no significant effect of age (from 4 to 16 mo) on HW/BW ratio, ANP or BNP mRNA, or ANP or BNP circulating levels in either males or females (13).
Circulating ANP levels are elevated in Npr1–/– mice
Circulating concentrations of ANP were also measured in the plasma of Npr1–/– mice and WT mice (Table 1). Plasma ANP was significantly higher in male Npr1–/– than WT mice (P = 0.005). Surprisingly, however, in female mice, there was no significant difference between Npr1–/– and WT control mice for plasma ANP. Male Npr1–/– mice had significantly greater levels of plasma ANP than female Npr1–/– mice (P = 0.03), whereas in WT mice, male and female plasma ANP concentrations were not significantly different.
ANP expression and IR are localized to areas of fibrosis
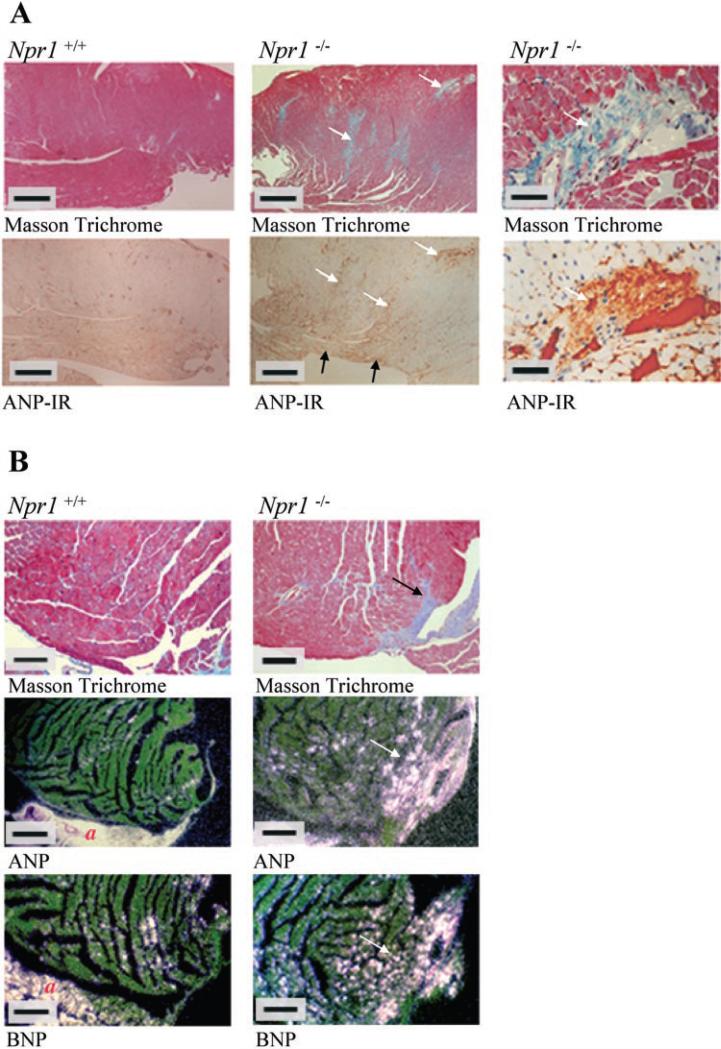
The presence of ANP-IR in female (Fig. 3) and male Npr1–/– and WT hearts was visualized using immunohistochemistry. In Npr1–/– hearts, intense ANP-IR was observed in the inner free LV wall (Fig. 3A), consistent with regions of ANP gene expression, as described previously (Fig. 1).
Fig. 3.
A: Masson trichrome staining (top) and ANP immunohistochemistry (bottom) of female Npr1–/– and WT hearts. Blue color with Masson trichrome staining indicates collagen deposition, which is characteristic of fibrosis. Brown staining indicates ANP immunoreactivity (IR) and can be seen colocalized with perivascular fibrosis. Contents of the blood vessels also stain brown due to IR of blood clots within the lumen. White arrows show regions of left ventricular fibrosis and the colocalization of ANP-IR to these regions. Black arrows show ANP-IR at nonfibrotic regions of the inner free wall of the left ventricle. Scale bars in left and middle represent 500 μM, and bars in right represent 50 μM. B: Masson trichrome staining (top) and dark-field photomicrographs of representative longitudinal sections through the left ventricles of female Npr1–/– and WT hearts hybridized with ANP and BNP. Blue color with Masson trichrome staining indicates collagen deposition, which is characteristic of fibrosis. Positive ANP and BNP gene expression is seen as bright silver grains above expressing cells. White arrows illustrate regions of ANP and BNP expression colocalized to areas of left ventricular fibrosis. Scale bars represent 200 μM. a, Atria.
In addition, a high-power examination of LV sections indicated diffuse patches of intense ANP-IR in Npr1–/– mice, and these were associated with regions of fibrosis, particularly in the LV free wall. This was confirmed by staining adjacent tissue blocks with Masson trichrome stain, which stains the collagen in fibrotic tissue blue. Regions of interstitial fibrosis were colocalized with ANP-IR (indicated by arrows in Fig. 3A). Examples of regions of intense ANP-IR colocalized with areas of perivascular fibrosis in the LV are shown in Fig. 3A, right. Colocalization of interstitial fibrosis with areas of ANP and BNP gene expression is shown in Fig. 3B. Fibrosis was more evident in the LV of female Npr1–/– mice than male Npr1–/– mice, paralleling the greater HW/BW and ANP and BNP mRNA levels quantified by the Taqman assay in female hearts, as described earlier.
ANP and BNP expression in embryos
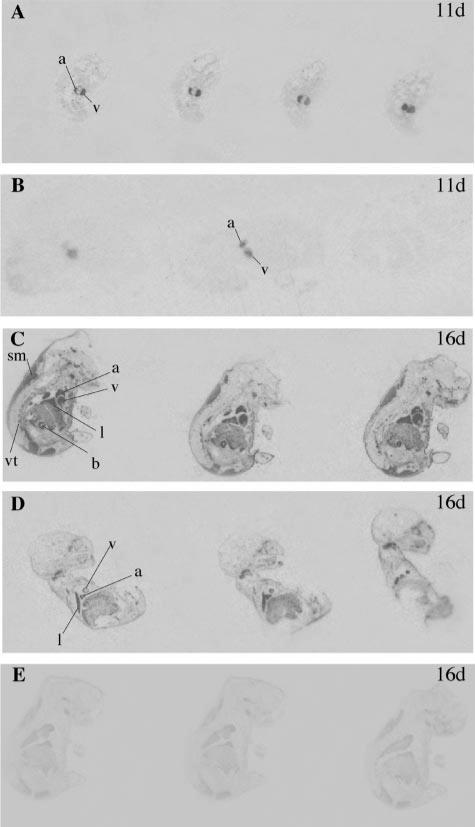
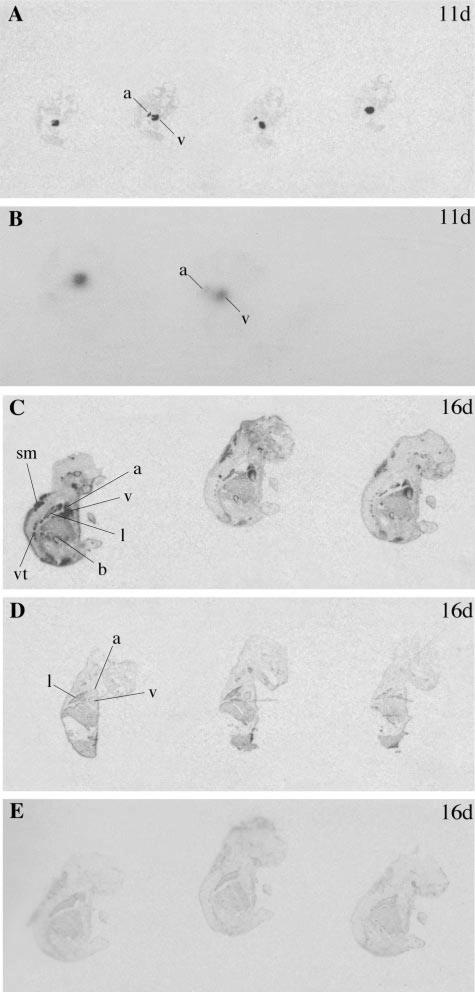
Expression of ANP and BNP was also examined in developing mouse embryos. At 11 days of gestation, strong expression of ANP (Fig. 4) and BNP (Fig. 5) could be seen in the developing heart, but was similar in Npr1–/– mice and control embryos. However, at 16 days gestation, Npr1–/– embryos showed increased cardiac expression of ANP and BNP compared with control embryos. Furthermore, from 16 days of gestation, ANP and BNP expression was also increased at extracardiac sites, including the lung, skeletal muscle, bladder, and vertebrae.
Fig. 4.
Representative autoradiographs of longitudinal sections through 11-day-old (11d) and 16-day-old (16d) Npr1–/– embryos (A and C) and WT embryos (B and D) hybridized with ANP and ANP control probe (E). Positive ANP expression is observed as darkened regions in the atrium (a), ventricle (v), lung (l), skeletal muscle (sm), bladder (b), and vertebrae (vt).
Fig. 5.
Representative autoradiographs of longitudinal sections through 11- and 16-day-old Npr1–/– embryos (A and C) and WT embryos (B and D) hybridized with BNP and BNP control probe (E). Positive BNP expression is observed as darkened regions (see Fig. 4 for abbreviations).
DISCUSSION
This study demonstrates that disruption of the receptor signaling pathway for the cardiac natriuretic peptides ANP and BNP results in augmented gene and protein expression of those peptides in the cardiac ventricles. This increased expression is highly correlated with the degree of cardiac hypertrophy. In addition, patches of ANP and BNP expression and IR were colocalized with regions of both interstitial and perivascular fibrosis in the ventricles. In contrast, the ANP and BNP expression seems to be less closely related to the level of blood pressure or the concentrations of these peptides in the circulation. These findings suggest that local factors associated with cardiac hypertrophy and fibrosis may be a major drive to the activation of ANP and BNP expression in adult cardiac ventricles.
Knowles et al. (13) showed that Npr1–/– mice have cardiac hypertrophy disproportionate to their increased blood pressure. Furthermore, when the blood pressure of these mice was maintained at control levels by chronic treatment with hypertensive drugs, the hypertrophy of Npr1–/– mice was not diminished. These results suggest that the NPR-A receptor system participates in regulating cardiac hypertrophy independent of blood pressure.
During hypertrophy, several biochemical and mechanical factors trigger a series of responses in myocardial cells in vitro, culminating in an increase in cell size and sarcomeric organization (26). These responses occur in a specific temporal sequence, with the triggering of the early gene cascade (i.e., c-jun, c-fos, c-myc, and egr-1) preceding activation of the embryonic repertoire, including ANP, α-skeletal actin, and β-myosin heavy chain. The ANP gene, as a representative of the embryonic repertoire, has been of particular interest in that reactivation of its expression in adult ventricular myocardium has become one of the most sensitive markers of hypertrophy (6). Furthermore, recent studies suggest that the natriuretic peptides may have a direct effect in regulating cardiac hypertrophy, because it has been reported that ANP inhibits cardiac hypertrophy in cultured cardiac myocytes (1, 11, 24) and that ANP induces apoptosis in cardiac myocytes in culture (28). This is supported by other mouse models of cardiac hypertrophy. For example, in transgenic mice with cardiac overexpression of a mutant α-myosin heavy chain gene (27), ANP mRNA in the LV increased approximately threefold and was found in regions of tissue pathology.
In addition to inhibiting cardiac hypertrophy, it has been proposed that all three natriuretic peptides, ANP, BNP, and CNP, suppress cardiac fibroblast growth (5). This raises the possibility that these peptides may function in a paracrine manner to modulate the development of cardiac fibrosis during cardiac hypertrophy. We (2) have shown that ANP is transiently expressed by fibroblasts during the formation of the fibrotic scar after myocardial infarction. In that study, treatment of cultured cardiac fibroblasts with transforming growth factor-β induced the expression of α-smooth muscle actin, characteristic of the transformation to myofibroblasts, and raised ANP concentrations in the medium. We have now demonstrated strong ANP and BNP mRNA and ANP protein expression in fibrotic tissue in two different animal models of cardiac fibrosis. It appears that, although ANP and BNP gene expression may be repressed in fibroblasts in normal physiology, transcription is activated in pathological states. In our previous study (2) of ovine myocardial infarction, ANP was colocalized to myofibroblasts. Thus we propose that ANP may be secreted on the phenotypic switch of fibroblasts to myofibroblasts, the cell type responsible for collagen deposition in the process of scar formation. We hypothesize that the release of ANP may inhibit the proliferation of fibroblasts and the deposition of collagen.
The regions of intense ANP and BNP expression observed along the endocardium of the LV free wall is likely to result from multiple stimuli. These include hypertrophy, hemodynamic overload, and regional mechanical stresses in response to elevated blood pressure in Npr1–/– mice compared with WT mice. However, a greater increase in ANP and BNP gene expression and ANP-IR was seen in the female Npr1–/– mice compared with male Npr1–/– mice, despite there being no significant difference in blood pressures of male versus female Npr1–/– mice. Gender-specific differences in ANP and BNP expression have been observed during the development of hypertension in humans and animals (7), and in that paper, it was suggested that estrogen may increase cardiac natriuretic peptide expression via activation of the renin-angiotensin system. Because tissue renin-angiotensin is implicated in both cardiac hypertrophy and fibrosis (7), this may provide a possible explanation for the marked increase in ANP and BNP gene expression and IR seen in female compared with male Npr1–/– mice.
Increased cardiac expression of ANP and BNP is initiated before birth in Npr1–/– mice, as demonstrated by in situ hybridization in embryos. Knowles et al. (13) reported that the hearts of Npr1–/– mice are enlarged at birth. Our examination of sections of 16-day-old embryos suggests that the hearts of Npr1–/– mice are larger than control mice aged as early as 16 days of gestation. These developing embryos are unlikely to have been exposed to high blood pressure in utero because the blood pressure in the fetus is governed by the maternal-fetal circulatory system via the placenta. This suggests that the increased ANP and BNP expression in the developing hearts of Npr1–/– mice may be activated by the hypertrophy. Thus a feedback loop may have started during development, with the deficiency of NPR-A pathways that would normally regulate the growth of cardiac myocytes leading to hypertrophy, and a consequent compensatory rise of ANP and BNP expression in the developing heart.
The ventricular expression of the natriuretic peptides was more closely related to heart weight than either blood pressure or circulating levels of ANP in this study, particularly with regard to the differences between male and female mice. Whereas blood pressure and its mechanical effect on the heart wall is one of the primary triggers for natriuretic peptide expression in normal physiology (8), local tissue factors may regulate the activation of the ventricular expression during the development of hypertrophy. The lack of correlation between ventricular levels of ANP and BNP mRNA and circulating peptide concentrations suggest that ANP and BNP secretion from the atria, which was not measured in this study, was making a greater contribution to plasma levels that the ventricular secretion.
In summary, this study provides evidence that hypertrophy itself may be activating ANP and BNP expression in the ventricles, independent of blood pressure and starting during development. Furthermore, areas of intense ANP and BNP expression in the ventricle were associated with regions of fibrosis, suggesting an intimate role between the fibrotic process and local natriuretic peptide production. Overall, this study suggests that within the ventricles, the cardiac peptides ANP and BNP participate in the complex interplay of local tissue factors involved in the process of myocyte hypertrophy and cardiac fibrosis, which appears to be independent of blood pressure and their secretion into the circulation.
Acknowledgments
The authors thank Jennifer Fox for technical assistance.
This work was supported by the Health Research Council of New Zealand and National Heart, Lung, and Blood Institute Grants HL-37001 (to O. Smithies) and HL-62845 (to N. Maeda).
REFERENCES
- 1.Calderone A, Thaik C, Takahashi N, Chang D, Colucci W. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest. 1998;101:812–818. doi: 10.1172/JCI119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron V, Rademaker M, Ellmers L, Espiner E, Nicholls M, Richards A. Atrial (ANP) and brain natriuretic peptide (BNP) expression after myocardial infarction in sheep: ANP is synthesized by fibroblasts infiltrating the infarct. Endocrinology. 2000;141:4690–4697. doi: 10.1210/endo.141.12.7847. [DOI] [PubMed] [Google Scholar]
- 3.Cameron VA, Aitken GD, Ellmers LJ, Kennedy MA, Espiner EA. The sites of gene expression of atrial, brain, and c-type natriuretic peptide in mouse fetal development: temporal changes in embryos and placenta. Endocrinology. 1996;137:817–824. doi: 10.1210/endo.137.3.8603590. [DOI] [PubMed] [Google Scholar]
- 4.Cameron VA, Nishimura E, Mathews LS, Lewis KA, Sawchenko PE, Vale WW. Hybridization histochemical localization of activin receptor subtypes in rat brain, pituitary, ovary and testis. Endocrinology. 1994;134:799–808. doi: 10.1210/endo.134.2.8299574. [DOI] [PubMed] [Google Scholar]
- 5.Cao L, Gardner D. Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension. 1995;25:227–234. doi: 10.1161/01.hyp.25.2.227. [DOI] [PubMed] [Google Scholar]
- 6.Day M, Schwartz D, Wiegand R, Stockman P, Brunnert S, Tolunay H, Currie M, Standaert D, Needleman P. Ventricular atriopeptin: unmasking of messenger RNA and peptide synthesis by hypertrophy or dexamethasone. Hypertension. 1987;9:485–491. doi: 10.1161/01.hyp.9.5.485. [DOI] [PubMed] [Google Scholar]
- 7.De Bold MLK. Estrogen, natriuretic peptides and the reninangiotensin system. Cardiovasc Res. 1999;41:524–531. doi: 10.1016/s0008-6363(98)00324-1. [DOI] [PubMed] [Google Scholar]
- 8.Espiner EA, Richards AM, Yandle TG, Nicholls MG. Natriuretic hormones. Endocrinol Metab Clin North Am. 1995;24:481–509. [PubMed] [Google Scholar]
- 9.Foy S, Crozier I, Richards A, Nicholls M, Turner J, Frampton C, Ikram H. Neurohumoral changes after acute myocardial infarction. Relationships with haemodynamic indices and effects of ACE inhibition. Eur Heart J. 1995;16:770–778. doi: 10.1093/oxfordjournals.eurheartj.a060995. [DOI] [PubMed] [Google Scholar]
- 10.Hama N, Itoh H, Shirakami G, Nakagawa O, Suga S, Ogawa Y, Masuda I, Nakanishi K, Yoshimasa T, Hashimoto Y, Yamaguchi M, Hori R, Yasue H, Nakao K. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation. 1995;92:1558–1564. doi: 10.1161/01.cir.92.6.1558. [DOI] [PubMed] [Google Scholar]
- 11.Horio T, Nishikimi T, Yoshihara F, Matsuo H, Takishita S, Kangawa K. Inhibitory regulation of hypertrophy by endogenous atrial natriuretic peptide in cultured cardiac myocytes. Hypertension. 2000;35:19–24. doi: 10.1161/01.hyp.35.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Hosoda K, Nakao K, Mudoyama M, Saito Y, Jougasaki G, Suga S, Ogawa Y, Yasue H, Imura H. Expression of brain natriuretic peptide gene in human heart: production in the ventricle. Hypertension. 1991;17:1152–1156. doi: 10.1161/01.hyp.17.6.1152. [DOI] [PubMed] [Google Scholar]
- 13.Knowles J, Esposito G, Mao L, Hagman J, Fox J, Smithies O, Rockman H, Maeda N. Pressure independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A deficient mice. J Clin Invest. 2001;107:975–984. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lainchbury J, Espiner E, Frampton C, Richards A, Yandle T, Nicholls M. Cardiac natriuretic peptides as predictors of mortality. J Intern Med. 1997;241:257–259. doi: 10.1046/j.1365-2796.1997.295134000.x. [DOI] [PubMed] [Google Scholar]
- 15.Logel J, Dill D, Leonard S. Synthesis of cRNA probes from PCR-generated DNA. Biotechniques. 1992;13:604–610. [PubMed] [Google Scholar]
- 16.Luchner A, Stevens T, Borgeson D, Redfield M, Wei C, Porter J, Burnett J. Differential atrial and ventricular expression of myocardial BNP during evolution of heart failure. Am J Physiol Heart Circ Physiol. 1998;274:H1684–H1689. doi: 10.1152/ajpheart.1998.274.5.H1684. [DOI] [PubMed] [Google Scholar]
- 17.Mukoyama M, Nakao K, Obata K, Jougasaki M, Yoshimura M, Morita E, Hosoda K, Suga S, Ogawa Y, Yaue H, Imura H. Augmented secretion of brain natriuretic peptide in acute myocardial infarction. Biochem Biophys Res Commun. 1991;180:431–436. doi: 10.1016/s0006-291x(05)81311-7. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimasa T, Uehira M, Matsuda S, Shiono S, Nishimoto H, Nakao K. Molecular cloning of the complementary DNA and gene that encode mouse brain natriuretic peptide and generation of transgenic mice that overexpress the brain natriuretic peptide gene. J Clin Invest. 1994;93:1911–1921. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliver P, Fox J, Kim R, Rockman H, Kim HS, Reddick R, Pandey K, Milgram S, Smithies O, Maeda N. Hypertension, cardiac hypertrophy and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrella M, Schwab T, O'Murchu B, Redfield M, Wei C, Edwards B, Burnett J. Cardiac atrial natriuretic factor during evolution of congestive heart failure. Am J Physiol Heart Circ Physiol. 1992;262:H1248–H1255. doi: 10.1152/ajpheart.1992.262.4.H1248. [DOI] [PubMed] [Google Scholar]
- 21.Saito Y, Nakao K, Arai H, Nishimura K, Okumura K, Obata K, Takemura G, Fujiwara H, Sugawara A, Yamada T, Itoh H, Mukoyama M, Hosoda K, Kawai C, Ban T, Yasue H, Imura H. Augmented expression of atrial natriuretic polypep-tide gene in ventricle of human failing heart. J Clin Invest. 1989;83:298–305. doi: 10.1172/JCI113872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidman C, Bloch K, Klein K, Smith J, Seidman J. Nucleotide sequence of the human and mouse atrial natriuretic peptide genes. Science. 1984;226:1206–1209. doi: 10.1126/science.6542248. [DOI] [PubMed] [Google Scholar]
- 23.Shimoike H, Iwai N, Kinoshita M. Differential regulation of natriuretic peptide genes in infarcted rat hearts. Clin Exp Pharmacol Physiol. 1997;24:23–30. doi: 10.1111/j.1440-1681.1997.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 24.Silberbach M, Gorenc T, Hershberger R, Stork P, Steyger P, Roberts C., Jr Extracellular signal-related protein kinase activation is required for the anti-hypertrophic effect of atrial natriuretic factor in neonatal rat ventricular myocytes. J Biol Chem. 1999;274:24858–24864. doi: 10.1074/jbc.274.35.24858. [DOI] [PubMed] [Google Scholar]
- 25.Simmons DM, Arriza JL, Swansen LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabelled single-stranded RNA probes. J Histotechnol. 1989;12:169–181. [Google Scholar]
- 26.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 27.Vikstrom K, Bohlmeyer T, Factor S, Leinwand L. Hypertrophy, pathology, and molecular markers of cardiac pathogenesis. Circ Res. 1998;82:773–778. doi: 10.1161/01.res.82.7.773. [DOI] [PubMed] [Google Scholar]
- 28.Wu C, Bishopric N, Pratt R. Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J Biol Chem. 1997;272:14860–14866. doi: 10.1074/jbc.272.23.14860. [DOI] [PubMed] [Google Scholar]
- 29.Yandle T, Fitzpatrick M, Espiner E, Richards A, Fisher S, Carne A. Ovine atrial natriuretic factor: sequence of circulating forms and metabolism in plasma. Peptides. 1991;12:279–283. doi: 10.1016/0196-9781(91)90012-e. [DOI] [PubMed] [Google Scholar]