Abstract
Background
The “New Western-style Diet” (NWD) characterized by high in fat and low in fiber, vitamin D, calcium and methyl donors - are considered as a risk factor for prostate cancer. Previous studies have shown that premalignant lesions of human prostate have decreased expression of the Retinoid X Receptor alpha (RXRα). This study was to determine the effect of diet in RXRα knockout mice in developing high grade prostate intraepithelial neoplasia (mPIN).
Methods
Male mice (N = 54) with or without the RXRα prostate null mutation were fed either NWD or AIN-76A control diet for 10 months; prostates were harvested at 11 months of age and examined for prostate mPIN.
Results
mPIN was seen in 79% of RXRα prostate null mice fed NWD (n=19), 30.8% RXRα prostate null mice fed AIN-76A (n=13), 42.9% RXRα wild type mice fed NWD (n=14), and 12.5% RXRα wild type mice fed AIN-76A (n=8). Unconditional Logistic analysis showed a significant joint effect of NWD and RXRα status in developing mPIN 26.3 (95% CI: 2.5-280), but interaction was not significant owing to the small sample size 1.6 (0.09-27.7, p=0.7441).
Conclusion
This study provides preliminary data to support a joint RXRα-diet effect in prostate carcinogenesis.
Keywords: prostate cancer, Retinoid X Receptor α, PIN, New Western Diet, Prostate-specific gene knockout
INTRODUCTION
With the success of the human genome project the opportunity exists to precisely study genetic, environmental, exogenous and endogenous risk factors contributing to the malignant phenotype. This is important for potentially pre screening individuals at risk (particularly family members) and designing chemoprevention strategies. However, this is a very complex process because environmental risk factors are variable and difficult to quantify during one’s life time. Populations with a large difference in disease incidence provide a unique human model to investigate the multiple parameters contributing to both gene and environment factors. Prostate cancer (PC) is the most commonly diagnosed cancer in Western and European countries [1] whereas the incidence of prostate cancer in Asian countries is substantially lower [2]. When Asians migrate to the Western world the incidence of prostate cancer approaches that of the indigenous population. Dietary patterns may increase risk for prostate cancer supported by epidemiological studies examining the risks of a “Western” style diet characterized by high intakes of high-fat foods, and low intakes of nutrient-rich food such as vegetables and whole grains [3]. While epidemiological evidence has suggested that such high fat/low nutrient diets may play a role in the development of prostatic malignancy [4], the direct experiment evidence for this is lacking.
Phenotypic protein expression studies in human confirm that RXRα is markedly decreased not only in prostate adenocarcinoma but also in precancerous high grade PIN [5]. A conditional RXRα knockout system that disrupts the RXRα gene in the prostatic epithelium [6] showed that prostate-specific RXRα-deficient mice developed multifocal hyperplasia at 4 months of age and had increased incidence of mouse high grade PIN (mPIN) among animals 10-15 months of age [7]. These studies demonstrated that loss of RXRα function results in preneoplastic lesions in the prostate which may promote PC development. This study aims to examine the combined effect of the New Western diet (NWD), a diet high in fat and low in fiber, vitamin D, calcium and methyl donors, and prostatic specific RXRα-deficiency in the development of mPIN.
MATERIALS AND METHODS
Mice and tissue preparation
Prostatic epithelium-specific Cre recombinase PB-Cre4 male and floxed RXRα female breeding mice were provided by Dr. P. Roy-Burman and mice with RXRα-deficient prostates were bred as previously described [5,7]. Offspring mice were genotyped by RT-PCR analysis to confirm the homozygous prostate-specific RXRα knockout as reported previously [7]. Mice were housed in the animal facility of the UCLA Center for Human Nutrition, which is accredited by the American Association for Accreditation of Laboratory Animal Care. From birth, all mice were fed a standard AIN-76A diet. At 5 weeks of age, mice were randomized into four groups: (i) wild type (WT) mice fed AIN-76A (n=8); (ii) WT mice fed NWD (n=14); (iii) RXRα null mice fed AIN-76A (n=13); (iv) RXRα null mice fed NWD (n=19). After feeding diets for an additional 42 weeks, mice were sacrificed at approximately 11 months of age. At necropsy the prostate was removed, dissected, and weighed. Prostates were fixed in 10% formalin, embedded in paraffin, cut to 4 μm tissue sections, and stained with H&E for histological examination. The study was approved by UCLA Chancellor’s Animal Research Committee (ARC).
Nutritional components of the diets
The nutritional compositions of the diets are shown in Table 1. AIN-76A is a mouse diet developed based on the standards of American Institute of Nutrition [8]. The NWD was developed based on nutrient densities by Newmark 2001 in which calcium, vitamin D, fat and phosphorus content are based on nutrient-density levels consumed by Western populations [9]. Compared to AIN-76A, the NWD has higher fat content, reduced calcium, vitamin D and fiber; it also has reduced levels of methyl donor nutrients (folic acid, methionine and choline) approximating the nutrient-density levels commonly consumed by Western populations [9]. The other dietary components (fiber, folic acid, methionine, cysteine and choline) have essentially equal nutrient-density level requirements in both humans and rodent diets [9]. The NWD modulated the levels of these components based on their potential to contribute to colon cancer in Western populations.
Table 1.
Diet composition
| Ingredients in % (wt) or amount (wt) | AIN-76A | NWD |
|---|---|---|
| Fat (corn oil), % | 5 | 20 |
| Calcium, mg/g | 5 | 0.5 |
| Vitamin D, IU/g | 1 | 0.11 |
| Phosphorus (PO4), mg/g | 4 | 3.6 |
| Fiber (cellulose), % | 5 | 2 |
| Folic acid, µg/g | 2 | 0.2 |
| DL- Methionine, % | 0.3 | - |
| L-Cysteine, % | - | 0.3 |
| Choline bitartrate, % | 0.2 | 0.12 |
| kcal/g (approximate) | 3.6 | 4.5 |
Pathological analysis of prostate
HE stained slides were reviewed by a board certified pathologist (Dr. Jianyu Rao) who specializes in genitourinary tract pathology and the diagnosis was further confirmed by another urological pathologist (Dr. Jiaoti Huang) who was not involved in the study. The PIN and cancer lesions were further verified by the two pathologists together by double scoping. Pathological review was performed in a blinded fashion without the knowledge of RXRα genotype and treatment status. The change of the epithelial tissue was described as normal, mouse high grade PIN (mPIN), or adenocarcinoma, using criteria as previously defined [10]. Immunohistochemical staining for p63 and Cytokeratin 5/6 (both antibodies from Abcam, Cambridge, MA, USA) was performed using standard immunohistochemical protocol of an automatic stainer (Dako North America, Inc. Carpinteria, CA). The positive control used was a clinically obtained cervical biopsy sample. The negative control used was omitting the primary antibody.
Statistical Analysis
Descriptive analyses were used to describe characteristics using frequency and percentage for both RXRα genotypes (normal=0, Null=1) and diet (AIN76A=0, NWD=1). Initially, Student t-test was used to compare means of two groups for continuous variables. Fisher’s Exact Test (two-tails) was used to determine whether mPIN progression from normal was associated with diet or RXRα genotypes.
In order to test hypotheses that NWD diet as well as RXRα null type are positively associated with the risk of the development of mPIN (mPIN=1, normal=0), crude and adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) for RXRα and diet were estimated using unconditional logistic regression models. Stratified analyses by RXRα or diet were performed and OR for each stratum-specific ORs are reported in Table 2.
Table 2.
Diet, RXRα genotypes and mouse high grade PIN (mPIN)
| Factors | mPIN (N, %) Normal (N,%) | Crude OR, 95%CI Adjusted OR*,95%CI | ||
|---|---|---|---|---|
| Diet | ||||
| AIN-76A | 5 (23.8) | 16 (76.2) | 1.0 | 1.0 |
| NWD | 21 (63.6) | 12 (36.4) | 5.6 (1.6-19.2) | 7.3 (1.9-28.2) |
| p-value | 0.0060 | 0.0038 | ||
| RXR α | ||||
| Normal | 7 (31.8) | 15 (68.2) | 1.0 | 1.0 |
| Null | 19 (59.4) | 13 (40.6) | 3.1 (1.0-9.8) | 4.4 (1.2-16.2) |
| p-value | 0.0500 | 0.0263 | ||
| Joint OR** | ||||
| RXRα Diet | OR | p-values | ||
| Normal AIN-76A | 1 (12.5) | 7 (87.5) | 1.0 | |
| Null AIN-76A | 4 (30.8) | 9 (69.2) | 3.1 (0.3-34.4) | 0.4964 |
| Normal NWD | 6 (42.9) | 8 (57.1) | 5.3 (0.5-54.9) | 0.7862 |
| Null NWD | 15 (79.0) | 4 (21.0) | 25.3 (2.5-280) | 0.0011 |
| Interaction*** | 1.6 (0.09-27.7) | 0.7441 | ||
| Stratified Analysis by RXR α | ||||
| Diet (RXRα Normal) | ||||
| AIN-76A | 1 (12.5) | 7 (87.5) | 1.0 | |
| NWD | 6 (42.9) | 8 (57.1) | 5.3 (0.5-54.9) | |
| p-value | 0.1930 | |||
| Diet (RXRα Null) | ||||
| AIN-76A | 4 (30.8) | 9 (69.2) | 1.0 | |
| NWD | 15 (79.0) | 4 (21.0) | 8.4 (1.7-42.4) | |
| p-value | 0.0110 | |||
| Stratified Analysis by Diet | ||||
| RXRα (AIN-76A diet) | ||||
| Normal | 1 (12.5) | 7 (87.5) | 1.0 | |
| Null | 4 (30.8) | 9 (69.2) | 3.1 (0.3-34) | |
| p-value | 0.6070 | |||
| RXRα (NWD diet) | ||||
| Normal | 6 (42.9) | 8 (57.1) | 1.0 | |
| Null | 15 (79.0) | 4 (21.0) | 5.0 (1.1-23.1) | |
| p-value | 0.0660 | |||
Adjusted ORs: ORs were estimated when both variables (diet and RXR were included in the same logistic regression model.
were included in the same logistic regression model.
Joint OR was estimated when RXR Null and NWD compared with RXR
Null and NWD compared with RXR Normal and AIN-76A diet
Normal and AIN-76A diet
Multiplicative interaction OR was estimated when both variables (diet and RXR□) and a product of both variables were all included in the same logistic regression model.
As shown in Table 2, the data were further analyzed by using a 2 x 4 table stratified by both main variables into four groups: RXRα normal and AIN-76A (baseline), RXRα null and AIN-76A (group 1), RXRα normal and NWD (group2), and RXRα null and NWD (group 3). Three dummy variables were created corresponding to three groups and included into the unconditional logistic regression model. The OR comparing group 3 with the baseline group is the OR for combined effect of both RXRα null genotype and new western diet (NWD).
In order to assess departure from multiplicative interaction, an interaction term was created using the two main variables with codes as described above. The likelihood ratio test with a p-value for heterogeneity was estimated by comparing the goodness of fit of a full logistic regression model including two main effect variables (RXRα and diet) with a full model with an interaction term (RXRα, diet, and a product term of both main variables). All statistical analyses were performed using SAS v.9.1 (SAS Institute, Cary, NC).
RESULTS
At the time of sacrifice, the average body weight of mice fed the NWD (43.3±5g) was significantly higher than the average body weight of mice fed the control AIN-76A diet (36.5±4.6g, P<0.05 by Student t-test). The body weight of WT mice fed AIN-76A (35.3±6.9 g) and RXRα prostate null mice fed AIN 76A (37.2±2.3 g) did not differ significantly and there was also no significant difference between WT and RXRα prostate null mice fed the NWD (43.0±3.9 g and 43.6±5.6 g, respectively, P>0.05 by Student t-test), indicating that the effect of the RXRα conditional mutation did not have an effect on overall gain in body weight.
Prostates from all groups were evaluated histologically as either normal (no areas of high grade PIN or cancer); mPIN (areas with high grade PIN); or cancer (areas with adenocarcinoma), using criteria for mPIN defined by Shappell et al [10]. The typical phenotypes of the mouse prostates are shown in Figure 1. Figure 1A to C shows the low magnification view of normal lateral glands, anterior prostate and ventral prostate, respectively. Figure 1D to F depict representative high magnification images of mPIN with either tufting or cribriform atypical epithelial proliferations with nuclear hyperchromasia and prominent nucleoli and occasional mitosis. Foci of invasive adenocarcinoma were seen in two of the 19 RXRα-knockout mice fed with NWD, but not other groups (Figure 1G to I for one mice and 1J to L for another mice). In both cases the tumor showed poorly formed acinar structures infiltrating the stromal tissue. There was diffuse background of mPIN in the surrounding glands. The tumor area characteristically showed loses of basal cell markers (negative p63 in tumor area but present in the adjacent mPIN area (Figure 1D)). CK5/6 stain showed the same results as that of the p63 staining with loss of basal cells in areas of suspicious for invasion and results are not presented.
Figure 1.
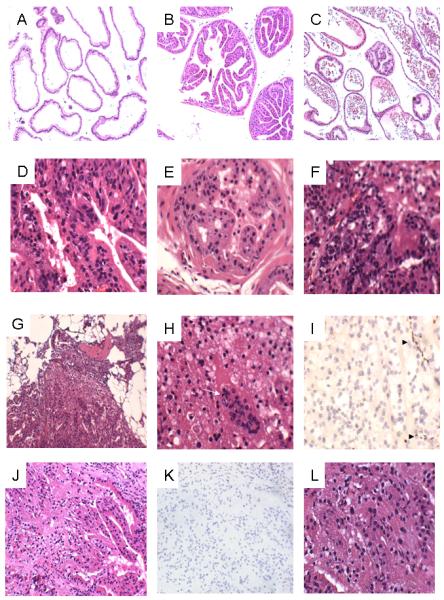
Representative H&E and IHC images. A. Normal lateral glands (100x); B. Normal anterior prostate (100x); C. Ventral prostate (100x); D. Diffuse mPIN in ventral prostate (100x); E. mPIN in anterior prostate (400x) F. Diffuse mPIN in lateral prostate, elsewhere areas of invasive cancer are seen (400x); G to I. The first invasive cancer at low (100x, G), high (400x, H-note mitotic figure indicated by white arrow) magnification and IHC for p63 (400x, I). Note the presence of basal cells in adjacent mPIN areas but absence in tumor areas (I, solid arrowhead); J to L, The second invasive cancer at low (100x, J), IHC for p63 (100x, K) and high (400x, L) magnifications.
The number of mice with normal, mPIN or PC is summarized in Figure 2 and Table 2. Prostates from wild-type mice fed the AIN-76A control diet were predominantly normal (87.5%), with a small group of mPIN (12.5%) and no PC was observed. Wild-type mice fed the NWD had 42.9% mPIN and no PC. In the RXRα null mice fed AIN-76A, mPIN was found in 30.8% and no PC was found. The RXRα deficient prostates from mice fed the NWD produced 15 mPIN (79.0%) and among these with mPIN, 2 PC were found (10.5%) in 19 mice.
Figure 2.
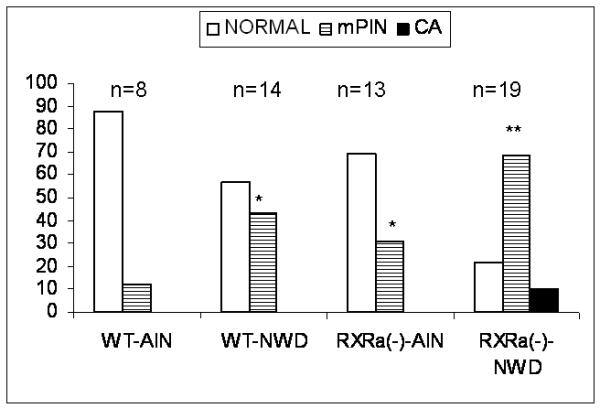
Percentage of prostate phenotypes according to genotype and diet. *P<0.05, **P<0.01comparing to WD-AIN by Chi-square test.
As shown in Table 2, the adjusted odds ratios (ORs) for mPIN were 7.3 (95%CI: 1.90-28.2) for NWD vs. AIN-76A, adjusting for RXRα genotype and 4.4 (95%CI: 1.2-16.2) for RXRα null vs. normal, when adjusting for diet. When stratified by RXRα status, the crude ORs for mPIN were 8.4 (95%CI: 1.7-42.4) for NWD vs. AIN-76A among RXRα null type and 5.3 (95%CI: 0.5-54.9) among RXRα normal group.
The joint OR for mPIN was 26.3 (95% CI: 2.5-280) when RXRα null with NWD group compared with the group of RXRα normal with control diet (AIN-76A). The large confidence interval of the joint odds ratio may be explained by small sample size. Thus, this result needs to be interpreted with caution. Departure from multiplicative interaction was assessed by using likelihood ratio test with p-value for heterogeneity of 0.74813 and the OR for interaction was 1.6 (0.09-27.7, p=0.7441), probably due to small sample size.
DISCUSSION
Our study provides preliminary experimental evidence for the combined effect of gene (RXRα deficiency)-environment (Western-style diet) in prostate cancer carcinogenesis. This study observed slightly higher background incidence of mPIN even in the WT-AIN-76Acontrol diet group (12.5%) than what has been reported in other studies [11]. This may be due to the advanced age (11-13 months) of the mice at the time of sampling in this study. However, even with advancing age, WT-AIN-76A diet group did not result in increased prostate cancer. Huang [7] found that incidence of low or high-grade PIN in RXRα null mice 10-15 months of age was 62 and 17% respectively, which was similar to our study. In addition, we observed about 12% more mPIN in WT-NWD group mice than in the RXRα null-AIN-76A group. Overall we found that RXRα knockout mice fed with a high fat, low nutrient diet developed significantly more precancerous lesions (mPIN) than control mice with normal RXRα and AIN-76A diet (79% versus 12.5%, respectively) (P=0.006 by two-tailed Fisher’s exact test). However, with the relatively small sample size studied one should be cautious with the findings, as reflected by rather large confidence intervals of the OR and an insignificant departure of multiplicative interaction.
The etiological factors leading to human PC are complex. The relationship between diet and PC risk remains unclear. Nutritional factors that may influence the disease include total energy intake, dietary fat, cooked meat, micronutrients and vitamins (carotenoids, retinoids, vitamins C, D and E), fruit and vegetable intake, minerals (calcium, selenium), and phytoestrogens (isoflavonoids, flavonoids, lignans) [12]. While some of these factors may act as protective factors and others may increase risk, overall the associations of these individual factors with prostate cancer development are rather weak and/or at times inconsistent from studies to studies. The complexity of identifying dietary risk factors for prostate cancer is highlighted by the findings of the Health Professionals Follow-Up Study (HPFS). This large prospective cohort study has been able to confirm a moderate lowered risk of consuming tomato products (presumably from lycopene) [13] and has also been able to isolate the contributor of increased risk from dietary fat as due to red meat, saturated and monounsaturated fat, and alpha-linolenic acid [14].
The New Western Diet contains increased dietary fat, with reduced calcium, vitamin D, folate, choline, methionine, B12, and fiber, to simulate human Western-style diets based on nutrient density in the diet and has been shown to induce adenomas and carcinoma in the colon of C57Bl/6 mice without carcinogenic exposure or targeted mutations [9]. A similar high-fat low-nutrient Western-style diet induces hyperproliferation of the mouse prostate after feeding for 16 weeks [11]. High-fat diets are associated with increased risk for aggressive prostate cancer [15] and several prospective cohort studies found that total energy intake is a risk factor for PC development and progression [16,17]. In our study, mice fed the NWD were exposed to a much higher fat diet and were significantly heavier than mice fed the control diet. High fat intake, or high energy, alone could have contributed to the increased and advanced mPIN found in our study. Dietary fat studies are difficult to control. Unless nutrients in the chow are balanced, animals fed ad libitum control their total caloric intake and will eat less of a high fat diet which may reduce intakes of other nutrients in the chow. The totality of the experimental data suggest that dietary fat per se does not affect the development or progressions of prostate cancer in rodent models but that fat in combination with as yet unknown concomitant dietary changes may enhance carcinogenesis [18]. The NWD has reduced levels of folate and other methyl donor constituents. Studies of dietary intake and blood levels of folate, methionine have generally found no associations with risk of prostate cancer although there is some evidence that high dietary intake of vitamin B12 are associated with increased risk. Recent studies suggest that supplemental dietary folate is associated with increased risk. High serum folate presumed to be from folic acid fortification in the diet is associated with increased cancer cell proliferation [19]. Folic acid supplementation was associated with increased risk of prostate cancer [20]. In contrast, dietary folate deficiency can block prostate cancer progression in the TRAMP model [21]. The effect of reduced folate in the NWD is unknown, but future dietary studies must account for the potential effects of folate intake.
While NWD may provide a useful simulating agent that can be used to study the effect of human Western-style diet with PC risk, the downside of that is one can not determine the relationship of individual nutrient (e.g., fat, folate, Vitamin B12, etc) within the mixture with prostate cancer. Individually these nutritional factors may all contribute to the risk of prostate cancer development, yet when combined one may argue that such association could be altered somehow. This is also a limitation of the study.
While Western-style diet may be a risk factor for PC, not all men consuming a Western-style diet develop PC. Genetic changes play an important role in PC carcinogenesis. Mice with the conditional disruption of the RXRα gene display increased proliferation and induction of PIN [7], whereas mice lacking both RXRβ and RXRγ are normal in terms of prostate morphology [22]. Decreased RXRα would result in deficient RAR/RXRα heterodimers and RXR/RXRα homodimers. Thus, in the model in which RXR functions as a transcriptionally active partner [23], this could result in functional cellular retinoid deficiency. Moreover, RXRα can mediate multiple signaling pathways in the prostate by dimerizing with other nuclear receptors, such as the peroxisomal proliferator-activated receptor-γ and vitamin D receptor, the ligands of which have been shown to inhibit prostatic cell growth [24,25]. Vitamin D is emerging as an important dietary factor that affects the incidence and progression of many malignancies including prostate cancer and the active form of vitamin D, 1,25-dihydroxycholecalciferol [1,25(OH)(2)D(3)], inhibits the growth and stimulates the differentiation of prostate cancer cells [26]. It may well be that RXRα and vitamin D act together in prostate cancer carcinogenesis, although the exact mechanisms remain to be elucidated. At the moment we do not have data to address the question of increasing vitamin D intake in the RXRα prostate null and the New Western Diet. A further study such as increasing vitamin D intake in the context of RXRα deficiency may be proposed to examine the interplay of vitamin D level, western diet, and RXRα in prostate cancer carcinogenesis.
Also to be determined are the exact mechanisms for the down regulation of RXRα in premalignant PIN and cancer. In human prostate, a highly heterogeneous pattern of RXRα protein expression, with some areas of low or no staining, was reported in 13 adenocarcinoma specimens by Zhong et al [27]. Mao et al [5] found RXRα protein expression was absent in basal and luminal secretory epithelial cells in 52% of human PIN specimens and that the basal cell expression of RXRα in PIN was significantly reduced compared to expression in basal cells of normal glands. That the loss of RXRα signal in PIN was a result of reduced RXRα expression in basal cells and not due to loss of basal cells was confirmed by positive Cytokeratin 903 (basal cell marker in human prostate tissue) stain in PIN. Loss of RXRα protein may indicate a premalignant alteration to basal cells along the pathway to neoplasia. Diet can influence the control of gene expression that might impact cancer development [28]. Dietary polyphenols have been shown to have cancer inhibition activities by reducing DNA hypermethylation properties [29, 30]. Green tea decreased CpG methylation in the promoter region of RXRα gene and increased RXRα protein levels in intestinal tumors in the azoxymethane-Apc Min/+ mouse [31]. While our study presented data to show the combined effect between RXRα and NWD in the development of mPIN in a mouse model, the direct link of such a combined effect for the initiation of human prostate cancer remains to be further determined. Additional studies are necessary to further explore the relationship between loss of RXRα expression and diet in prostate carcinogenesis. Prostate tissues from the study have been retained for additional studies which will include examination of markers for cellular proliferation and markers for prostate neoplasia, and larger sized animal experiments will be performed to validate the findings from this preliminary study.
In summary, both the NWD and RXRα prostate-specific deficiency showed increased risk of developing mPIN. However, there is a possible synergistic effect for developing mPIN when both genetic and dietary factors are present. Additional studies are needed to further explore the relationship between loss of RXRα expression and diet in prostate carcinogenesis.
Acknowledgements
We thank Dr. Jiaotin Huang for reviewing the histological sections. This study is supported in part by the UCLA Clinical Nutrition Research Unit Pilot/Feasibility Grant (P30 CA 42710), the NIH Cancer Epidemiology Training Grant (CA T32 CA09142), NCI Cancer Education and Career Development Program (5-R25-CA87949), the NCI Career Development (K07 CA98880), the NCE Division of Cancer Prevention Bio-Active Nutrient Gene Expression Omnibus (BANGEO) Grant (G.E.M), and NIH grant RO1 CA59705 (P. Roy-Burman). The UCLA Center for Nutritional Research, UCLA Division of Cancer Prevention and Control Research, UCLA School of Public Health and the Jonsson Comprehensive Cancer Center provided additional funding.
Footnotes
Disclosure Statement: All authors have no financial or personal relationships with other people or organizations that could inappropriately influence the work.
REFERENCES
- 1.Bracarda S, de Cobelli O, Greco C, et al. Cancer of the prostate. Crit Rev Oncol Hematol. 2005;56:379–96. doi: 10.1016/j.critrevonc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Park S, Bae J, Nam BH, et al. Etiology of cancer in Asia. Asian Pac J Cancer Prev. 2008;9:371–80. [PubMed] [Google Scholar]
- 3.Ma RW, Chapman K. A systematic review of the effect of diet in prostate cancer prevention and treatment. J Hum Nutr Diet. 2009;22:187–99. doi: 10.1111/j.1365-277X.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Patten CL, de Boer JG, Guns ES Tomlinson. Diet and dietary supplement intervention trials for the prevention of prostate cancer recurrence: a review of the randomized controlled trial evidence. J Urology. 2008;180:2314–21. doi: 10.1016/j.juro.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 5.Mao GE, Reuter VE, Cordon-Cardo C, et al. Decreased retinoid X receptor-α protein expression in basal cells occurs in the early stage of human prostate cancer development. Cancer Epidemiol Biomarkers Prevention. 2004;3:383–90. [PubMed] [Google Scholar]
- 6.Wu X, Wu J, Huang J, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–9. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Powell WC, Khodavirdi AC, et al. Prostatic intraepithelial neoplasia in mice with conditional disruption of the retinoid X receptor alpha allele in the prostate epithelium. Cancer Research. 2002;62:4812–19. [PubMed] [Google Scholar]
- 8.American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies: Report of the American Institute of Nutrition ad hoc committee on standards for nutritional studies. J Nutr. 1977;107:1424–34. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 9.Newmark HL, Yang K, Lipkin M, et al. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–75. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 10.Shappell SB, Thomas GV, Roberts RL, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Research. 2004;64:2270–305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 11.Xue L, Yang K, Newmark H, et al. Induced hyperproliferation in epithelial cells of mouse prostate by a Western-style diet. Carcinogenesis. 1997;18:995–9. doi: 10.1093/carcin/18.5.995. [DOI] [PubMed] [Google Scholar]
- 12.Schmid HP, Fischer C, Engeler DS, et al. Nutritional aspects of primary prostate cancer prevention. Recent Results Cancer Res. 2011;188:101–7. doi: 10.1007/978-3-642-10858-7_8. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Rimm EB, Liu Y, et al. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–8. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Rimm EB, Colditz GA, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–9. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 15.Ambrosini GL, Fritschi L, de Klerk NH, et al. Dietary patterns identified using factor analysis and prostate cancer risk: a case control study in Western Australia. Ann Epidemiol. 2008;18:364–70. doi: 10.1016/j.annepidem.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Kristal AR. Diet and trend in prostate-specific antigen: inferences for prostate cancer risk. J Clin Oncol. 2002;20:3570–71. doi: 10.1200/JCO.2002.20.17.3570. [DOI] [PubMed] [Google Scholar]
- 17.Andersson SO, Wolk A, Bergstrom R. Energy, nutrient intake and prostate cancer risk: a population-based case-control study in Sweden. Int J Cancer. 1996;68:716–22. doi: 10.1002/(SICI)1097-0215(19961211)68:6<716::AID-IJC4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Bosland MC, Oakley-Girvan I, Whittemore AS. Dietary fat, calories, and prostate cancer risk. J Natl Cancer Inst. 1999;91:489–91. doi: 10.1093/jnci/91.6.489. [DOI] [PubMed] [Google Scholar]
- 19.Tomaszewski JJ, Cummings JL, Parwani AV. Increased cancer cell proliferation in prostate cancer patients with high levels of serum folate. Prostate. 2011;71:1287–93. doi: 10.1002/pros.21346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomeized clinical trial. J Natl Cancer Inst. 2009;101:432–5. doi: 10.1093/jnci/djp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bistulfi G, Foster BA, Karasik E, et al. Dietary folate deficiency blocks prostate cancer progression in the TRAMP model. Cancer Prev Res. 2011;4:1825–34. doi: 10.1158/1940-6207.CAPR-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krezel W, Dupe V, Mark M, et al. RXRγ null mice are apparently normal and compound RXRα+/-/RXRβ-/-/RXRγ-/-mutant mice are viable. Proc Natl Acad Sci USA. 1996;93:9010–4. doi: 10.1073/pnas.93.17.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minucci S, Leid M, Toyama, et al. Retinoid X receptor (RXR) within the RXR-retinoic acid receptor heterodimer binds its ligand and enhances retinoid-dependent gene expression. Mol Cell Biol. 1997;17:644–55. doi: 10.1128/mcb.17.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangelsdorf DJ, Umesono K, Evans RM. The retinoid receptors. In: Sporn MB, Roberts AB, Goodman DS, editors. The retinoids: biology, chemistry and medicine. Raven Press; New York: 1994. pp. 319–50. [Google Scholar]
- 25.Kubota T, Koshizuka K, Williamson EA, et al. Ligand for peroxisome proliferator-activiated receptor γ (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Research. 1998;58:3344–52. [PubMed] [Google Scholar]
- 26.Peehl DM, Skowronski RJ, Leung GK, et al. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994;54:805–10. [PubMed] [Google Scholar]
- 27.Zhong C, Yang S, Huang J, et al. Aberration in the expression of the retinoid receptor, RXR alpha, in prostate cancer. Cancer Biol Ther. 2003;2:179–84. doi: 10.4161/cbt.2.2.281. [DOI] [PubMed] [Google Scholar]
- 28.Ross SA. Evidence for the relationship between diet and cancer. Exp Oncol. 2010;32:137–42. [PubMed] [Google Scholar]
- 29.Li LC. Epigenetics of prostate cancer. Front Biosci. 2007;12:3377–97. doi: 10.2741/2320. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Xu X. Diet, epigenetic, and cancer prevention. Adv Genet. 2010;71:237–55. doi: 10.1016/B978-0-12-380864-6.00008-0. [DOI] [PubMed] [Google Scholar]
- 31.Volate SR, Muga SJ, Issa AY, et al. Epidenetic modulation of the retinoid X receptor alpha by green tea in the azoxymethane-Apc Min/+ mouse model of intestinal cancer. Mol Carcinog. 2009;48:920–33. doi: 10.1002/mc.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]


