Abstract
Background
The onset of sarcoidosis is thought to be seasonal, particularly Lofgren’s syndrome. However, there are conflicting data on seasonality by country and by radiographic stage.
Objective
The objective of this study was to determine if there is seasonality of the diagnosis of sarcoidosis in outpatients in the United States.
Methods and Results
Using time series methods, we performed a retrospective analysis of 3750 incident cases of sarcoidosis in the Veteran’s Health Administration national outpatient claims database (2000–2007). We did not find overall seasonality in the occurrence of new sarcoidosis in United States Veterans (p=0.9860), even after we subdivided the United States by northern (p=0.6824) and southern regions (p=0.4588).
Conclusion
The lack of seasonality in this study indicates that season is not a dominant factor in complex gene-environment-host interaction that precedes presentation of new sarcoidosis cases in the United States Veteran population.
Introduction
Sarcoidosis is a systemic inflammatory disease characterized by the formation of noncaseating granulomas, with over 90% of patients having lung involvement.(1) Although the disease was first described over 100 years ago, the etiology and natural history are still unclear. The immune response is clearly a T-helper 1 mediated response, and it is thought that new cases are caused by either a persistent unknown antigen perpetuating the granulomatous inflammation or a defect in regulatory T cells that prevents normal resolution of granulomas.(2)
Investigating seasonal and regional patterns of sarcoidosis is important to further understand potential environmental factors that may be etiologic triggers. Previous studies in Europe, Turkey, New Zealand, and Japan have suggested seasonal peaks of sarcoidosis presentations in the early spring, especially for new cases of Lofgren’s syndrome.(3–7) However, there are few published reports specifically analyzing seasonality nationally in the United States (U.S.). Because sarcoidosis is uncommon, it is difficult to investigate seasonality based on single center data. The purpose of this study was to determine if seasonal trends of sarcoidosis exist in the U.S., using appropriate methods for dealing with autocorrelated data. In addition, because seasonality has previously varied by latitude and hemisphere, we tested for seasonal trends in both the northern and southern latitudes of the U.S.(5–6)
Methods
All data from October 2000 to June 2007 were extracted from the computerized Veterans Health Administration (VHA) national outpatient care claims database. The VHA maintains one of the largest integrated health care systems in the United States. All patient identifiers were encrypted. The study was in compliance with the Helsinki declaration and was approved by the Institutional Review Board ethical committee at the University of Iowa, IRB# 200504802 (Iowa City, Iowa, United States). Informed consent was deemed not necessary as all information was de-identified. Sarcoidosis cases were identified by the International Classification of Diseases, Ninth Revision, Clinical Modification code, 135. Cases were included if they were 18 years or older. To select for patients who utilize the system on a regular basis, cases had at least one primary care visit in the year prior to diagnosis. To select for incident cases, potential cases were excluded if they had a diagnosis of sarcoidosis in the one year prior to the index date. The geographical location of patients with sarcoidosis was obtained by identifying the longitude and latitude of the patient’s zip code. The ZIPCodeWorld database was used for mapping the zip code to its corresponding longitude and latitude.(8) The patients were divided into two groups: north with latitude greater than or equal to 35 and south with latitude less than 35 (approximately below the state of Tennessee and Los Angeles, California).
To investigate potential seasonality, we first inspected the sample autocorrelation function (ACF) and box plots against each month for the nationwide sarcoidosis series, and subsequently, for the two sub-series stratified by latitude (north and south). A test for seasonality was conducted by determining whether or not the autocorrelation at lag 12 was statistically different from zero. We further explored the seasonal pattern of nationwide sarcoidosis incidence using a time series regression model. A dichotomous variable, indicating the suspected peak months of February through May was included in the model as an exogenous covariate. An autoregressive moving average (ARMA) structure was employed to account for the serial correlation in the residual process. Model specification and diagnostics were performed based on the sample ACF, partial ACF (PACF), and extended ACF (EACF). The Ljung-Box test was conducted to check whether or not the residuals from the final model appear to be white noise. In a similar fashion, we built time series regression models for the two sub-series of sarcoidosis incidences in the south and north.
All statistical analyses were performed using R version 2.11.1 (R Foundation for Statistical Computing). The funding source did not have involvement in study design, data analysis, writing, or submission of the manuscript.
Results
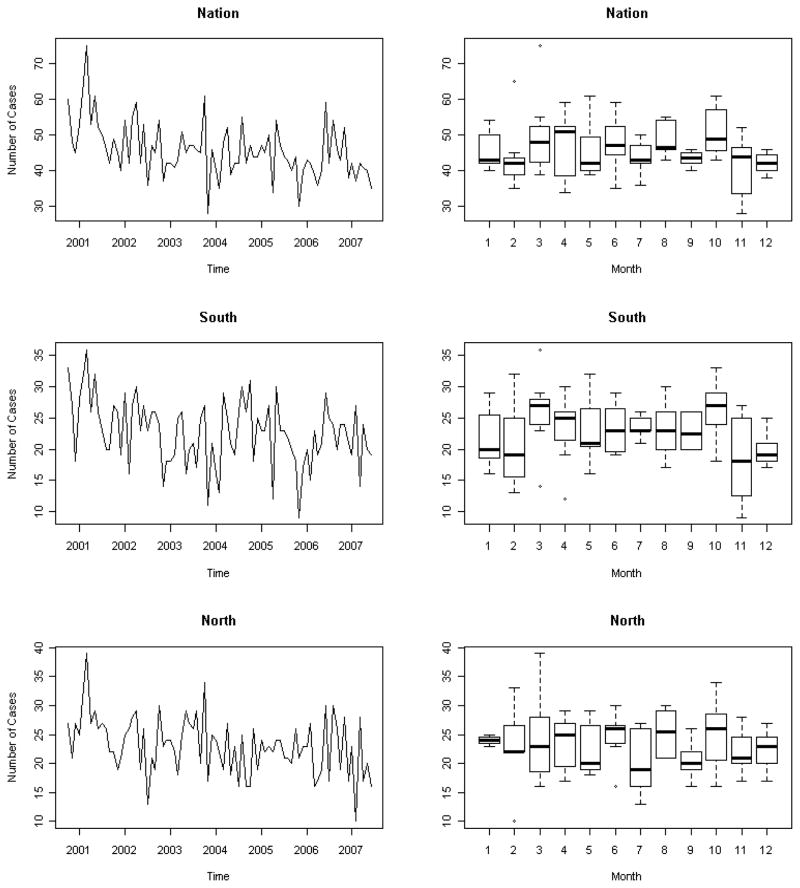
Of the 3791 subjects included in the study, 86% were male. Racial demographics indicate that 39% of patients were black, 25% white, 2% Hispanic, 0.3% Asian, and 33% of the subjects had unknown race. Both median and mean age was 50 years old (range 21 to 86 years). Over the study period, the time series plots and box plots against each month did not show any apparent seasonality for sarcoidosis incidence (Figure 1). None of the autocorrelations at lag 12 was significant (nation: p=0.9860; south: p=0.9864; north: p=0.9947).
Figure 1.
Time series and box plots for sarcoidosis incidences in the nation (p=0.9860), south (p=0.9864), and north (p=0.9947) from October 2000 to June 2007, indicating no evidence of seasonality in sarcoidosis diagnoses in the United States Veteran population.
Our final time series regression model for the nationwide sarcoidosis series contained a first-order autoregressive component (p < 0.0001), a first-order moving average component (p < 0.0001), and the dichotomous variable (p = 0.3979) that indicated the suspected peak months. The final model showed no lack of fit based on the Ljung-Box test (p = 0.3031) and an inspection of the ACF and PACF of the residuals. We did not find any significant seasonality for the two sub-series (north: p = 0.6824; south: 0.4588) using the same model structure.
Discussion
In this study, we did not find a significant seasonal pattern in sarcoidosis diagnoses in the United States Veteran population. A subanalysis also investigated trends in both southern and northern latitudes to assess how distance from the equator may affect seasonality. However, we did not detect any statistically significant trends, even after using several different descriptive and inferential approaches.
Previous studies of the seasonality of sarcoidosis have varied by country, presentation, age and gender. In Turkey, Greece, Finland, and Croatia, nonspecific peaks in overall diagnoses have been noted in spring months.(4,6,9) Conversely, our data compliments the New Zealand study from Wilsher, et. al., which found no evidence of seasonality except for patients with erythema nodosum and/or arthralgia.(6) In our study, the U.S. Veteran population may reflect a different demographic or manifestation of sarcoidosis that is not seasonal. Previous study has shown that seasonality is more prominent in women and in younger populations.(10) Thus, seasonality may not be apparent in the U.S. Veterans, as the demographic is primarily male with an older average age of presentation. Furthermore, over time, the Veteran population has noted a decrease in hospitalizations due to sarcoidosis, potentially attributable to improved respiratory precautions and increased vigilance toward prevention of inhalational exposures. Thus, the military population may reflect a different subgroup of disease and exposures.(11) Finally, our cohort may include a greater proportion of patients with previously undiagnosed disease compared to other studies, given the older average age in our predominately male cohort. These clinical variations in populations may indicate a differing etiology, exposure, or presentation of the military population, limiting the generalization of prior data from other populations.
Our study has several limitations. First, pathologic and radiologic data were not available to confirm diagnosis, stratify by sarcoidosis phenotype, or confirm incidence of cases. Second, any lag in diagnosis from onset of symptoms may dampen a potential seasonal effect, particularly if the lag is variable by region. Third, it may be that sarcoidosis is a manifestation of a number of different diseases or exposures, which is why the triggering factor has historically been so elusive. In this respect, it may also be that different clinical phenotypes, such as Lofgren’s (which typically has spontaneous regression) may represent a different disease or host susceptibility than someone with chronic progressive sarcoidosis, and therefore, the phenomenon should not be analyzed as one uniform disease process.
Despite these limitations, our study includes a large national sample, allowing us to use appropriate time series and seasonal modeling techniques for autocorrelated data. We have analyzed the data using three separate approaches to ensure that a seasonal stimulus was not missed due to our choice of statistical methodology. We were also able to assess seasonality by latitude. To our knowledge, this study is one of the first to specifically address seasonality of new sarcoidosis cases in the U.S. with this magnitude of sample size.
Conclusion
The absence of proof is not the proof of absence, but our results indicate that season is not a dominant factor in the development of sarcoidosis in the U.S. Veteran population. Rather, seasonal exposure may reflect one part of a complex gene-environment-host interaction that precedes presentation of the disease. In order to further study this phenomenon, a large, national registry is urgently needed to evaluate these trends, particularly to analyze environmental and seasonal effects according to various phenotypes.
Acknowledgments
Research Funding Support: This work was supported by the National Institutes of Health, Grant 1KL2RR024980: Institute of Clinical and Translational Science, University of Iowa. The funding source did not have involvement in study design, data analysis, writing, or submission of the manuscript.
Footnotes
Conflicting Interests: The author(s) declare that they have no competing interests.
Contributor Information
Alicia K. Gerke, Email: alicia-gerke@uiowa.edu.
Fan Tang, Email: fan-tang@uiowa.edu.
Ming Yang, Email: ming-yang@uiowa.edu.
Joseph E. Cavanaugh, Email: joe-cavanaugh@uiowa.edu.
Philip M. Polgreen, Email: philip-polgreen@uiowa.edu.
References
- 1.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 2.Gerke AK, Hunninghake G. The immunology of sarcoidosis. Clin Chest Med. 2008;29(3):379–90. vii. doi: 10.1016/j.ccm.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Poukkula A, Huhti E, Lilja M, Saloheimo M. Incidence and clinical picture of sarcoidosis in a circumscribed geographical area. Br J Dis Chest. 1986;80(2):138–47. doi: 10.1016/0007-0971(86)90034-3. [DOI] [PubMed] [Google Scholar]
- 4.Demirkok SS, Basaranoglu M, Akbilgic O. Seasonal variation of the onset of presentations in stage 1 sarcoidosis. Int J Clin Pract. 2006;60(11):1443–50. doi: 10.1111/j.1742-1241.2005.00773.x. [DOI] [PubMed] [Google Scholar]
- 5.Hosoda Y, Hiraga Y, Odaka M, et al. A cooperative study of sarcoidosis in Asia and Africa: analytic epidemiology. Ann N Y Acad Sci. 1976;278:355–67. doi: 10.1111/j.1749-6632.1976.tb47046.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilsher ML. Seasonal clustering of sarcoidosis presenting with erythema nodosum. Eur Respir J. 1998;12(5):1197–9. doi: 10.1183/09031936.98.12051197. [DOI] [PubMed] [Google Scholar]
- 7.Alilovic M, Peros-Golubicic T, Tekavec-Trkanjec J, Smojver-Jezek S, Liscic R. Epidemiological characteristics of sarcoidosis patients hospitalized in the Uuniversity Hospital for Lung Diseases “Jordanovac” (Zagreb, Croatia) in the 1997–2002 period. Coll Antropol. 2006;30(3):513–7. [PubMed] [Google Scholar]
- 8. [Accessed March 2011];The ZipCodeWorld Database. http://www.zipcodeworld.com/
- 9.Glennas A, Kvien TK, Melby K, et al. Acute sarcoid arthritis: occurrence, seasonal onset, clinical features and outcome. Br J Rheumatol. 1995;34(1):45–50. doi: 10.1093/rheumatology/34.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Demirkok SS, Basaranoglu M, Akinci ED, Karayel T. Analysis of 275 patients with sarcoidosis over a 38 year period; a single-institution experience. Respir Med. 2007;101(6):1147–54. doi: 10.1016/j.rmed.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Gorham ED, Garland CF, Garland FC, Kaiser K, Travis WD, Centeno JA. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975–2001. Chest. 2004;126(5):1431–8. doi: 10.1378/chest.126.5.1431. [DOI] [PubMed] [Google Scholar]