Abstract
AIM: To detect the effects of DNA vaccines in combination with duck IFN-γ gene on the protection of ducks against duck hepatitis B virus (DHBV) infection.
METHODS: DuIFN-γ cDNA was cloned and expressed in COS-7 cells, and the antiviral activity of DuIFN-γ was detected and neutralized by specific antibodies. Ducks were vaccinated with DHBpreS/S DNA alone or co-immunized with plasmid expressing DuIFN-γ. DuIFN-γ mRNA in peripheral blood mononuclear cells (PBMCs) from immunized ducks was detected by semi-quantitative competitive RT-PCR. Anti-DHBpreS was titrated by enzyme-linked immunosorbent assay (ELISA). DHBV DNA in sera and liver was detected by Southern blot hybridization, after ducks were challenged with high doses of DHBV.
RESULTS: DuIFN-γ expressed by COS-7 was able to protect duck fibroblasts against vesicular stomatitis virus (VSV) infection in a dose-dependent fashion, and anti-DuIFN-γ antibodies neutralized the antiviral effects. DuIFN-γ in the supernatant also inhibited the release of DHBV DNA from LMH-D2 cells. When ducks were co-immunized with DNA vaccine expressing DHBpreS/S and DuIFN-γ gene as an adjuvant, the level of DuIFN-γ mRNA in PBMCs was higher than that in ducks vaccinated with DHBpreS/S DNA alone. However, the titer of anti-DHBpreS elicited by DHBpreS/S DNA alone was higher than that co-immunized with DuIFN-γ gene and DHBpreS/S DNA. After being challenged with DHBV at high doses, the load of DHBV in sera dropped faster, and the amount of total DNA and cccDNA in the liver decreased more significantly in the group of ducks co-immunized with DuIFN-γ gene and DHBpreS/S DNA than in other groups.
CONCLUSION: DHBV preS/S DNA vaccine can protect ducks against DHBV infection, DuIFN-γ gene as an immune adjuvant enhances its efficacy.
Keywords: Duck IFN-γ, DHBV, DNA vaccine, Immune adjuvant
INTRODUCTION
An estimated 350 million people are chronically infected with hepatitis B virus (HBV). Cytotoxic T lymphocytes (CTLs) play a critical role in the clearance of HBV from patients by lysis of virus-infected hepatocytes[1]. However, IFN-γ and TNF-α have been shown to inhibit HBV replication in HBV transgenic mice, without lysing infected hepatocytes[2,3]. An in vitro model in which lymphocytes from HBsAg positive patients are co-cultured with HBV replicating hepatocytes, shows that the secretion of IFN-γ from lymphocytes inhibits HBV replication[4]. Consequently, in addition to enhancing the cytotoxicity of CTLs, IFN-γ also mediates the non-cytolytic inhibition of HBV replication, and shows its significance in the host defense against HBV infection and recovery from virus infection.
Recombinant woodchuck IFN-γ up-regulates MHC class I molecule transcription, but hardly inhibits or clears woodchuck hepatitis virus replicating intermediates and RNAs in persistently infected woodchuck primary hepatocytes[5]. However, treatment of primary duck hepatocytes with recombinant duck IFN-γ can inhibit DHBV replication in a dose-dependent manner[6]. The role of IFN-γ in hepadnavirus infection is still controversial.
DHBV and HBV share common genomic and structural features, with an envelope surrounding a spherical nucleocapsid that contains a similar viral DNA genome in terms of size, structure, and organization. This is why DHBV-infected ducks have become a useful animal model to explore the molecular mechanism of host defense against HBV infection[7-9]. Animals immunized with antigen and IFN-γ gene develop Th1-biased immune responses, and it has been reported that IFN-γ, as a genetic adjuvant has stronger effects on the nature of immune response in neonates than in adults[10]. To date, whether IFN-γ inhibits DHBV replication in vivo has not been reported. Whether DNA vaccine protects ducks against DHBV infection is still controversial[11-14]. To test the function of IFN-γ as an immune adjuvant and to validate the efficacy of DHBV vaccines, ducks were immunized with the mixture of plasmid expressing DHBV preS/S protein and IFN-γ. The results support the view that DuIFN-γ inhibits DHBV replication and increases the protective efficacy of DNA vaccines expressing DHBV preS/S protein.
MATERIALS AND METHODS
Animals and cells
Cherry valley ducks (Anas platyrhynchos) were supplied by Breeding Center of Shanghai Institute of Veterinary Medical Sciences, China. Duck embryo fibroblasts were prepared as previously described[15] and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 2 mmol/L glutamine, 100 U/mL penicillin and streptomycin, supplemented with 2% duck sera (prepared from DHBV-free ducks and filtered through a 0.22 μm membrane) and 8% fetal calf sera (FCS). COS-7 cells were cultured in DMEM containing 10% FCS. LMH-D2, a chicken hepatoma cell line and a gift from Dr. William S. Mason (Fox Chase Cancer Center), was incubated in DMEM/F12 medium supplemented with 2% chicken sera and 8% FCS.
Separation of duck PBMCs and amplification of DuIFN-γ mRNA by RT-PCR
Blood was taken from adult ducks and diluted by an equal volume of heparinized PBS. PBMCs were separated with Ficoll as described previously[16] 5×106 PBMCs in 1 mL of RPMI 1 640 supplemented with 10% FCS were transferred into 24-well plates and stimulated with PHA at 10 mg/mL for 24 h. Total RNA was extracted from PBMCs with TRIzol reagent (Gibco BRL, USA) and 4 μg of total RNA was reverse transcribed into cDNA by Superscript IITM and hexamer random primer (Gibco BRL). The sequences of sense and anti-sense primers are 5-CGCCGGATCCAT-GACTTGCCAGACCTACTGCTT-3 and 5-CGCCG-ATATCTCATTAACATCTGCATCTCTTTGG-3. cDNA of DuIFN-γ was amplified for 30 cycles at 94 °C for 5 min, 94 °C for 1 min, 54 °C for 1 min, and at 72 °C for 2 min.
Expression of recombinant DuIFN-γ in COS-7 cells
For expression of recombinant DuIFN-γ, the amplified DuIFN-γ DNA fragment was cloned into eukaryotic expression vector pcDNA3.1 (Invitrogen) at the EcoRV/BamHI sites. Transfection was performed by the method of calcium phosphate precipitation. At 72 h post-transfection, the culture supernatant were harvested and centrifuged to remove the cell debris.
Titration of recombinant DuIFN-γ
Duck embryo fibroblasts were seeded into 96-well plates and incubated in the presence of serial four-fold dilution of DuIFN-γ in the supernatant. After 12 h incubation, the cells were challenged with VSV at 100 TCID50 for 36 h. Under microscope, the cytopathic effects (CPE) were observed and MTT was added for another 8 h. MTT could be degraded by mitochondrial enzymes and developed blue color. Consequently, the optical density could indicate the course of virus infection and cellular pathogenesis[17]. DuIFN-γ titers were expressed as reciprocals of the dilutions that resulted in 50% protection against virus-induced cell CPE. The role of DuIFN-γ in protecting cells against virus infection also was confirmed by specific IgG of anti-DuIFN-γ provided by Dr. C.J. Burrel (University of Adelaide, Australia).
Treatment of LMH-D2 cells with DuIFN-γ
LMH-D2 cells were seeded at 1×106 cells/flask at 37 °C in 50 mL/L CO2, and incubated with 10% of recombinant DuIFN-γ supernatant (titrated as 47-48) or the same volume of supernatant derived from transfected pcDNA3.1 vector. After the culture supernatant was collected, fresh DMEM/F12 was refilled everyday or every other day, the collected culture supernatant was centrifuged at 5 000 r/min×10 min, and 5 μL of the supernatant was spotted onto positively charged nylon membranes and the DHBV DNA was detected by dot hybridization.
Subcloning of DHBV-preS/S gene into eukaryotic expression vector and detection of gene expression in COS-7 cells
The full length of DHBV genome clone (pCMV-DHBV9) was also a gift from Dr. William S. Mason. DHBV preS/S gene was subcloned into pcDNA3.1 with AflII/EcoRI restriction sites introduced by PCR primers. The sequences of sense and antisense primers are 5-CGCCTTAAGA-TGGGGCAACATCCAGCAAAATC-3 and 5-CGCG-AATTCAGTTTATTCTTATTCCTAACTCTT-3, respectively. The subcloned DHBV preS/S gene was transfected into COS-7 cells by calcium phosphate precipitation. The expressed protein was detected by indirect immunofluorescence (IMF) staining with mAb against DHBV-preS/S protein as previously described[11]. A reporter gene secreting alkaline phosphatase (SEAP)[18] was transfected into COS-7 cells simultaneously for detection of the efficacy.
Vaccination protocol
Three-day-old ducks were vaccinated intramuscularly in the quadriceps anterior muscle. DHBpreS/S DNA and DuIFN-γ DNA were extracted with a kit (QIAGEN Mega DNA extracted kit) following the manufacturer’s instructions. All ducks were grouped. Group 1 was vaccinated with plasmid pcDNA3.1-DHBpreS/S at 200 μg/duck, group 2 with DHBpreS/S DNA at 200 μg and plasmid pcDNA3.1-DuIFN-γ at 200 μg/duck, group 3 was injected with an equal volume of PBS. The same procedure for DNA vaccination was repeated after 4 wk.
Detection of mRNA of DuIFN-γ in PBMCs after being stimulated with DHBsAg by competitive RT-PCR
Competitive RT-PCR method has been described[19]. Briefly, β-actin gene functioned as a housekeeping gene. Competitive internal control (IC) was constructed by deleting the partial segment of DuIFN-γ cDNA using a pair of specific PCR primers. The sequences of sense and anti-sense primers are 5-GGATGTAGCTGATGGCAATCC-3 and 5-TCATTAACATCTGCATCTCTTTGGGACAGTTCC-ACGAGGTC-3, respectively. The sequence of anti-sense primer for amplification of the target segment of DuIFN-γ cDNA is 5-TCATTAACATCTGCATCTCTTTGG-3 (partial sequences of anti-sense primer for IC), and the sequence of sense primer is the same as the one used for constructing IC. After peripheral blood was separated using Ficoll, PBMCs were transferred into 24-well plates at 5×106 cells/well, and then stimulated with purified DHBsAg at 1 μg/mL for 24 h. IC (1.25×10-5 μg/mL) was used to quantify the samples. The conditions of amplification were at 94 °C for 4 min, 94 °C for 1 min, 60 °C for 45 s, at 72 °C for 1 min. In the next five cycles, the temperature for annealing was decreased at 1 °C per cycles and another 25 cycles were performed at 94 °C for 1 min, 55 °C for 45 s, 72 °C for 1 min, at 72 °C for 10 min.
Serological assay
DHBV preS protein was expressed in E.coli and 100 μL of purified protein (1 μg/mL) was coated on 96-well microdilution plates at 4 °C overnight. Non-specific sites were blocked with 200 μL skim milk (Shanghai Guangming Milk Co., Ltd) in PBS-0.05% Tween 20. The plates were incubated with 2-fold serially diluted serum sample and then with 100 μL of HRP-goat anti-duck immunoglobulin Y at 1/1 000 dilution (KPL). The color was developed by substrate of TMB system in darkness for 15 min and terminated by 50 μL 2 N H2SO4. The optical density at 450 nm (A450) was read on an automatic ELISA reader. The antibody titer in each serum sample was defined as the highest serum dilution that resulted in S/N>3 (S for A450 of serum sample, N for A450 of control normal duck serum sample at the same dilution).
Viral challenge and DHBV DNA assay
All vaccinated ducks were boosted again at wk 7, vaccinated ducks were challenged with DHBV at a high-titer dosage (1.0×1010 DHBV DNA genomes/mL) at wk 13. The volume for injection was adjusted according to the body weight of ducks: volume (mL) = weight (g) ×7%×10%. The ducks were cannulated via the jugular vein. Blood samples were collected before virus challenge and at 1, 5, 15, 30, 45, 60, 90, 120 min post-challenge. After being challenged with virus for 5 d, the ducks were killed and livers were taken out for extraction of DHBV DNA. DHBV DNA in sera and liver was detected by Southern blot as previously described[20].The whole DHBV genome from pCMV-DHBV9 was used as DNA template for producing specific probes labeled by [α-32P] dCTP with a random primer.
RESULTS
Expression of recombinant DuIFN-γ in vitro and biological assay
Monolayer of duck embryo skin fibroblast cells was incubated with the supernatant from recombinant plasmid pcDNA3.1-DuIFN-γ transfected COS-7 cells, and then infected with VSV at 100 TCID50. The results indicated that the supernatant from pcDNA3.1-DuIFN-γ transfected cell culture could protect the duck embryo fibroblast cells against VSV infection, while the supernatant from pcDNA3.1 transfected cells showed no antiviral effect (Figure 1A). The protective effect was dose-dependent. The antiviral effect of DuIFN-γ in the supernatant was titrated in a series of four-fold dilution, and titer was expressed as 47-48 (Figure 2 and Table 1). The antiviral activity of the supernatant from pcDNA3.1-DuIFN-γ transfected cells could be neutralized by anti-DuIFN-γ (Figure 1B), indicating that recombinant DuIFN-γ played a role in the control of viral infection.
Figure 1.
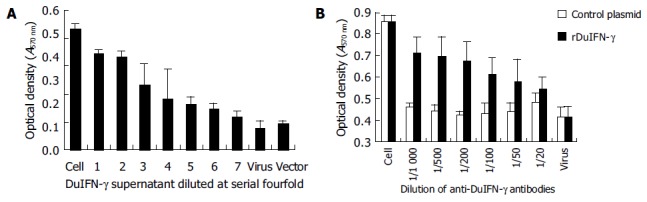
Titration of recombinant DuIFN-g by MTT (A) and neutralization of antiviral activity by anti-DuIFN-g (B).
Figure 2.
Morphology of normal duck fibroblasts (A) and cytopathic effect (CPE) of duck fibroblasts when infected with VSV (B).
Table 1.
CPE inhibition assay to quantify recombinant DuIFN-γ
| IFN-γ (Diluted by log4) | CPE (Four wells) | Average of CPE | Inhibition of CPE | Accumulation of inhibitory CPE | Accumulation of CPE | Inhibition of CPE | Inhibition of CPE (%) |
| 1 | 0, 0, 0, 0 | 0.00 | 4.00 | 27.25 | 0.00 | 27.25/27.25 | 100 |
| 2 | 0, 0, 0, 0 | 0.00 | 4.00 | 23.25 | 0.00 | 23.25/23.25 | 100 |
| 3 | 1, 0, 0, 0 | 0.25 | 3.75 | 19.25 | 0.25 | 19.25/19.50 | 98.7 |
| 4 | 0, 1, 0, 0 | 0.25 | 3.75 | 15.5 | 0.5 | 15.50/16.00 | 96.9 |
| 5 | 1, 0, 1, 0 | 0.5 | 3.5 | 11.75 | 1.00 | 11.75/12.75 | 92.2 |
| 6 | 1, 1, 2, 3 | 1.75 | 2.25 | 8.25 | 2.75 | 8.25/11.00 | 75 |
| 7 | 1, 2, 2, 1 | 1.5 | 2.5 | 6.00 | 4.25 | 6.00/10.25 | 58.5 |
| 8 | 2, 1, 2, 3 | 2.00 | 2.00 | 3.5 | 6.25 | 3.50/9.75 | 35.9 |
| 9 | 3, 3, 3, 2 | 2.75 | 1.25 | 1.5 | 9.00 | 1.50/10.50 | 14.3 |
| 10 | 3, 4, 4, 4 | 3.75 | 0.25 | 0.25 | 12.75 | 0.25/13.00 | 1.9 |
Inhibition of the DHBV replication by DuIFN-γ
For investigation of anti-DHBV effect of recombinant DuIFN-γ, LMH-D2 cells continuously producing DHBV, were incubated with the supernatant from pcDNA3.1-DuIFN-γ transfected cells, culture fluid was collected everyday or every other day. DHBV in the supernatant was spotted on the nylon membrane and detected by hybridization with a DHBV DNA probe. The results showed that the amount of DHBV DNA decreased dramatically 1 d after treatment with DuIFN-γ and the anti-DHBV effect lasted for 4 d, while no anti-DHBV effect of recombinant DuIFN-γ on the supernatant from pcDNA3.1 transfected cell culture was observed. After treatment for 4 d, no apparent difference was observed between cells treated with the recombinant DuIFN-γ and the control supernatant (Figure 3). The results demonstrated that the recombinant DuIFN-γ temporally inhibited the DHBV replication in LMH-D2 cells.
Figure 3.
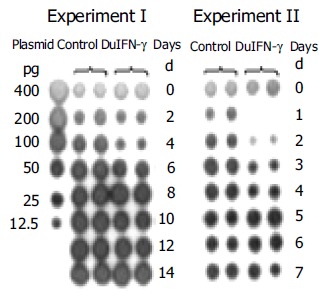
Effects of recombinant DuIFN-g on release of DHBV DNA from LMH-D2 cells.
Effects of DuIFN-γ as adjuvant of DNA vaccine on DuIFN-γ mRNA transcription in duck PBMCs
Three-day-old ducks were immunized with plasmids expressing DHBV-preS/S gene alone (group 1) or expressing DHBV-preS/S gene and DuIFN-γ DNA (group 2), or PBS alone (group 3), and boosted at wk 4 and 7 after the first immunization. The blood was taken and separated PBMCs were incubated with purified DHBsAg at 1 μg/mL for 24 h. The cellular total RNA was extracted and DuIFN-γ mRNA was quantified by competitive RT-PCR. The ducks co-vaccinated with plasmids expressing DHBV-preS/S and DuIFN-γ showed a higher ratio of DuIFN-γ T/IC (1.56±0.08) than those immunized with plasmid expressing DHBV-preS/S alone (the ratio is 1.29±0.09), or normal ducks (0.92±0.06) 3 wk after immunization (P<0.05) (Table 2 and Figure 4). The effects were more significant at wk 5 and 7 post-immunization (P<0.01), the ratios of DuIFN-γ T/IC were 2.51±0.11 and 1.81±0.10 in group 2, 1.96±0.17 and 1.26±0.11 in group 1, 1.33±0.11 and 0.94±0.04 in group 3 at wk 5 and 7, respectively. These results suggested that DuIFN-γ mRNA transcription of duck PBMCs was enhanced by DuIFN-γ as an immuno-adjuvant co-immunized with DNA vaccines.
Table 2.
Detection of DuIFN-γ mRNA of PBMCs from ducks after immunization (mean±SD)
| Immunized groups | Ratio of DuIFN-γ/IC (weeks after first vaccination) | |||
| 1 wk | 3 wk | 5 wk | 7 wk | |
| Group 1 (DHBpreS/S DNA) | 1.10±0.06 | 1.29±0.09ab | 1.96±0.17ab | 1.26±0.11ab |
| Group 2 | ||||
| (DHBpreS/S DNA+DuIFN-γ) | 1.15±0.08 | 1.56±0.08b | 2.51±0.11b | 1.81±0.10b |
| Group 3 (PBS) | 1.04±0.03 | 0.92±0.06 | 1.33±0.11 | 0.94±0.04 |
P<0.05 vs group 2;
P<0.01 vs group 3.
Figure 4.
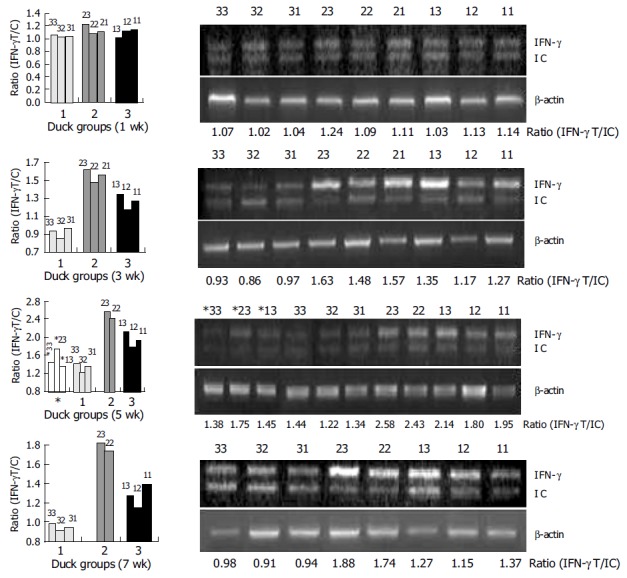
Detection of DuIFN-g mRNA of PBMCs from ducks after immunization.
Effects of DuIFN-γ as an adjuvant of DNA vaccine on anti-DHBpreS antibody responses in ducks
Anti-DHBpreS antibody responses in immunized ducks were detected by ELISA after the first vaccination (Figure 5), the titers of anti-DHBpreS antibody increased gradually after boost at wk 4 and 7. The titer of anti-DHBpreS in ducks vaccinated with DHBV preS/S DNA alone, gradually increased to 27.4-210.6 from wk 4 to wk 13, and was higher than that in ducks co-immunized with plasmid expressing DuIFN-γ and DHBV preS/S DNA, whose titer of anti-DHBpreS was 25.6-28.8 from wk 4 to wk 13. The results showed that DHBV vaccine expressing DHBV preS/S induced antibody responses, but DuIFN-γ DNA down-regulated the elicitation of IgY against DHBV preS.
Figure 5.
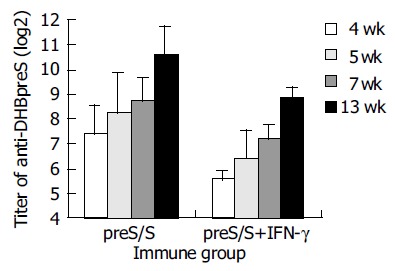
Anti-DHBpreS antibody responses following DNA immunization of DHBV-free ducks.
Clearance of viremia in vaccinated-ducks after being challenged with DHBV
To investigate the role of DuIFN-γ as an adjuvant of DNA vaccines in protecting ducks against DHBV infection, 6 wk after the second boost, the vaccinated ducks were challenged with a high dose of DHBV according to their body weight (7.0×1010/kg). The peripheral blood was collected from ducks at different time points after being challenged to detect DHBV DNA. The results showed that ducks co-vaccinated with plasmids expressing DuIFN-γ and DHBV preS/S had a stronger competence to remove DHBV from bloodstream than ducks immunized with DHBV preS/S DNA alone (Figure 6). After DHBV challenge, more than 50% of DHBV DNA in the blood of co-vaccinated ducks was removed in 5 min, whereas DHBV DNA in the ducks immunized with DHBV preS/S DNA alone decreased only a little in 45 min, and DHBV in both groups was undetectable at the last observation time point (120 min after virus challenge). As a viral infection control, the ducks immunized with PBS did not show significant viremia clearance in 120 min. We further detected the DHBV in liver 5 d after the challenge. Although total and ccc DHBV DNA could be detected in the liver, DHBV DNA was much lower in the ducks co-immunized with DuIFN-γ DNA than that in ducks injected only with DHBV preS/S DNA or PBS. The results indicated that DNA vaccine expressing DHBV preS/S protein enhanced the ability of ducks to exclude the DHBV from bloodstream and remove the DHBV from liver. The capability of ducks was much more significantly upregulated by co-immunization with DuIFN-γ DNA than simple immunization with DHBV preS/S DNA (Figure 7).
Figure 6.

Exclusion of DHBV from bloodstream in DNA-vaccinated ducks following challenge with viruses.
Figure 7.
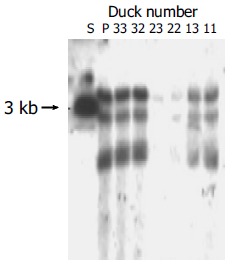
Detection of DHBV cccDNA in ducks’ liver tissues after being challenged with high dose of DHBV. S: Full length fragment of DHBV genome, 3.0 kb. P: Positive control ducks infected DHBV.
DISCUSSION
IFN-γ plays a critical role in the host defense against virus infection. It was reported that IFN-γ regulates immune responses and induces strong Th1 biased responses, which are weak in persistent infection and immune tolerance[21-23]. Transfer of specific CTLs to HBV transgenic mice could inhibit HBV replication in a non-cytolytic way and the CTL capacity of inhibiting HBV infection contributes to IFN-γ and TNF-α[2,3]. The efficacy of IFN-γ in inhibiting HBV replication is also observed by Suri et al[4] Lu et al[5] showed that woodchuck IFN-γ in vitro upregulates the expression of MHC class I molecules, but does not inhibit the formation of WHV intermediates. Schultz and Chisari[6] reported that duck IFN-γ inhibits DHBV replication in a dose-dependent fashion. In the present study, we found that recombinant duck IFN-γ could inhibit the release of DHBV from LMH-D2 cells, suggesting that duck IFN-γ can inhibit DHBV replication.
It has been reported that cytokines function as an efficient immuno-adjuvant, when animals are immunized with DNA vaccines[24-26] but it is unknown whether DuIFN-γ gene also enhances the activity of DNA vaccine in protecting ducks against DHBV infection. Whether DNA vaccine expressing DHBV preS/S protein elicits neutralizing antibodies is still controversial. Triyatni et al[11] reported that, DNA vaccine expressing DHBV preS/S induces high titer anti-DHBV preS/S, but shows less protection against virus infection than plasmid pCI/Amp expressing DHBV S protein. Plasmids expressing DHBV preS/S elicits high neutralizing antibodies and the protection could be transferred to offsprings through specific IgY[12-14]. We have also proved the protective efficacy of DNA vaccine expressing DHBV preS/S, and showed that it could promote DuIFN-γ mRNA transcription of duck PBMCs and quicken the removal of virus from bloodstream and inhibit virus replication in liver, after being challenged with high titer viruses, when the plasmid expressing DuIFN-γ functioned as an immuno-adjuvant.
Little is known about the specific mechanism of DuIFN-γ enhancing the protective capacity of DNA vaccine. Since the titer of anti-DHBpreS elicited by DHBV preS/S DNA vaccine was down-regulated by DuIFN-γ in the present study, the affinity of antibody against antigen may be enhanced and more Th1 cytokines are induced to take effects synergistically. Furthermore, the number and killing capacity of specific cytotoxic lymphocytes could also increase. Further studies are necessary to clarify these important issues.
Thermet et al[27], have shown that DHBV preS/S DNA vaccine has some protective activity and early administration of anti-DHBV drugs such as lamivudine, significantly increases the protection and clears virus from chronic infection patient. However, it was not the case in our experiments and no significant differences were observed in virus-carriers between non-vaccinated and vaccinated ducks, including the ducks co-administered with plasmid expressing DuIFN-γ (data not shown). Many factors may affect the efficacy of vaccines, such as pathway of vaccination, DNA dosage and protocol. It is possible that the antigens in different pathways of vaccination recruit different antigen presenting cells. If the co-stimulatory signals are lost, immune tolerance but not viral clearance occurs[28-30]. When animals are vaccinated at different growth phases, their ability to respond to antigens is significantly different. Also the distribution of the same antigen may affect the pathway of antigen presentation, which might be the reason, why viral antigen in circulating bloodstream cannot be cleared by host itself but DNA vaccines expressing antigen induces the efficacy of immune protection and exclude the viruses, when host is re-infected. Consequently, there is a long way to go for defining the molecular mechanism of DNA immunization and developing efficient vaccines.
Footnotes
Supported by the Major State Basic Research Development Program of China, 973 Program, No. G2002CB512803; the National Natural Science Foundation of China, No. 30070693; the Science and Technology Foundation of Shanghai, No. 02DJ14002
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Bertoletti A, Maini M, Williams R. Role of hepatitis B virus specific cytotoxic T cells in liver damage and viral control. Antiviral Res. 2003;60:61–66. doi: 10.1016/j.antiviral.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 3.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 4.Suri D, Schilling R, Lopes AR, Mullerova I, Colucci G, Williams R, Naoumov NV. Non-cytolytic inhibition of hepatitis B virus replication in human hepatocytes. J Hepatol. 2001;35:790–797. doi: 10.1016/s0168-8278(01)00215-x. [DOI] [PubMed] [Google Scholar]
- 5.Lu M, Lohrengel B, Hilken G, Kemper T, Roggendorf M. Woodchuck gamma interferon upregulates major histocompatibility complex class I transcription but is unable to deplete woodchuck hepatitis virus replication intermediates and RNAs in persistently infected woodchuck primary hepatocytes. J Virol. 2002;76:58–67. doi: 10.1128/JVI.76.1.58-67.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz U, Chisari FV. Recombinant duck interferon gamma inhibits duck hepatitis B virus replication in primary hepatocytes. J Virol. 1999;73:3162–3168. doi: 10.1128/jvi.73.4.3162-3168.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cova L, Zoulim F. Duck hepatitis B virus model in the study of hepatitis B virus. Methods Mol Med. 2004;96:261–268. doi: 10.1385/1-59259-670-3:261. [DOI] [PubMed] [Google Scholar]
- 8.Jilbert AR, Botten JA, Miller DS, Bertram EM, Hall PM, Kotlarski J, Burrell CJ. Characterization of age- and dose-related outcomes of duck hepatitis B virus infection. Virology. 1998;244:273–282. doi: 10.1006/viro.1998.9095. [DOI] [PubMed] [Google Scholar]
- 9.Jilbert AR, Kotlarski I. Immune responses to duck hepatitis B virus infection. Dev Comp Immunol. 2000;24:285–302. doi: 10.1016/s0145-305x(99)00079-8. [DOI] [PubMed] [Google Scholar]
- 10.Pertmer TM, Oran AE, Madorin CA, Robinson HL. Th1 genetic adjuvants modulate immune responses in neonates. Vaccine. 2001;19:1764–1771. doi: 10.1016/s0264-410x(00)00388-1. [DOI] [PubMed] [Google Scholar]
- 11.Triyatni M, Jilbert AR, Qiao M, Miller DS, Burrell CJ. Protective efficacy of DNA vaccines against duck hepatitis B virus infection. J Virol. 1998;72:84–94. doi: 10.1128/jvi.72.1.84-94.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rollier C, Sunyach C, Barraud L, Madani N, Jamard C, Trepo C, Cova L. Protective and therapeutic effect of DNA-based immunization against hepadnavirus large envelope protein. Gastroenterology. 1999;116:658–665. doi: 10.1016/s0016-5085(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 13.Rollier C, Charollois C, Jamard C, Trepo C, Cova L. Maternally transferred antibodies from DNA-immunized avians protect offspring against hepadnavirus infection. J Virol. 2000;74:4908–4911. doi: 10.1128/jvi.74.10.4908-4911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rollier C, Charollois C, Jamard C, Trepo C, Cova L. Early life humoral response of ducks to DNA immunization against hepadnavirus large envelope protein. Vaccine. 2000;18:3091–3096. doi: 10.1016/s0264-410x(00)00130-4. [DOI] [PubMed] [Google Scholar]
- 15.Schultz U, Köck J, Schlicht HJ, Staeheli P. Recombinant duck interferon: a new reagent for studying the mode of interferon action against hepatitis B virus. Virology. 1995;212:641–649. doi: 10.1006/viro.1995.1522. [DOI] [PubMed] [Google Scholar]
- 16.Long JE, Huang LN, Wang WY, Cheng MJ, Wen YM, Yuan ZH, Qu D. Cloning and Expression of Chinese Duck Interferon-gamma Gene. ShengWu HuaXue Yu ShengWu WuLi XueBao (Shanghai) 2001;33:707–712. [PubMed] [Google Scholar]
- 17.Berg K, Hansen MB, Nielsen SE. A new sensitive bioassay for precise quantification of interferon activity as measured via the mitochondrial dehydrogenase function in cells (MTT-method) APMIS. 1990;98:156–162. doi: 10.1111/j.1699-0463.1990.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 18.Berger J, Hauber J, Hauber R, Geiger R, Cullen BR. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- 19.Long JE, Huang LN, Qin ZJ, Wang WY, Cheng MJ, Qu D. Quantitation of IFN-γmRNA in duck PBMC and Its Application. Zhonghua Chuanranbing Zazhi. 2003;23:529–533. [Google Scholar]
- 20.Moraleda G, Wu TT, Jilbert AR, Aldrich CE, Condreay LD, Larsen SH, Tang JC, Colacino JM, Mason WS. Inhibition of duck hepatitis B virus replication by hypericin. Antiviral Res. 1993;20:235–247. doi: 10.1016/0166-3542(93)90023-c. [DOI] [PubMed] [Google Scholar]
- 21.Tsai SL, Sheen IS, Chien RN, Chu CM, Huang HC, Chuang YL, Lee TH, Liao SK, Lin CL, Kuo GC, et al. Activation of Th1 immunity is a common immune mechanism for the successful treatment of hepatitis B and C: tetramer assay and therapeutic implications. J Biomed Sci. 2003;10:120–135. doi: 10.1007/BF02256004. [DOI] [PubMed] [Google Scholar]
- 22.Infante-Duarte C, Kamradt T. Th1/Th2 balance in infection. Springer Semin Immunopathol. 1999;21:317–338. doi: 10.1007/BF00812260. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson Brown PM, Neuman MG. Immunopathogenesis of hepatitis C viral infection: Th1/Th2 responses and the role of cytokines. Clin Biochem. 2001;34:167–171. doi: 10.1016/s0009-9120(01)00210-7. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki S, Takeshita F, Xin KQ, Ishii N, Okuda K. Adjuvant formulations and delivery systems for DNA vaccines. Methods. 2003;31:243–254. doi: 10.1016/s1046-2023(03)00140-3. [DOI] [PubMed] [Google Scholar]
- 25.Leclercq S, Harms JS, Oliveira SC. Enhanced efficacy of DNA vaccines against an intracellular bacterial pathogen by genetic adjuvants. Curr Pharm Biotechnol. 2003;4:99–107. doi: 10.2174/1389201033489892. [DOI] [PubMed] [Google Scholar]
- 26.Chow YH, Chiang BL, Lee YL, Chi WK, Lin WC, Chen YT, Tao MH. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–1329. [PubMed] [Google Scholar]
- 27.Thermet A, Rollier C, Zoulim F, Trepo C, Cova L. Progress in DNA vaccine for prophylaxis and therapy of hepatitis B. Vaccine. 2003;21:659–662. doi: 10.1016/s0264-410x(02)00575-3. [DOI] [PubMed] [Google Scholar]
- 28.Kiefer F, Vogel WF, Arnold R. Signal transduction and co-stimulatory pathways. Transpl Immunol. 2002;9:69–82. doi: 10.1016/s0966-3274(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 29.Dennert G. Elimination of virus-specific cytotoxic T cells in the liver. Crit Rev Immunol. 2002;22:1–11. [PubMed] [Google Scholar]
- 30.Sebille F, Vanhove B, Soulillou JP. Mechanisms of tolerance induction: blockade of co-stimulation. Philos Trans R Soc Lond B Biol Sci. 2001;356:649–657. doi: 10.1098/rstb.2001.0842. [DOI] [PMC free article] [PubMed] [Google Scholar]


