Abstract
AIM: To characterize the alpha-fetoprotein (AFP) positive and negative hepatocellular carcinoma (HCC) samples.
METHODS: Thirty-seven paraffin-embedded human HCC samples were analyzed by immunohistochemistry for the following antigens: AFP, β-catenin, p53, CD44, MSH-2, MLH-1, and HNF-4. The tumors were divided into two groups based on the AFP expression. The immunophenotypic data and important clinical parameters were studied between the two groups.
RESULTS: Twenty-one of the thirty-seven examined HCCs were AFP positive. Seven with nuclear p53 staining were AFP positive, while seven tumors with nuclear β-catenin staining were AFP negative. CD44 staining and high histological tumor grade were more frequent among the AFP-positive HCCs. The other immunophenotypical and clinical parameters did not show statistically significant difference in their distribution between the AFP positive and negative samples.
CONCLUSION: AFP expression in HCC correlates with unfavorable prognostic factors, while nuclear β-catenin positivity is more common among the AFP-negative liver tumors. This observation supports the microarray data on in vivo human tumors.
Keywords: Hepatocellular carcinoma, Alpha-fetoprotein, p53, β-catenin, CD44
INTRODUCTION
Alpha-fetoprotein (AFP) is one of the earliest recognized oncofetal markers[1]. It is produced in large amount by the fetal liver, but its expression reduces sharply at birth. AFP is synthesized by most hepatoblastomas and approximately half of hepatocellular carcinomas (HCC), and widely used in differential diagnosis and follow-up of patients with liver tumors, but so far no correlation has been found between the clinical behavior and AFP production in HCC.
DNA microarray analysis may result in the discovery of new tumor markers and can re-evaluate the known tumor parameters. Two independent teams studied the gene expression profile of human HCC cell lines and divided the cell lines into two groups, based on their gene expression pattern. AFP expression is highly correlated with the molecular subtypes of HCC[2,3]. This observation led us to study the AFP expression in HCC samples and to compare it with other immunophenotypic and clinical features of the tumor.
MATERIALS AND METHODS
Thirty-seven recently diagnosed HCC cases were collected. Formalin-fixed and paraffin-embedded tumor samples (19 needle biopsies and 18 resected tumors) were immunohis-tochemically stained. The primary antibodies used were: AFP: DAKO (A 0008), MLH-1: Bio PharMingen (554072), MSH-2: Bio PharMingen (556349), β-catenin: Sigma (C2206), CD44: R&D (BBA10), HNF-4: Santa Cruz (SC 6556), p53: DAKO (M 3566). The reaction was visualized using Vector Laboratories’ Elite kit (Burlingame, CA, USA), DAB was used as a chromogen. Since AFP expression was frequently focal, all tumors were taken as positive when any reliable staining was detected. In case of p53 and β-catenin, tumors with at least 20% nuclear staining were deemed as positive. CD44 staining resulted in a more or less diffuse stromal reaction in positive cases. Tumor grade and histological subtype were evaluated on H&E-stained sections. Grading was done as previously described by Edmondson and Steiner[4]. The tumors were classified histologically into pseudoglandular, trabecular, sheet-like, and mixed pattern groups. The clinical parameters studied were age and gender of the patients, the presence of cirrhosis and etiological factors (HCV, HBV, alcoholism, etc.). Unfortunately, no serum AFP value was available for half of the patients; therefore, it was not included in our study. Since the majority of the cases were diagnosed in the last 2 years, their survival was not analyzed. Statistical analysis was done by χ2 and Student’s t-test.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s guidelines (permission number: TUKEB 156/2003).
RESULTS
The tumors were divided into two groups, based on the AFP staining and other parameters. Twenty-one of the thirty-seven examined HCCs were AFP positive (Figure 1A), which was comparable to previous data in the literature. No correlation was found between the AFP staining and age/gender of the patients, etiological factors, presence or absence of cirrhosis, histological subtype of the tumor, MLH-1, MSH-2, HNF-4 staining (data not shown). However, four of the parameters showed statistically significant (P<0.05) difference between the AFP positive and negative HCCs (Tables 1 and 2). Seven p53-positive tumors (Figure 1B) were positive for AFP, while seven nuclear β-catenin-positive tumors (Figure 1C) AFP negative. Nineteen of the twenty-one AFP-positive tumors were positive for CD44 (Figure 1D), while only 3 of the 17 AFP-negative tumors were positive for this adhesion molecule. Statistical comparison resulted in a significant difference between the tumor grades (P<0.05). The AFP-positive tumors had a higher statistical value.
Figure 1.
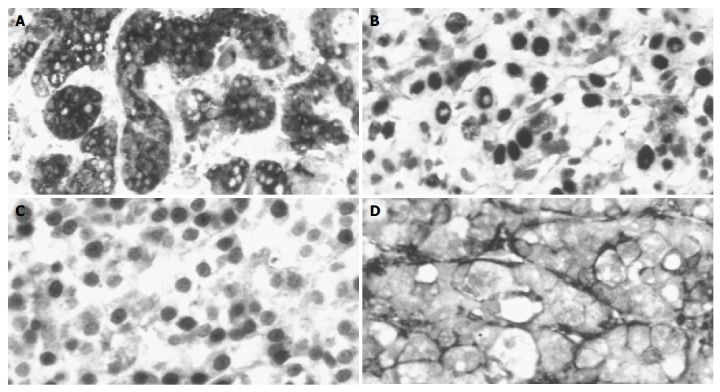
Immunohistochemical reactions on tumor specimens. A: Trabecular HCC, strong cytoplasmic AFP staining; B: Nuclear staining with p53 antibody in tumor cells; C: b-Catenin nuclear staining in tumor cells; D: CD44 immunoreactivity in tumor stroma.
Table 1.
AFP negative cases
| Case number | B-catenin1 | p531 | CD442 | grade |
| 1 | - | - | - | 2 |
| 2 | + | - | - | 1 |
| 3 | - | - | - | 2 |
| 4 | - | - | + | 2 |
| 6 | + | - | - | 2 |
| 7 | - | - | - | 2 |
| 10 | + | - | - | 2 |
| 11 | + | - | - | 2 |
| 14 | + | - | - | 2 |
| 16 | - | - | + | 1 |
| 18 | + | - | - | 1 |
| 19 | + | - | - | 1 |
| 29 | - | - | - | 2 |
| 34 | - | - | + | 2 |
| 36 | - | - | - | 1 |
| 37 | - | - | - | 2 |
| 16 cases | 7/16 | 0/16 | 3/16 |
Positivity indicates more than 20% nuclear staining.
Positivity indicates diffuse stromal staining.
Table 2.
AFP positive cases
| Case number | B-catenin1 | p531 | CD442 | Grade |
| 5 | - | - | + | 2 |
| 8 | - | - | - | 2 |
| 9 | - | + | - | 2 |
| 12 | - | - | + | 3 |
| 13 | - | - | + | 3 |
| 15 | - | - | + | 4 |
| 17 | - | + | + | 3 |
| 20 | - | - | + | 2 |
| 21 | - | + | + | 3 |
| 22 | - | - | + | 3 |
| 23 | - | + | + | 2 |
| 24 | - | + | + | 2 |
| 25 | - | - | + | 2 |
| 26 | - | - | + | 2 |
| 27 | - | + | + | 3 |
| 28 | - | - | + | 2 |
| 30 | - | - | + | 2 |
| 31 | - | - | + | 3 |
| 32 | - | - | + | 3 |
| 33 | - | - | + | 2 |
| 35 | - | + | + | 3 |
| 21 cases | 0/21 | 7/21 | 19/21 |
Positivity indicates more than 20% nuclear staining.
Positivity indicates diffuse stromal staining.
DISCUSSION
We compared the distribution of several parameters between AFP positive and negative HCCs and found that some of them were significantly different between the two groups. All the p53-positive tumors were stained with AFP antibody and the CD44 positivity was also more common in this group.
β-catenin nuclear staining occurred exclusively in the AFP-negative tumors and the tumor grade was significantly lower in this group, which is in agreement with the reports of Kawai et al[2] and Lee and Thorgeirsson[3]. There are some sporadic observations that AFP is more frequently expressed in poorly differentiated HCCs[5-8].
We found that three unfavorable prognostic markers were correlated with positive AFP. Tumor grade is not as meaningful in HCCs as in other tumors (e.g., prostate carcinoma), but shorter survival has been described in poorly differentiated tumors[9]. Nuclear p53 staining indicates increased protein half-life, which is usually the consequence of point mutation. Therefore, we can assume that tumors with p53 nuclear staining carry p53 mutation. It is well documented that p53 mutation or nuclear accumulation is a valuable marker for predicting the poor prognosis of HCC patients[10,11]. CD44, a widely distributed integral membrane protein, has been implicated in tumor invasion and development of metastasis. It was reported that upregulation of CD44 in liver tumors is associated with a shorter survival and p53 overexpression[12,13].
β-catenin nuclear staining is observed in AFP-negative liver carcinomas. Nuclear accumulation of this protein indicates the increased activity of Wnt signal pathway[14]. In cases of HCC, this is most frequently caused by β-catenin mutation[15,16]. The significance of nuclear β-catenin positivity in HCC is controversial. Suzuki et al[17] have described nuclear expression in poorly differentiated tumors. Van Nhieu et al[18] reported that the higher proliferative index and poor outcome correlate with nuclear β-catenin-stained HCCs, but others could not confirm the role of β-catenin in the progression of HCC[19]. In fact, Hsu et al[20] and Mao et al[21] have found that the nuclear expression of β-catenin is present in a subgroup of HCCs with a favorable prognosis. Laurent-Puig et al[22] demonstrated that nuclear β-catenin staining occurs in HCC with a low AFP serum level, but there is high chromosomal instability, frequent p53 mutations, and high AFP level in tumors without β-catenin mutation. A similar conclusion can be found in studies by Calvisi et al[23,24]. They suggested the existence of two major genetic pathways of neoplastic development in the liver. The first was characterized by disruption of Wnt signaling, including β-catenin mutation and a low rate of loss of heterozygocity. The Wnt signaling is intact in the second group of tumors, but they could detect chromosomal instability in them. AFP expression was more common in the second group. Mao et al[21] also described that nuclear β-catenin expression is less frequently associated with serum AFP elevation. Unfortunately, the serum AFP values were not available in all the cases in our study, but our results had a close correlation with immunohistochemical staining.
Among the parameters we studied, only CD44 was detected in microarray experiments as differentially expressed between the AFP positive and negative HCC cell lines[2]. This is not surprising, since the difference in p53 and β-catenin is not transcriptionally regulated.
In brief, the three clearly unfavorable prognostic markers of p53 nuclear staining, CD44 expression, and high grade are more common in AFP-producing HCCs, while one potentially favorable parameter, nuclear β-catenin staining occurs in AFP-negative tumors, indicating that AFP can be used as a prognostic marker in patients with liver tumors.
Footnotes
Supported by the National Science Foundation of Hungary, No. OTKA 42674
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 1990;12:1420–1432. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 2.Kawai HF, Kaneko S, Honda M, Shirota Y, Kobayashi K. alpha-fetoprotein-producing hepatoma cell lines share common expression profiles of genes in various categories demonstrated by cDNA microarray analysis. Hepatology. 2001;33:676–691. doi: 10.1053/jhep.2001.22500. [DOI] [PubMed] [Google Scholar]
- 3.Lee JS, Thorgeirsson SS. Functional and genomic implications of global gene expression profiles in cell lines from human hepatocellular cancer. Hepatology. 2002;35:1134–1143. doi: 10.1053/jhep.2002.33165. [DOI] [PubMed] [Google Scholar]
- 4.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Brumm C, Schulze C, Charels K, Morohoshi T, Klöppel G. The significance of alpha-fetoprotein and other tumour markers in differential immunocytochemistry of primary liver tumours. Histopathology. 1989;14:503–513. doi: 10.1111/j.1365-2559.1989.tb02186.x. [DOI] [PubMed] [Google Scholar]
- 6.Peng SY, Lai PL, Chu JS, Lee PH, Tsung PT, Chen DS, Hsu HC. Expression and hypomethylation of alpha-fetoprotein gene in unicentric and multicentric human hepatocellular carcinomas. Hepatology. 1993;17:35–41. [PubMed] [Google Scholar]
- 7.Fujioka M, Nakashima Y, Nakashima O, Kojiro M. Immunohistologic study on the expressions of alpha-fetoprotein and protein induced by vitamin K absence or antagonist II in surgically resected small hepatocellular carcinoma. Hepatology. 2001;34:1128–1134. doi: 10.1053/jhep.2001.29202. [DOI] [PubMed] [Google Scholar]
- 8.Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, Poovorawan Y. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302–308. doi: 10.1097/00004836-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chedid A, Ryan LM, Dayal Y, Wolf BC, Falkson G. Morphology and other prognostic factors of hepatocellular carcinoma. Arch Pathol Lab Med. 1999;123:524–528. doi: 10.5858/1999-123-0524-MAOPFO. [DOI] [PubMed] [Google Scholar]
- 10.Sugo H, Takamori S, Kojima K, Beppu T, Futagawa S. The significance of p53 mutations as an indicator of the biological behavior of recurrent hepatocellular carcinomas. Surg Today. 1999;29:849–855. doi: 10.1007/BF02482774. [DOI] [PubMed] [Google Scholar]
- 11.Heinze T, Jonas S, Kärsten A, Neuhaus P. Determination of the oncogenes p53 and C-erb B2 in the tumour cytosols of advanced hepatocellular carcinoma (HCC) and correlation to survival time. Anticancer Res. 1999;19:2501–2503. [PubMed] [Google Scholar]
- 12.Endo K, Terada T. Protein expression of CD44 (standard and variant isoforms) in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, p53 expression and patient survival. J Hepatol. 2000;32:78–84. doi: 10.1016/s0168-8278(00)80192-0. [DOI] [PubMed] [Google Scholar]
- 13.Washington K, Telen MJ, Gottfried MR. Expression of cell adhesion molecule CD44 in primary tumors of the liver: an immunohistochemical study. Liver. 1997;17:17–23. doi: 10.1111/j.1600-0676.1997.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 14.Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terris B, Pineau P, Bregeaud L, Valla D, Belghiti J, Tiollais P, Degott C, Dejean A. Close correlation between beta-catenin gene alterations and nuclear accumulation of the protein in human hepatocellular carcinomas. Oncogene. 1999;18:6583–6588. doi: 10.1038/sj.onc.1203051. [DOI] [PubMed] [Google Scholar]
- 16.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki T, Yano H, Nakashima Y, Nakashima O, Kojiro M. Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J Gastroenterol Hepatol. 2002;17:994–1000. doi: 10.1046/j.1440-1746.2002.02774.x. [DOI] [PubMed] [Google Scholar]
- 18.Nhieu JT, Renard CA, Wei Y, Cherqui D, Zafrani ES, Buendia MA. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol. 1999;155:703–710. doi: 10.1016/s0002-9440(10)65168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vona G, Estepa L, Béroud C, Damotte D, Capron F, Nalpas B, Mineur A, Franco D, Lacour B, Pol S, et al. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology. 2004;39:792–797. doi: 10.1002/hep.20091. [DOI] [PubMed] [Google Scholar]
- 20.Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol. 2000;157:763–770. doi: 10.1016/s0002-9440(10)64590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao TL, Chu JS, Jeng YM, Lai PL, Hsu HC. Expression of mutant nuclear beta-catenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J Pathol. 2001;193:95–101. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH720>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Laurent-Puig P, Legoix P, Bluteau O, Belghiti J, Franco D, Binot F, Monges G, Thomas G, Bioulac-Sage P, Zucman-Rossi J. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology. 2001;120:1763–1773. doi: 10.1053/gast.2001.24798. [DOI] [PubMed] [Google Scholar]
- 23.Calvisi DF, Factor VM, Loi R, Thorgeirsson SS. Activation of beta-catenin during hepatocarcinogenesis in transgenic mouse models: relationship to phenotype and tumor grade. Cancer Res. 2001;61:2085–2091. [PubMed] [Google Scholar]
- 24.Calvisi DF, Factor VM, Ladu S, Conner EA, Thorgeirsson SS. Disruption of beta-catenin pathway or genomic instability define two distinct categories of liver cancer in transgenic mice. Gastroenterology. 2004;126:1374–1386. doi: 10.1053/j.gastro.2004.02.014. [DOI] [PubMed] [Google Scholar]


