Abstract
Dysregulation of Akt, PTEN, Drg-1, Cx-26, and L-plastin expression appear to be important in the progression of various cancers. Their expression in bladder cancer has not been well characterized. To assess the expression of these genes and their relationship to the outcome of bladder cancer, we used a bladder cancer tissue microarray (TMA) of 251 transitional cell carcinomas. We quantitated immunohistochemical staining of each protein using both automated and manual methods and correlated the expression levels with the clinicopathologic characteristics of the tumor and patient survival. Overall, the results from both automated and manual analyses were similar. We found a significant correlation between the expression of PTEN, Cx-26 and L-plastin with known clinically important pathologic features of bladder cancer (tumor grade, stage, and growth pattern). Aberrant localization patterns of Cx-26 and Drg-1 were observed in bladder tumors. There was also a significant correlation in expression among pAkt, PTEN, and L-plastin. Although the expression of these genes correlated with factors known to be associated with patient outcome, none of them was an independent predictor of progression-free or overall survival.
Keywords: bladder neoplasms, tumor markers, tissue microarray analysis
INTRODUCTION
Recent data suggest that alterations in the expression of pAkt (phosphorylated Akt), PTEN (phosphatase and tensin homologue deleted on chromosome 10), Drg-1 (differentiation related gene 1), Cx-26 (Connexin-26), and L-plastin genes contribute to the malignant phenotype observed in various cancers [1–5]. We determined the expression levels of this panel of genes in a bladder cancer tissue microarray and correlated gene expression with tumor pathological features and progression-free and overall survival.
The oncoprotein Akt, a serine/threonine kinase, is important in regulating key processes involved in tumor growth and progression. Immunohistochemical (IHC) studies of the phosphorylated active form, pAKT, demonstrated that the expression of pAkt is increased in advanced prostate, breast, and ovarian cancers [6–8]. We have found pAkt expression is upregulated in several human bladder cancer cell lines including UM-UC-3 and T24 [9]. Inhibition of Akt signaling can result in growth inhibition in vitro [10].
PTEN is a tumor-suppressor gene that encodes a phosphatase upstream of Akt in the phosphatidylinositol-3-kinase (PI3K) pathway [2]. PTEN is shown to be mutated or deleted in 14–23% of invasive bladder cancers [11]. This phenomenon is also seen in vitro with the loss of PTEN expression in some human bladder cancer cell lines [9,11,12]. Restoration of PTEN in these cell lines leads to apoptosis and growth inhibition. The loss of PTEN expression combined with the overexpression of pAkt can result in tumorigenesis [13].
Drg-1 (also known as Cap43) is associated with the pAkt and PTEN signaling pathways. Drg-1 has been found to have metastatic suppressor activity in breast, colon and prostate cancer cells and suppress tumorgenecity of bladder cancer cells in nude mice [14]. Inhibition of PTEN leads to downregulation of Drg-1, and treatment with an Akt inhibitor restores Drg-1 expression. This suggests Drg-1 is regulated by PTEN in a pAKT dependent manner. IHC analysis has shown that the expressions of PTEN and Drg-1 are correlated in prostate and breast cancers [1].
Cx-26 is a component of the gap junction intercellular communication (GJIC) network, which provides intercellular communication necessary for cell growth, proliferation, and differentiation. The loss of expression of connexins and gap junction assembly has been found in a variety of tumors [15,16]. Cx-26 has been shown to play a role in bladder cancer [17,18], and DNA microarray analysis of bladder cancer cell lines with Cx-26 exogenous expression documents changes in the Akt and PTEN signaling pathways (data not published).
L-plastin is an actin-bundling protein that is overexpressed in many malignant human solid tumors [3,4,19]. L-plastin is upregulated in prostate cancer and has been suggested to be a potential marker for metastasis in colorectal cancer [4,20]. We identified L-plastin as a potentially important gene in bladder cancer through gene expression profiling (data not published).
To assess the role of these markers in bladder cancer, we interrogated a bladder cancer tissue microarray (TMA) to determine their expression in this cancer and the association of their expression with tumor grade (grades 1–3), stage (carcinoma in situ [Tis], Ta, T1–T4), growth pattern (papillary vs. nonpapillary), and patient survival.
MATERIALS AND METHODS
TMA assembly and IHC analyses
The bladder cancer TMA was assembled as previously described [21] and consisted of 251 bladder cancer specimens. The tumor types examined were nonpapillary invasive G3 (121), papillary invasive G3 (19), papillary noninvasive G3 (26) and papillary noninvasive G2 (85). IHC staining was performed using antibodies to pAkt (Ser473), clone 736E11 (Cell Signaling, Danvers, MA), PTEN, clone PN37 (Zymed Corp., South San Francisco, CA), Drg-1, clone Z44.WE (Zymed Corp.), Cx-26, clone CX-1E8 (Zymed Corp.), and L-plastin, clone LPL4A.1 (Neomarkers, Fremont, Ca). Conditions for each marker were optimized for use on the DAKO Autostainer (DAKO, Carpinteria, CA). Mouse IgG was used as a negative control. Normal ureters were used as positive controls for PTEN, Drg-1, and Cx-26. Breast carcinoma was used as a positive control for pAkt, and colon carcinoma was used as a positive control for L-plastin. The TMA slides were deparaffinized and rehydrated. Antigen retrieval was performed by incubation in citrate buffer (Biogenex, San Ramon, CA) in a steam bath for 20 minutes. Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide/methanol for 10 min at room temperature. The slides were blocked for 30 minutes using serum blocking solution, 1.5% serum in phosphate-buffered saline (PBS). The slides were then stained for 60 minutes with primary antibody diluted in serum blocking solution [pAKT (1:100), PTEN (1:100), Drg-1 (1:100), Cx-26 (1:100), and L-plastin (1:50)]. The slides were then washed in PBS and incubated for 30 minutes with biotinylated secondary antibody in serum blocking solution at room temperature. The slides were washed again with PBS and incubated for 30 minutes with avidin-biotin-peroxidase complex using a DAKO LSAB+ kit (DAKO, Carpinteria, CA). Color was developed with diaminobenzidine, and the sections were then counterstained with hematoxylin, dehydrated, and mounted.
The TMA slides were scored using two methods: automated digital analysis (Ariol SL-50; Applied Imaging, San Jose, CA) and manually through visual assesssment. Automated imaging analysis utilized the Ariol algorithm which measures the total brown stain regardless of cellular localization. The total stained area was expressed in pixels. The total integrated optical density was expressed in arbitrary optical density units. For statistical analysis, all cases displaying total integrated optical density (mean ± Standard Error) were rated on a scale of 0–3: negative staining (the absence of stain) = 0, weak staining = 1, intermediate staining = 2, and strong staining = 3. Visual assessment was performed and correlated with the automated measurements. The counting criteria and software settings were identical for all slides [22]. The TMA slides were also manually scored by visual assessment with three independent researchers blinded to clinicopathologic information. Discrepancies were resolved by discussion. Overall staining was rated on a scale of 0–3: negative staining (the absence of stain) = 0, weak staining = 1, intermediate staining = 2, and strong staining = 3. Each tissue core was examined by a Pathologist to score the presence of tumor. Specimens in which less than 30% of the tissue contained cancer (~50 cores) were excluded from analysis.
Statistical analysis
All statistical analyses were performed by our Biostatistics and Data Management group (Genitourinary SPORE Core Resources). Descriptive analyses, expressed in plots and tables, were generated for exploratory purposes. Spearman’s rank correlation coefficients were calculated to evaluate correlations between gene expressions. The Chi-square test was used to test the associations between markers and the clinicopathologic characteristics (tumor grade, stage, and growth pattern). Progression-free survival (PFS) and overall survival (OS) intervals were estimated using the Kaplan-Meier method and were calculated from the date of diagnosis of bladder cancer to the date of recurrence, death, or last follow-up. Patients in whom the disease did not recur after cystectomy and those in whom the tumor did not progress (i.e., no increase in disease stage) were censored for PFS. The log-rank test was performed to compare the survival distributions associated with clinicopathologic characteristics, the different levels of gene expression, or with different combinations of clinicopathologic characteristics and gene expression. The Cox proportional hazards model was fitted to determine the association of OS with clinicopathologic characteristics and gene expression. All statistical tests used a two-sided 0.05 level of significance. The analyses were performed using S-plus 2000 (Insightful, Inc., Seattle, WA) and Statistica software version 6.0 (StatSoft, Inc., Tulsa, OK).
RESULTS
Drg-1 and Cx-26 have aberrant staining patterns in bladder tumors
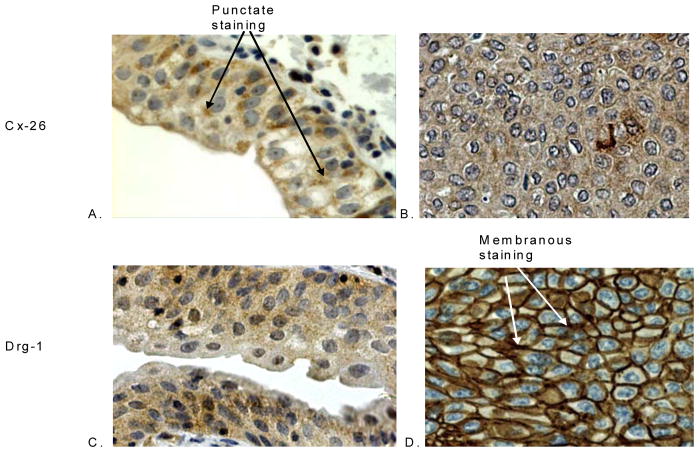
Figure 1 shows the staining patterns of pAkt, PTEN, Drg-1, Cx-26, and L-plastin in normal urothelium and the negative (0) and positive (1, 2, and 3) staining patterns of each marker in bladder cancer. With the exception of pAkt, all of the proteins decorated normal urothelium. In normal urothelium, PTEN localization was cytoplasmic. Bladder cancers exhibited mostly cytoplasmic PTEN expression along with some nuclear staining. pAkt expression was heterogeneous, with cytoplasmic and some nuclear staining noted in some cancers. The expression of Drg-1 was observed in the cytoplasm of normal urothelium and in tumors. In addition, membranous staining was observed in 20% of the tumors (data not shown). Cx-26 expression in normal urothelium was homogeneous with some punctate staining, whereas in cancer tissue Cx-26 exhibited diffuse cytoplasmic staining with some heterogeneity. Figure 2 illustrates the aberrant localizations of Cx-26 and Drg-1 among some of the bladder cancer specimens. L-plastin exhibited cytoplasmic localization in both normal and malignant tissues.
Figure 1. Immunhistochemical staining of human bladder cancer tissue microarray.
Both the positive and negative staining patterns of pAkt, PTEN, Drg-1, Cx-26, and L-plastin are shown on bladder carcinomas. The staining of normal urothelium is also observed.
Figure 2. Immunhistochemical localization pattern of Cx-26 and Drg-1 of human bladder cancer tissue microarray.
Cx-26 staining is punctate in normal urothelium (A) and diffuse and cytoplasmic in bladder cancer (B). Drg-1 is found in the cytoplasm of normal urothelium (C) and is membranous in bladder cancer tissues (D).
The expression of PTEN, Cx-26, and L-plastin is significantly associated with the clinicopathologic characteristics of bladder cancer
Both manual and automated analysis found the expression of PTEN, Cx-26, and L-plastin to be significantly associated with known clinically important pathologic features of bladder cancer (tumor grade, stage, and growth pattern). The associations of the clinicopathologic characteristics with the expressions of pAkt, PTEN, Drg-1, Cx-26, and L-plastin based on manual assessment are shown in Table 1. P values with statistical significance identified by automated analysis but not by manual enumeration is shown in brackets. The expression of pAkt was not significantly associated with tumor grade or stage in either analysis. However, pAkt expression was found to be significant in growth pattern (P = .001) by automated assessment. PTEN expression was highest in low grade, superficial, and papillary tumors. The expression of PTEN was significantly associated with tumor grade (P = .0002), stage (P = .0003), and growth pattern (P = .0001). Neither manual analysis nor automated analysis found a statistically significant difference between the distributions of strong and weak Drg-1 staining among bladder tumors. Cx-26 expression was highest in high grade, invasive, and nonpapillary tumors (P = .0002, P = .008, and P = .001, respectively). L-plastin expression was found to be significant in tumor grade and growth pattern by both analyses and in stage (P = .001) by automated assessment. Its expression was significantly associated with tumor grade (P = .035) and growth pattern (P = .047).
Table 1.
Clinicopathological characteristics associated with pAkt, PTEN, Drg-1, Cx-26, and L-Plastin in bladder carcinomas determined by manual assessment P values calculated by chi-square test of independence. P values with statistical significance in automated analysis but not by manual enumeration is shown in brackets.
| Marker | Score | 0 | 1 | 2+3 | Total | P Value |
|---|---|---|---|---|---|---|
| pAkt | Histologic Grade | |||||
| Low Grade 1–2 | 38 (63%) | 17 (28%) | 5 (8%) | 60 | ||
| High Grade 3 | 72 (65%) | 33 (30%) | 5 (5%) | 110 | P = .603 | |
| Stage | ||||||
| Stage Tis, Ta-T1 | 49 (59%) | 29 (35%) | 5 (6%) | 83 | P = .400 | |
| Stage T2–T4 | 46 (70%) | 17 (26%) | 3 (5%) | 66 | ||
| Growth Pattern | ||||||
| Papillary | 54 (59%) | 32 (35%) | 5 (6%) | 91 | P = .209 [P = .001] | |
| Nonpapillary | 56 (71%) | 18 (23%) | 5(6%) | 79 | ||
|
| ||||||
| PTEN | Histologic Grade | |||||
| Low Grade 1–2 | 4 (7%) | 5 (8%) | 50 (85%) | 59 | P = .0002 | |
| High Grade 3 | 6 (6%) | 42 (39%) | 60 (55%) | 108 | ||
| Stage | ||||||
| Stage Tis, Ta-T1 | 4 (5%) | 12 (15%) | 66 (80%) | 82 | P = .0003 | |
| Stage T2–T4 | 6 (9%) | 27 (42%) | 32 (49%) | 65 | ||
| Growth Pattern | ||||||
| Papillary | 4 (4%) | 14 (16%) | 72 (80%) | 90 | P = .0001 | |
| Nonpapillary | 6 (8%) | 33 (43%) | 38 (49%) | 77 | ||
|
| ||||||
| Drg-1 | Histologic Grade | |||||
| Low Grade 1–2 | 7 (12%) | 22 (39%) | 28 (49%) | 57 | P = .216 | |
| High Grade 3 | 20 (19%) | 28 (26%) | 59 (55%) | 107 | ||
| Stage | ||||||
| Stage Tis, Ta-T1 | 9 (11%) | 26 (33%) | 45 (56%) | 80 | P = .688 | |
| Stage T2–T4 | 10 (16%) | 18 (29%) | 35 (55%) | 63 | ||
| Growth Pattern | ||||||
| Papillary | 11 (13%) | 28 (32%) | 48 (55%) | 87 | P = .373 | |
| Nonpapillary | 16 (21%) | 22 (29%) | 39(50%) | 77 | ||
|
| ||||||
| Cx-26 | Histologic Grade | |||||
| Low Grade 1–2 | 11(19%) | 19 (32%) | 29 (49%) | 59 | P =.0002 | |
| High Grade 3 | 2(2%) | 29 (27%) | 78 (71%) | 109 | ||
| Stage | ||||||
| Stage Tis, Ta-T1 | 12 (15%) | 25 (30%) | 45 (55%) | 82 | P = .008 | |
| Stage T2–T4 | 1 (2%) | 16 (25%) | 48 (73%) | 65 | ||
| Growth Pattern | ||||||
| Papillary | 13(14%) | 27(30%) | 50 (56%) | 90 | P = .001 | |
| Nonpapillary | 0(0%) | 21(27%) | 57(73%) | 78 | ||
|
| ||||||
| L-plastin | Histologic Grade | |||||
| Low Grade 1–2 | 8 (14%) | 24(42%) | 25 (44%) | 57 | ||
| High Grade 3 | 28 (26%) | 50(48%) | 27 (26%) | 105 | P = .035 | |
| Stage | ||||||
| Stage Tis, Ta-T1 | 13 (16%) | 37 (46%) | 30 (38%) | 80 | P = .239 [P < .001] | |
| Stage T2–T4 | 17(27%) | 27 (44%) | 18 (29%) | 62 | ||
| Growth Pattern | P = .047 | |||||
| Papillary | 15 (17%) | 38 (43%) | 35 (40%) | 88 | ||
| Nonpapillary | 21 (28%) | 36 (49%) | 17 (23%) | 74 | ||
The correlations of the expression of these markers based on manual analysis are illustrated in Table 2. P values with statistical significance identified by automated analysis but not by manual numeration is shown in bracketsr. We identified positive correlations in the expression of pAkt and L-plastin (Spearman’s correlation coefficient = .245, P = .002) and pAkt and PTEN (Spearman’s correlation coefficient = .380, P < .0001). We also identified positive correlations in the expression of PTEN and L-plastin (Spearman’s correlation coefficient = .395, P < .0001) and between Drg-1 and L-plastin (Spearman’s correlation coefficient = .158, P < .047). The only difference found between manual and automated analysis was among the correlation of Drg-1 with pAkt (P = .002). None of these markers were found to be independent predictors of Overall Survival or Progression-Free Survival by either analysis (data not shown).
Table 2.
Spearman’s rank correlation between markers. P values with statistical significance in automated analysis but not by manual enumeration is shown in brackets.
| pAkt | PTEN | L-plastin | Drg-1 | |
|---|---|---|---|---|
| pAkt | .380 (P < .0001) | .245 (P = .002) | .146 (P = .063) [P = .002] | |
| Cx-26 | .094 (P = .227) | .011 (P = .884) | −.003 (P = .967) | −.029 (P = .710) |
| Drg-1 | .111 (P = .160) | .158 (P = .047) | ||
| PTEN | .395 (P < .0001) |
DISCUSSION
While data suggest pAkt, PTEN, Cx-26, Drg-1, and L-plastin appear to be important in some cancers [1–5], there is limited information about their roles in bladder cancer. We found the associations between the expression of PTEN, Cx-26, and L-plastin with known clinically important pathologic features of bladder cancer (tumor grade, stage, and growth pattern) to be statistically significant both by automated and manual methods. There were correlations found among the expression of pAkt, PTEN and L-plastin. In addition, we observed aberrant localization patterns of Drg-1 and Cx-26 in some bladder tumors.
There were some differences between the automated and manual analysis of pAkt, PTEN, Drg-1, Cx-26, and L-plastin in bladder cancer. The automated assessment found significance between pAkt and growth pattern and L-plastin and stage not identified through manual analysis. There was also a positive correlation of pAkt and Drg-1 observed by automated but not manual analysis. The variations in data between the automated and manual analysis is due to differences in scoring criteria. Although the automated method reduced variability among scoring, it also obscured specimens with heterogeneous or weak nuclear staining. For example, in some tissues, pAkt expression was scored as negative (0) by automated analysis, but manually the same tissues were scored as weakly stained (1). Overall, the results from both analyses were similar.
The PI3K/Akt signaling pathway is important in several cancers. Immunohistochemical studies have shown that the expression of pAkt, an oncogene, is increased in advanced human prostate, breast, and ovarian cancers [6–8]. Wu, et al. found high pAkt expression in about half of 20 bladder cancer specimens by western analysis [11]. In that study similar pAkt expression was found in both low grade and high grade tumors. Our IHC analysis also found the expression pattern of pAkt to be similar in both low and high grade tumors. However, the expression of pAkt was heterogeneous and sometimes nuclear in bladder cancer specimens. PTEN has been shown to be mutated or deleted in some invasive bladder cancers [11]. Furthermore, a decrease in PTEN expression has been found in approximately 10% of superficial and invasive bladder carcinomas [23]. We found that PTEN expression was highest in low grade, superficial, and papillary tumors, supporting the role of PTEN as a tumor suppressor in bladder cancer. Our analysis of PTEN expression in normal urothelium was limited to the cytoplasm, but some bladder cancers exhibited both cytoplasmic and nuclear PTEN expression. A nuclear pattern of PTEN staining is thought to be related to carcinogenesis [24]. Recently data has shown that hyperplastic urothelium and papillary bladder tumors from mice exhibited both pAkt and PTEN expression [25]. Despite the expression of PTEN, protein function was inactivated by hyperphosphorylation. This finding provides a possible explanation for the positive correlation we identified between pAkt and PTEN.
Drg-1 is a downstream target of PTEN and is Akt-dependent [1]. Recent data suggest that Drg-1 may suppress metastasis in prostate cancer [1]. Neither analysis performed identified a significant correlation of decreased Drg-1 expression with high-grade or invasive bladder cancer. The expression of Drg-1 was mostly cytoplasmic with some membranous localization noted in 20% of the tumors. We did not observe membranous staining for Drg-1 in the normal tissues. The significance of this finding remains a matter for future studies.
Loss of Cx-26 has been reported in breast cancer and other tumors [26–29] and is thought to play a role in tumorigenesis. In vitro, Cx-26 is expressed in normal urothelial cells but is lost in some human bladder cancer cell lines [18]. Gene expression analysis of melanoma cell lines found Cx-26 mRNA expression upregulated in metastatic and invasive cell lines [30]. In our IHC analysis, Cx-26 expression was highest in high grade, invasive, and nonpapillary tumors with an altered expression pattern. Kannczuga-Koda, et al. found aberrant expression of Cx-26 in colorectal cancers by IHC [31]. They found punctate staining of Cx-26 in normal colon mucosa and heterogeneous cytoplasmic staining in colon carcinomas. We observed homogeneous Cx-26 expression in normal urothelium with some punctate staining. Cx-26 exhibited diffuse cytoplasmic staining with some heterogeneity in bladder tumors. The aberrant localization of Cx-26 may contribute to tumorigenesis through the loss of intercellular communication via gap junctions. Our TMA analysis demonstrated that aberrant Cx-26 overexpression is significantly associated with adverse clinically important pathologic features.
L-plastin is an actin-binding protein that was initially found in mature normal leukocytes. Western blot and reverse transcription-polymerase chain reaction analyses have demonstrated L-plastin expression in more than 90% of epithelial neoplastic cells but not in normal epithelial cells [32]. This protein is also expressed in breast myoepithelial cells [3]. We found L-plastin expression in normal and malignant urothelium. L-plastin staining was significantly associated with tumor grade and tumor growth. However, similar to an observation made with breast cancer tissues[3], we did not find L-plastin to be an independent predictor of Patient survival.
Our findings show that expression of PTEN, Cx-26, and L-plastin are significantly associated with the clinicopathologic characteristics of bladder cancer (tumor grade, stage, and growth pattern) but are not significantly related to patient survival. The expression of pAkt, PTEN and L-plastin positively correlate in bladder cancer. In addition, we observed aberrant localization patterns of Cx-26 and Drg-1 in bladder tumors. The roles of these proteins in bladder cancer biology remain to be elucidated.
Acknowledgments
This work was supported in part by NCI grant CA91846 and NHI grant CA016672. The authors would like to thank Delores Richards, Leslie Nesbitt, Carol Johnston, and Jolanta Bondaruk for their help with this project.
Abbreviations
- GJIC
gap junction intercellular communication
- IHC
immunohistochemical
- Cx-26
Connexin-26
- PBS
phosphate-buffered saline
- PI3K
phosphatidylinositol-3-kinase
- TMA
tissue microarray
- pAkt
phosphorylated Akt
- Drg-1
differentiation related gene 1
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
References
- 1.Bandyopadhyay S, Pai SK, Hirota S, et al. PTEN up-regulates the tumor metastasis suppressor gene Drg-1 in prostate and breast cancer. Cancer Res. 2004;64(21):7655–7660. doi: 10.1158/0008-5472.CAN-04-1623. [DOI] [PubMed] [Google Scholar]
- 2.Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16(8):797–803. doi: 10.1097/01.cad.0000173476.67239.3b. [DOI] [PubMed] [Google Scholar]
- 3.Lapillonne A, Coue O, Friederich E, et al. Expression patterns of L-plastin isoform in normal and carcinomatous breast tissues. Anticancer Res. 2000;20(5A):3177–3182. [PubMed] [Google Scholar]
- 4.Otsuka M, Kato M, Yoshikawa T, et al. Differential expression of the L-plastin gene in human colorectal cancer progression and metastasis. Biochem Biophys Res Commun. 2001;289(4):876–881. doi: 10.1006/bbrc.2001.6047. [DOI] [PubMed] [Google Scholar]
- 5.Sheen IS, Jeng KS, Wang PC, et al. Are gap junction gene connexins 26, 32 and 43 of prognostic values in hepatocellular carcinoma? A prospective study. World J Gastroenterol. 2004;10(19):2785–2790. doi: 10.3748/wjg.v10.i19.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51(2):181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Bose S, Chandran S, Mirocha JM, Bose N. The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol. 2006;19(2):238–245. doi: 10.1038/modpathol.3800525. [DOI] [PubMed] [Google Scholar]
- 8.Slipicevic A, Holm R, Nguyen MT, Bohler PJ, Davidson B, Florenes VA. Expression of activated Akt and PTEN in malignant melanomas: relationship with clinical outcome. Am J Clin Pathol. 2005;124(4):528–536. doi: 10.1309/YT58WWMTA6YR1PRV. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Koul D, Davies MA, Liebert M, Steck PA, Grossman HB. MMAC1/PTEN inhibits cell growth and induces chemosensitivity to doxorubicin in human bladder cancer cells. Oncogene. 2000;19(47):5406–5412. doi: 10.1038/sj.onc.1203918. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez-Escrig JL, Kelly JD, Neal DE, King SM, Davies BR. Evaluation of the therapeutic potential of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in preclinical models of bladder cancer. Clin Cancer Res. 2004;10(14):4874–4884. doi: 10.1158/1078-0432.CCR-04-0034. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Obata T, Khan Q, Highshaw RA, de Vere White R, Sweeney C. The phosphatidylinositol-3 kinase pathway regulates bladder cancer cell invasion. BJU International. 2004;93(1):143–150. doi: 10.1111/j.1464-410x.2004.04574.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Grossman HB. In vivo gene therapy of human bladder cancer with PTEN suppresses tumor growth, downregulates phosphorylated Akt, and increases sensitivity to doxorubicin. Gene Ther. 2003;10(19):1636–1642. doi: 10.1038/sj.gt.3302056. [DOI] [PubMed] [Google Scholar]
- 13.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22(14):2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 14.Ulrix W, Swinnen JV, Heyns W, Verhoeven G. The differentiation-related gene 1, Drg1, is markedly upregulated by androgens in LNCaP prostatic adenocarcinoma cells. FEBS Lett. 1999;455(1–2):23–26. doi: 10.1016/s0014-5793(99)00845-5. [DOI] [PubMed] [Google Scholar]
- 15.Oyamada Y, Oyamada M, Fusco A, Yamasaki H. Aberrant expression, function and localization of connexins in human esophageal carcinoma cell lines with different degrees of tumorigenicity. J Cancer Res Clin Oncol. 1994;120(8):445–453. doi: 10.1007/BF01191797. [DOI] [PubMed] [Google Scholar]
- 16.Terzaghi-Howe M, Chang GW, Popp D. Emergence of undifferentiated rat tracheal cell carcinomas, but not squamous cell carcinomas, is associated with a loss of expression of E-cadherin and of gap junction communication. Carcinogenesis. 1997;18(11):2043–2050. doi: 10.1093/carcin/18.11.2043. [DOI] [PubMed] [Google Scholar]
- 17.GEE JT, MOTOYOSHI, GROSSMAN H, BARTON* Connexin 26 is Abnormally Expressed in Bladder Cancer. Journal of Urology. 2003;169(3):1135–1137. doi: 10.1097/01.ju.0000041954.91331.df. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Grossman HB. Connexin 26 gene therapy of human bladder cancer: induction of growth suppression, apoptosis, and synergy with Cisplatin. Hum Gene Ther. 2001;12(18):2225–2236. doi: 10.1089/10430340152710568. [DOI] [PubMed] [Google Scholar]
- 19.Lin CS, Lau A, Yeh CC, Chang CH, Lue TF. Upregulation of L-plastin gene by testosterone in breast and prostate cancer cells: identification of three cooperative androgen receptor-binding sequences. DNA Cell Biol. 2000;19(1):1–7. doi: 10.1089/104454900314654. [DOI] [PubMed] [Google Scholar]
- 20.Zheng J, Rudra-Ganguly N, Miller GJ, Moffatt KA, Cote RJ, Roy-Burman P. Steroid hormone induction and expression patterns of L-plastin in normal and carcinomatous prostate tissues. Am J Pathol. 1997;150(6):2009–2018. [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Tuziak T, Hu L, et al. Alterations in transcription clusters underlie development of bladder cancer along papillary and nonpapillary pathways. Lab Invest. 2005;85(4):532–549. doi: 10.1038/labinvest.3700250. [DOI] [PubMed] [Google Scholar]
- 22.Rosen DG, Wang L, Atkinson JN, et al. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005;99(2):267–277. doi: 10.1016/j.ygyno.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 23.Koksal IT, Yasar D, Dirice E, et al. Differential PTEN Protein Expression Profiles in Superficial versus Invasive Bladder Cancers. Urologia Internationalis. 2005;75(2):102–106. doi: 10.1159/000085933. [DOI] [PubMed] [Google Scholar]
- 24.Chung JH, Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65(18):8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 25.Mo L, Zheng X, Huang HY, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. J Clin Invest. 2007;117(2):314–325. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li GY, Lin HH, Tu ZJ, Kiang DT. Gap junction Cx26 gene modulation by phorbol esters in benign and malignant human mammary cells. Gene. 1998;209(1–2):139–147. doi: 10.1016/s0378-1119(98)00039-0. [DOI] [PubMed] [Google Scholar]
- 27.Muramatsu A, Iwai M, Morikawa T, et al. Influence of transfection with connexin 26 gene on malignant potential of human hepatoma cells. Carcinogenesis. 2002;23(2):351–358. doi: 10.1093/carcin/23.2.351. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Grossman HB. Connexin 26 induces growth suppression, apoptosis and increased efficacy of doxorubicin in prostate cancer cells. Oncol Rep. 2004;11(2):537–541. [PubMed] [Google Scholar]
- 29.Ito A, Koma Y, Uchino K, et al. Increased expression of connexin 26 in the invasive component of lung squamous cell carcinoma: significant correlation with poor prognosis. Cancer Lett. 2006;234(2):239–248. doi: 10.1016/j.canlet.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 30.Ito A, Watabe K, Koma Y, Kitamura Y. An attempt to isolate genes responsible for spontaneous and experimental metastasis in the mouse model. Histol Histopathol. 2002;17(3):951–959. doi: 10.14670/HH-17.951. [DOI] [PubMed] [Google Scholar]
- 31.Kanczuga-Koda L, Sulkowski S, Koda M, Sulkowska M. Alterations in connexin26 expression during colorectal carcinogenesis. Oncology. 2005;68(2–3):217–222. doi: 10.1159/000086777. [DOI] [PubMed] [Google Scholar]
- 32.Park T, Chen ZP, Leavitt J. Activation of the leukocyte plastin gene occurs in most human cancer cells. Cancer Res. 1994;54(7):1775–1781. [PubMed] [Google Scholar]