Introduction
Osteoarthritis (OA) is the most common degenerative joint disease affecting articular cartilage and other joint tissues, resulting in serious morbidity and large socioeconomic impact (1–3). In the US, 27 million people experienced OA in 2005 (4). The total cost attributed to arthritis and other rheumatic conditions in the US was 128 billion dollars in 2003 (5,6). Due to an aging population and increasing obesity rates, OA will become even more prevalent in the next decades with an estimated 67 million Americans expected to be diagnosed with arthritis by 2030 (7). The final treatment option for OA is joint replacement, which is associated with complications, limited durability, and contributes to the high costs of OA.
Consequently, current OA research focuses on strategies that may be disease modifying, such as disease-modifying osteoarthritis drugs, chondrocyte implantation, stem cell therapy, or high tibial osteotomy (8–10). Attention is also focused on measures that may prevent or delay OA onset, including lifestyle interventions. These strategies all target OA at an early stage when they are most effective, thereby avoiding or delaying joint replacement.
In order for therapeutic approaches to OA to be successful, monitoring of their effectiveness with accurate, sensitive, and objective imaging techniques is essential. Clinical OA measurements, such as pain, remain important, but are subjective and correlate poorly with disease severity (11,12). Radiography is used to stage and follow OA, but it cannot depict articular cartilage directly and correlates weakly with cartilage damage on arthroscopy (13,14). Furthermore, radiography is incapable of detecting early OA and subtle OA changes (15,16), and often does not correlate with OA symptoms (17,18).
Magnetic resonance imaging (MRI) is useful in OA because cartilage and other joint structures can be assessed for morphologic changes more reliably than on radiography (19,20). Several semiquantitative MRI scoring systems for OA have been developed, such as the Knee Osteoarthritis Scoring System (21), the Whole-Organ Magnetic Resonance Imaging Score (22), the Boston Leeds Osteoarthritis Knee Score (23), and the MRI Osteoarthritis Knee Score (24,25). Cartilage segmentation followed by thickness or volume measurements increase sensitivity further (26,27). Morphologic changes, however, are not always present in early-stage OA and may not change significantly with disease progression. In addition, conflicting reports have been published on the association between cartilage defects on morphologic MRI and pain or other OA symptoms (28,29). Therefore, MRI methods that rely on morphology may fail to capture early OA onset or subtle changes in disease severity (30). Because of the limitations of radiography and traditional MRI methods, there is need for new imaging modalities to not only advance our understanding of the pathogenesis of OA, but to provide novel end points to accelerate development of therapies. In the future, use of imaging to identify asymptomatic individuals in a “pre-OA” state may identify individuals for whom therapeutic intervention could prevent the subsequent development of OA.
Novel imaging techniques that enable measurement of the biochemical composition of cartilage rather than its morphology show promise to fulfill this need (31,32). These techniques offer numerical outcome measures that can be used as imaging biomarkers for cartilage quality in research on OA and other joint diseases. In addition, they may enhance understanding of the pathogenesis of OA. A recent report suggests that quantitative MRI may correlate better with knee pain in early OA (33). The majority of quantitative imaging techniques for cartilage composition are MRI-based, but recently computed tomography (CT) arthrography was also shown to be a suitable imaging modality for this purpose (34). In addition, quantitative imaging techniques are also applied to other tissues, such as menisci (35–37) and intervertebral discs (38,39). Most research, however, has been performed on articular cartilage of human knee joints.
This article presents the principles of quantitative imaging techniques for cartilage composition of the human knee, followed by a description of the most commonly used techniques, their advantages and limitations, as well as reported applications.
Principles of quantitative cartilage imaging
Hyaline articular cartilage is largely acellular as chondrocytes constitute only 4% of its wet weight (40). The main components of hyaline cartilage are water (65–85%), the extracellular matrix consisting of type II collagen (15–20%), and proteoglycans (PGs) (3–10%) (41,42) (Figure 1A). Hyaline cartilage can be subdivided in 3 layers: the superficial, middle, and deep layer. In healthy cartilage, PG density and the orientation of the collagen fibers vary by location within the cartilage layer and regionally within the joint (41,43–45) (Figure 1A).
Figure 1.
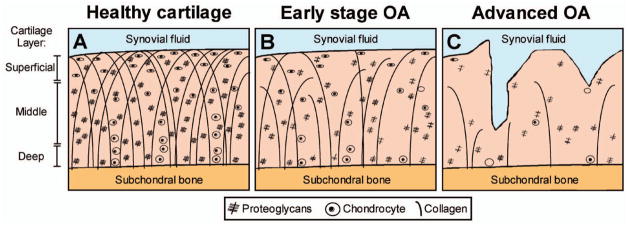
Schematic representation of articular cartilage composition and morphology in healthy (A), early-stage osteoarthritic (B), and advanced-stage osteoarthritic cartilage (C). In healthy cartilage (A), the orientation and density of the collagen fibers varies by location within the cartilage layer and regionally within the joint. Relative to the articular surface, their prevailing orientation is parallel in the superficial layer, oblique in the transitional (middle) layer, and perpendicular in the deep radial zone. Similarly, the concentration of proteoglycans varies according to location and is highest in the middle layer. In early osteoarthritis (OA) (B), proteoglycans and glycosaminoglycans leak from the cartilage and the collagen fibers change in size and orientation. These initial disease processes occur without macroscopic alterations in cartilage morphology. When OA progresses (C), morphologic changes (thinning and defects) of the cartilage appear. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/acr.22316/abstract.
PGs mainly consist of glycosaminoglycans (GAGs) that are negatively charged due to ionized sulfate and carboxyl groups. These strong negative electrostatic charges, collectively responsible for the so-called fixed-charge density, are important contributors to the structure and biomechanical properties of articular cartilage (40). They allow GAG molecules to be fixed to the extracellular matrix and attract positive ions that attract water molecules, resulting in a swelling pressure of cartilage. This tendency to expand is counteracted by the surrounding collagen mesh-work and this balance between swelling pressure and collagen tension contributes to the tremendous tensile and compressive strength of hyaline cartilage under normal physiologic conditions (40–42,46).
It has been shown that in the early stage of OA and other hyaline cartilage diseases, PGs and GAGs leak from the cartilage and the collagen fibers change in size and orientation, allowing more water and less restricted water diffusion into the cartilage. These initial disease processes occur without macroscopic alterations in cartilage morphology (31,32) (Figure 1B). When cartilage disease progresses, morphologic changes (thinning and defects) of the cartilage appear (Figure 1C). Recently, MRI techniques have been introduced that are capable of measuring changes in cartilage composition before morphologic changes occur. These techniques all provide quantitative measures that correlate with collagen and PG content. Some techniques are believed to specifically correlate with the GAG component of PG, whereas other techniques correlate with water content and with collagen content and orientation. Several MRI methods have been proposed, each providing different outcome measures that can be used as imaging biomarkers.
The quantitative MRI techniques for cartilage composition require postprocessing algorithms to derive the outcome measure in the specific cartilage region of interest. Consequently, the underlying mathematic modeling may impact the quality and reliability of the biomarker. For example, it has been demonstrated that calculation of T1 relaxation times in quantitative cartilage MRI is improved by applying an automated registration algorithm (47,48).
In addition to the various MRI techniques, it has been shown that CT provides a quantitative outcome measure for cartilage biochemical status (34). Specific features and selected applications of quantitative MRI and CT techniques for cartilage composition are discussed below. Outcome measures, clinically relevant biochemical correlates, reported typical outcome values for healthy and OA-diseased cartilage, and advantages and disadvantages of each technique are summarized in Table 1.
Table 1.
Outcome measures, clinically relevant biochemical correlates, reported ranges for healthy and OA-diseased cartilage in literature, and advantages and disadvantages per quantitative imaging technique*
| Technique | Clinically relevant biochemical correlate | Outcome measure | Reported outcome range†
|
References, reported outcome range | Advantage | Disadvantage | |
|---|---|---|---|---|---|---|---|
| Healthy cartilage | OA cartilage‡ | ||||||
| dGEMRIC§ | GAG | T1 relaxation time | 600–800 ms | 300–600 ms | 52, 57, 59, 64, 169, 172 | Currently best validated indirect GAG measurement | Use of contrast agent with associated risks Long examination time |
| T2 mapping | Collagen content and orientation | T2* relaxation time | 30–45 ms | 40–60 ms | 92, 98, 99, 105, 173–175 | Correlation with collagen content and orientation without need for exogenous contrast agent | May possibly detect degenerative cartilage changes at a later stage than GAG specific techniques Magic angle effect |
| T1rho mapping | PG/GAG | T1rho relaxation time | 30–50 ms | 40–80 ms | 92, 98, 99, 174, 176 | Advocated to be sensitive to PG depletion without need for exogenous contrast agent | Exact biochemical correlate still controversial High RF power required, thus limited by SAR |
| Ultrashort TE | Collagen content and orientation | T2* relaxation time | 20–30 ms | NR | 148, 177 | Enables visualization of deep cartilage layers, osteochondral junction, and subchondral bone | Long scan times Several technical MRI challenges |
| gagCEST# | GAG | CEST asymmetry | 4–15% | NR | 152–154 | Direct measure of GAG content without need for exogenous contrast agent | Technically complex Requires high magnetic field strength Sophisticated postprocessing tools required |
| Sodium MRI¶ | GAG | Sodium (23NA) signal intensity | 10–35 | NR | 152, 154, 162, 178 | Strong correlation with GAG content without need for exogenous contrast agent | Demanding MRI hardware requirements: (ultra) high field scanners, special RF coils Limited spatial resolution Long examination time |
| CT arthrography | GAG | X-ray attenuation | NR | NR | NA | Enables quantitative cartilage imaging in subjects with MRI contra-indications Short examination time (seconds instead of minutes) Detailed information on subchondral bone |
Use of intraarticular contrast with associated risks Use of ionizing radiation Limited information on other soft tissues |
OA = osteoarthritis; dGEMRIC = delayed gadolinium-enhanced magnetic resonance imaging (MRI) of cartilage; GAG = glycosaminoglycan; PG = proteoglycan; RF = radio frequency; SAR = specific absorption rate; TE = time to echo; NR = range not reported for in vivo human knee cartilage in the published literature; gagCEST = GAG-specific chemical exchange saturation transfer; CT = computed tomography; NA = not applicable.
Ranges are reported for human in vivo (patellar, femoral, or tibial plateau) cartilage of the knee examined with 3.0T MRI equipment unless indicated otherwise.
All stages of OA according to Kellgren and Lawrence grading (179) were included in the OA cartilage outcome range.
Reported dGEMRIC outcome range for double dose (0.2 mmole/kg) intravenous contrast agent administration.
Reported outcome range acquired at 7.0T instead of 3.0T.
Quantitative MRI techniques for cartilage composition
Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC)
The dGEMRIC mechanism makes use of the repulsive force between a negatively charged contrast agent (gadopentate dimeglumine) and negative charges on the GAGs, resulting in the contrast agent accumulating in cartilage inversely with GAG content (49). The outcome parameter is T1 relaxation time (usually averaged for the pixels in a region of interest: the dGEMRIC index). T1 relaxation time is reduced by the contrast agent and consequently is lower in areas with decreased GAG content compared to healthy cartilage (Figure 2A). The contrast agent is commonly administered intravenously using a double dose (0.2 mmole/kg [50]), after which the joint is exercised to promote contrast distribution into the synovium and joint cavity. For knees, a 90-minute delay between contrast injection and image acquisition is usually applied to achieve a semi-equilibrium state between contrast agent and cartilage (51). It has been reported that precontrast imaging is unnecessary (52,53). Variations on the dGEMRIC protocol have been reported, with the contrast injected intraarticularly (54,55), the contrast agent dose changed (50,56), and the length of delay optimized for other joints (56). Image acquisition is aimed at calculating the dGEMRIC index in the cartilage, and several MRI pulse sequences can be applied (57–59) with variable flip angles (60) or inversion times (57). Recently, time-efficient 3-dimensional (3-D) pulse sequences were applied that allowed coverage of the entire joint (57–59).
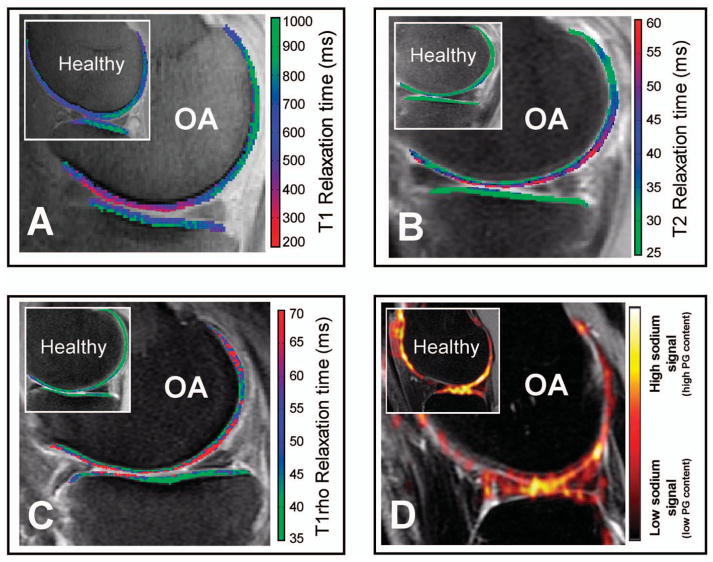
Figure 2.
Delayed gadolinium-enhanced magnetic resonance imaging (MRI) of cartilage (dGEMRIC), T2 mapping, T1rho mapping, and sodium imaging differentiate between healthy and early-stage knee osteoarthritis (OA). Sagittal slices through the center of the medial or lateral tibiofemoral compartment of knee OA patients and healthy volunteers (insets) acquired using different quantitative MRI techniques (all images acquired in different subjects). None of the early OA patients showed clear abnormalities on the conventional MRI sequences (not shown). A, dGEMRIC color map shows a clear decrease in T1 relaxation times (purple/red) in early OA, representing loss of glycosaminoglycans. B, T2 mapping demonstrates increased T2 relaxation times (blue/purple/red) in early OA due to disorganization of the collagen matrix and increase in water content. C, T1rho mapping shows increased T1rho relaxation times (red) in a patient with moderate knee OA. D, Sodium MRI detects less sodium signal in OA compared to healthy knee cartilage (inset). PG = proteoglycan. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/acr.22316/abstract.
dGEMRIC has been validated largely with in vitro experiments, demonstrating a good correlation with GAG content. In vivo validation studies of dGEMRIC, however, are lacking, except for one study by Watanabe et al in which the difference between T1 relaxation time before and after contrast administration was found to correlate with GAG content of biopsied cartilage determined by liquid chromatography in 9 patients after autologous chondrocyte implantation (ACI) (61). When performed according to a standardized protocol, reproducibility of dGEMRIC was found to be good in several studies in healthy and OA subjects (62–64). It has been shown recently that dGEMRIC is associated with change in cartilage thickness over time (65).
dGEMRIC has been applied in studies on the influence of anterior cruciate ligament (ACL) tear (66,67), femoroacetabular impingement (68), and developmental dysplasia of the hip (69,70) on cartilage quality and OA pathogenesis. It was also used to study the OA disease-modifying potential of oral (71) and intraarticular medication (72), high tibial osteotomy (73), and weight reduction (74), and to followup cartilage repair with (matrix-associated) ACI in the knee (75) and ankle (76). In the field of rheumatoid arthritis, dGEMRIC has been used to visualize early cartilage damage in finger joints (77) and to assess the therapeutic effect of tumor necrosis factor inhibitors (78).
dGEMRIC is regarded as the best available imaging tool for indirect GAG measurement in vivo. There are, however, disadvantages of dGEMRIC mainly related to the contrast agent that increases costs and is potentially dangerous for patients with impaired renal function. Total examination time is extremely long due to the required delay between contrast administration and image acquisition (Table 1). Finally, rate and degree of contrast accumulation in cartilage may be influenced by factors other than cartilage GAG content, such as collagen content and orientation (79,80), pharmacokinetics (81), and type and duration of the exercise (56,82).
T2 mapping
T2 mapping is regarded as the best method to measure collagen content (measured by signal intensity) and orientation (expressed by anisotropy) (83,84). In healthy cartilage the collagen network exhibits low signal intensity on T2-weighted MRI. In the earliest stages of cartilage disease, damage occurs to the cartilage matrix, causing loss of collagen content as well as a disorganization in its orientation, reflected in elevated T2 relaxation times (85) (Figure 2B).
Image acquisition in T2 mapping is usually performed using multiple spin-echo sequences at different echo times (83). Several recent alternatives have been proposed that yield full joint coverage within a relatively short acquisition time (86–89). In addition, double-echo steady-state was recently introduced as a time-efficient 3-D T2 mapping sequence with simultaneous acquisition of apparent diffusion coefficient values in cartilage that provide a measure of water diffusion (90,91).
In a recent multicenter multivendor study by Mosher et al, reproducibility of T2 mapping was found to be moderate to high in OA patients and normal controls (92). Previous validation studies of T2 mapping in vitro (84,93,94) and in vivo (85,95,96) have demonstrated that T2 mapping is a reliable method for quantification of early changes in collagen content and orientation. Although many authors have suggested T2 mapping to specifically correlate with collagen (83), some studies have also found a relationship with PG content (97–99).
T2 mapping has been used frequently as an outcome measure in clinical studies. Examples in the knee include T2 mapping to assess cartilage quality after ACL reconstruction (100), and cartilage repair procedures such as ACI (101) and microfracture (102,103). Abnormal T2 relaxation times have been reported to correlate with knee malalignment (104), meniscal pathology (105), and progression of knee OA (106). In the hip, T2 mapping was shown to be an indicator of early cartilage degeneration in patients with hip dysplasia (107) and slipped capital femoral epiphysis (108), whereas in the ankle it was mainly used to followup cartilage repair (109–111). T2 mapping is also incorporated as an imaging biomarker in large-scale epidemiologic studies on knee OA, such as the Osteoarthritis Initiative (33,112,113) and followup measurements of the Rotterdam Study (114,115).
T2 mapping shows high correlation with collagen content and orientation without the need for contrast administration (Table 1). Therefore, it provides information on cartilage quality that may be complementary to GAG-specific techniques. Drawbacks include magic angle effects (116) that may render T2 relaxation times inaccurate in certain areas of the joint (117). Another potential disadvantage is the fact that PG depletion has been suggested to occur earlier in the OA disease process than collagen loss by several reports (118–120). Therefore, T2 mapping may possibly detect cartilage changes later than GAG-specific techniques. It has been suggested that isolated PG depletion from cartilage may be reversible and more amenable to repair than cartilage with a structurally damaged collagen matrix (83).
T1rho mapping
T1rho mapping, also described as relaxation in the rotating frame, uses a constant radio frequency field referred to as a “spin lock” pulse to change relaxation rates of water associated with large macromolecules in cartilage. As T1rho relaxation time is sensitive to protons associated with PG and GAG, several authors have suggested a direct relationship between T1rho relaxation time and PG/GAG concentration (121). Decreased GAG content may lead to increased mobile proton density in bulk water and increased T1rho relaxation times (Figure 2C).
Reproducibility of T1rho mapping was found to be moderate for the femorotibial joint, but substantially better for patellar cartilage regardless of OA stage (92,122). The inverse relationship between T1rho relaxation time and PG/GAG content was found in several in vitro bovine cartilage experiments (123,124) and ex vivo human cartilage specimen validation studies (97,125), but the strength of the reported correlations varies (97,123,126,127). Several recent in vivo studies have also reported inconsistent correlation coefficients (range 0.2–0.61) for the relationship between T1rho relaxation times and GAG measurements (98,99,128). These differences between in vitro and ex vivo results compared to the in vivo results may be caused by the differences between preclinical and clinical T1rho sequences used for these studies (129).
Applied in clinical knee OA research, elevated T1rho relaxation times were found to correlate with age (130) and clinical OA scores (131), as well as predicted OA progression after 2 years followup (106). After ACL tears, T1rho relaxation times in femorotibial cartilage were found to be significantly increased generally (67) and in specific areas overlying bone marrow edema–like lesions (132), as well as in patients following ACL reconstruction (133). T1rho was also shown to be feasible for monitoring of cartilage repair procedures such as microfracture (102) and mosaicplasty (134). In several studies by Souza and colleagues, T1rho was sensitive to alterations in knee cartilage composition due to various loading conditions, such as jumping tasks (135), acute loading (136), and unloading (137). T1rho relaxation times were found to be decreased immediately following 30 minutes of treadmill running (138), and elevated 48 hours and 3 months after marathon running (139). Outside the knee, T1rho was able to detect biochemical differences in acetabular cartilage between normal and cam-type femoroacetabular impingement hips (140).
Although the exact biochemical correlate in terms of cartilage biochemical composition is still debated, T1rho has been advocated as a method to noninvasively measure cartilage GAG content without a contrast agent (Table 1). A disadvantage of T1rho is the large radio frequency power applied that may cause tissue heating at higher field strengths (122,141).
Ultrashort echo time (UTE)
Important musculoskeletal tissues such as cortical bone, tendons, ligaments, menisci, and the deepest layers of articular cartilage have short intrinsic T2/T2* relaxation times and produce little or no signal on most conventional T2-weighted sequences (142). UTE MRI applies 20–50-fold shorter TEs so that signals from short T2/T2* relaxation time tissues can be detected (143,144). This enables visualization of the deepest cartilage layers, including the calcified zone (Figure 3). Furthermore, UTE offers the possibility to study the osteochondral junction and subchondral bone structure that both are believed to play a role in the pathogenesis of OA and cartilage damage (145). Novel UTE sequences also enable T1 and T1rho measurements in deep cartilage layers (146) or yield constant signal intensity data sets (147). Reproducibility of UTE T2* mapping of human articular cartilage in vivo was reported as good in a study of 11 asymptomatic subjects (148).
Figure 3.
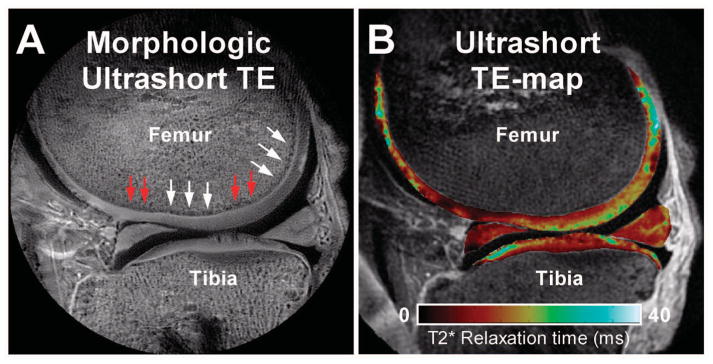
Morphologic and quantitative ultrashort echo time (TE) images of the knee. Ultrashort TE morphologic (A) and quantitative T2* (B) magnetic resonance images of a cadaveric knee (male donor, age 77 years). Note that the deepest layer of articular cartilage is clearly visible as a line of high signal intensity (A, white arrows), along with focal areas of diminished signal intensity (A, red arrows) that may suggest abnormality of the deep region. (Courtesy of Christine Chung, University of California, San Diego).
Although UTE is a promising method that offers assessment of the entire cartilage layer, the technology is still evolving and there have been no reports on its application as an outcome measure in clinical studies. Disadvantages include lengthened scan times and several other challenges related to MRI technique and physics that can reduce image quality (149) (Table 1).
GAG-specific chemical exchange saturation transfer (gagCEST)
Specific MRI sequences acquire independent signals from 2 water pools: free water and macromolecule-bound water. Interactions between these water pools result in a decrease of free water signal, the extent of which can be analyzed and used as a measure of macromolecule content in certain tissues. This MRI effect is called magnetization transfer (MT) (150). In cartilage, MT occurs between water bound to the collagen fibers and free water (151).
CEST is an adaptation of MT featuring selective magnetic saturation of exchangeable protons of specific molecules. Applied in cartilage, hydroxyl residues of GAG are excited to provide a direct measurement of GAG content (gagCEST) (151). The outcome measure in gagCEST is usually expressed as an asymmetry value or percentage, whereby lower percentages indicate less GAGs in the cartilage matrix.
The technique has been validated in vitro by comparing the results of gagCEST in healthy versus GAG-depleted cartilage and to sodium signal (151). In vivo, results of gagCEST have been compared to sodium MRI of healthy and diseased cartilage (152), and the difference between gagCEST outcomes at 3T and 7T have been assessed (153). In clinical research, gagCEST has been primarily used to investigate effects of knee cartilage repair strategies (152,154) (Figure 4). A recent cadaver study by Schmitt et al also suggested feasibility of gagCEST in the ankle (155).
Figure 4.
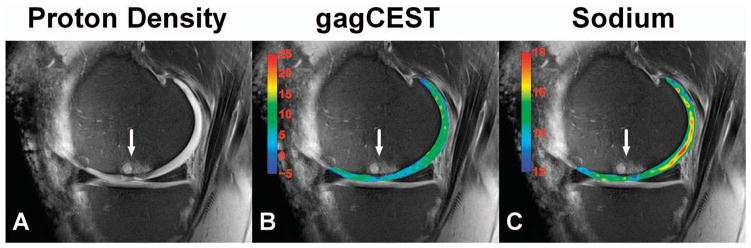
Glycosaminoglycans (GAGs)–specific chemical exchange saturation transfer (gagCEST) and sodium magnetic resonance imaging (MRI) to followup cartilage repair. Proton density (A), gagCEST (B), and sodium (C) MRIs acquired at 7T of a patient 8.7 years after autologous osteochondral transplantation (white arrow). The color overlay in (B) represents the gagCEST asymmetries in percentages (the lower the values, the less GAGs are present in the cartilage). The color overlay in (C) represents the sodium signal-to-noise ratio values (the lower the values, the less GAGs are present in the cartilage). The transplantation region (white arrow) clearly contains less GAGs compared to the posterior femoral cartilage (B and C). The CEST image (B) has a relatively high spatial resolution compared to the sodium image (C), which makes the technique promising as an outcome measure for cartilage GAG content in future research. (Courtesy of Benjamin Schmitt and Siegfried Trattnig, Medical University of Vienna, MR Center of Excellence, Vienna, Austria [154]).
GagCEST is a promising technique to quantitatively measure GAG depletion of cartilage in early cartilage disease. GagCEST is applicable on 7T and 3T MR systems (152–155), but drawbacks of gagCEST include its technical complexity and need of sophisticated postprocessing tools. Although gagCEST is feasible at 3T MR systems, acquisition is very difficult compared to 7T MR systems and might not be possible at all on 1.5T scanners due to different relaxation properties of water at different MR field strengths (152,153) (Table 1).
Sodium MRI
Unlike previously discussed MRI techniques that rely on proton (1H) imaging, sodium MRI measures sodium (23Na) signal (156). As positive sodium ions are associated with negatively charged GAGs of the extra-cellular matrix (157), sodium MRI outcomes correlate with GAG content. GAG loss from cartilage in early cartilage degeneration is accompanied by reduced sodium concentration resulting in decreased sodium MRI signal (158) (Figure 2D).
Sodium imaging has been validated in vitro, demonstrating its sensitivity and specificity in detecting small differences in GAG concentration (157,159). It has been used both in vitro and in vivo to assess its potential to measure cartilage GAG content (49,160).
Despite good reproducibility in healthy volunteers and OA patients (161,162), sodium MRI is used infrequently as an outcome measure of cartilage GAG content in clinical research, due to several limitations, i.e., less favorable magnetic properties and a much lower concentration render sodium signal-to-noise ratio and in-plane resolution low unless the magnetic field strength is increased (158), special transmit and receive coils are used, and acquisition time is prolonged (163) (Table 1).
Nevertheless, several groups have applied sodium MRI in clinical studies to determine GAG content, mainly for cartilage repair assessment (152,154,164,165) (Figure 4C). Recently, fluid-suppressed sodium MRI at 7.0T was shown to distinguish healthy subjects from OA patients more accurately than conventional sodium MRI sequences (166).
Quantitative CT arthrography
Interest in quantitative CT arthrography to measure cartilage quality has recently increased (167,168). Like dGEMRIC, quantitative CT arthrography, also referred to as delayed quantitative CT arthrography, uses the inverse relation between a negatively charged contrast agent (ioxaglate) and GAG content in cartilage (34). The quantitative outcome of CT arthrography is the radiographic attenuation measured in Hounsfield units and is higher in GAG-depleted compared to healthy cartilage (Figure 5). In CT arthrography, the contrast agent is injected intraarticularly and is also used for cartilage delineation and segmentation (168,169). After contrast injection, the patient actively moves the joint to promote contrast distribution throughout the joint cavity. The reported delay between contrast administration and CT acquisition varies (34,169), but is shorter than dGEMRIC (51).
Figure 5.
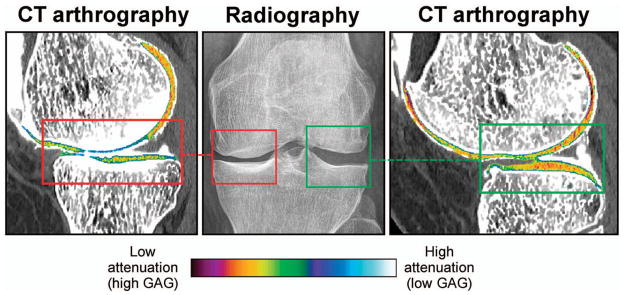
Quantitative computed tomography (CT) arthrography detects Glycosaminoglycans (GAGs) reduction in osteoarthritis. Representative image of a knee with medial joint space narrowing (red box, middle panel) and a normal lateral joint space (green box, middle panel). CT arthrography clearly shows higher radiographic attenuation values, indicating less GAGs in the medial knee compartment (red box, left panel) compared to the lateral tibiofemoral compartment (green box, right panel) (34). Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/acr.22316/abstract.
CT arthrography in humans has been validated in a recent ex vivo study in which radiographic attenuation on CT arthrography was correlated with GAG content in cartilage determined by a reference test (34). A recent in vivo study showed that CT arthrography correlates well with dGEMRIC T1 relaxation times in patients with knee symptoms (169). The reproducibility of CT arthrography has not been investigated yet, probably due to the relatively high amount of ionizing radiation used by most groups to obtain CT arthrography. It was demonstrated, however, that accurate measurement of overall cartilage quality is feasible with low-radiation dose CT arthrography protocols (170).
CT arthrography needs further optimization before it may serve as an outcome measure in clinical research. Despite ionizing radiation, the technique has some important advantages over MRI: short acquisition time, relatively wide availability, low costs, and high in-plane resolution. Its ability to simultaneously acquire high-resolution information on cartilage and the underlying bone may be utilized to study the proposed role of subchondral bone in the pathogenesis of OA and cartilage damage (171) (Table 1).
Discussion and future perspectives
Quantitative MRI and CT techniques that measure cartilage composition in early disease stages are available. All techniques have a great potential to evolve further and be implemented increasingly in clinical research on OA. Even an application in patient care for specific clinical decision-making scenarios is foreseeable in the near future. Most techniques need thorough validation of in vivo measurements against in vitro reference standards, and studies comparing different techniques within the same subject are lacking. Future research should focus on larger-scale validation and comparison of these techniques. Quantitative imaging research needs to establish correlations with clinical symptoms and predictive value for clinical outcomes. These techniques will play a pivotal role in future research on and development of next-generation therapeutics for OA and other cartilage diseases. Ultimately, imaging could be used to guide clinical decision making by identifying individuals in a “pre-OA” disease state, for which intervention with a future disease-modifying therapy could prevent the development of OA.
Significance & Innovations.
There is great need for imaging to advance our understanding of the pathogenesis of osteoarthritis (OA), to provide end points to accelerate development of therapeutics, and to ultimately guide clinical decision making.
A variety of innovative magnetic resonance imaging (MRI) and computed tomography techniques to measure cartilage composition are available, correlating with cartilage matrix elements (collagen and proteoglycans/glycosaminoglycans) that are affected in early disease stages.
The role of quantitative imaging techniques in OA is emerging because they detect cartilage disease at earlier stages than radiography and conventional MRI, and provide outcome measures that can be used as imaging biomarkers in clinical research.
Quantitative imaging techniques for cartilage composition are likely to play a pivotal role in future research and development of disease-modifying therapy for arthritis.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Gold had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Oei, van Tiel, Gold.
Acquisition of data. Oei, van Tiel, Gold.
Analysis and interpretation of data. Oei, van Tiel, Robinson, Gold.
Dr. Gold has received consultant fees, speaking fees, and/or honoraria (less than $10,000 each) from Isto, Zimmer, and Boston Scientific, and has received research support from GE Healthcare.
References
- 1.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research [review] Clin Orthop Relat Res. 2004;427 (Suppl):S6–15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 2.Hermans J, Koopmanschap MA, Bierma-Zeinstra SM, van Linge JH, Verhaar JA, Reijman M, et al. Productivity costs and medical costs among working patients with knee osteoarthritis. Arthritis Care Res (Hoboken) 2012;64:853–61. doi: 10.1002/acr.21617. [DOI] [PubMed] [Google Scholar]
- 3.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. for the National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56:1397–407. doi: 10.1002/art.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions: United States 2003. MMWR Morb Mortal Wkly Rep. 2007;56:4–7. [PubMed] [Google Scholar]
- 7.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–9. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 8.Brouwer RW, Raaij VT, Bierma-Zeinstra SM, Verhagen AP, Jakma TS, Verhaar JA. Osteotomy for treating knee osteoarthritis. Cochrane Database Syst Rev. 2007;3:CD004019. doi: 10.1002/14651858.CD004019.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Bos PK, van Melle ML, van Osch GJ. Articular cartilage repair and the evolving role of regenerative medicine. Open Access Surg. 2010;3:109–22. [Google Scholar]
- 10.Hunter DJ. Pharmacologic therapy for osteoarthritis: the era of disease modification. Nat Rev Rheumatol. 2011;7:13–22. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- 11.Cubukcu D, Sarsan A, Alkan H. Relationships between pain, function and radiographic findings in osteoarthritis of the knee: a cross-sectional study. Arthritis. 2012;2012:984060. doi: 10.1155/2012/984060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65:363–72. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kijowski R, Blankenbaker D, Stanton P, Fine J, de Smet A. Correlation between radiographic findings of osteoarthritis and arthroscopic findings of articular cartilage degeneration within the patellofemoral joint. Skeletal Radiol. 2006;35:895–902. doi: 10.1007/s00256-006-0111-7. [DOI] [PubMed] [Google Scholar]
- 14.Kijowski R, Blankenbaker DG, Stanton PT, Fine JP, de Smet AA. Radiographic findings of osteoarthritis versus arthroscopic findings of articular cartilage degeneration in the tibiofemoral joint. Radiology. 2006;239:818–24. doi: 10.1148/radiol.2393050584. [DOI] [PubMed] [Google Scholar]
- 15.Guermazi A, Roemer FW, Burstein D, Hayashi D. Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Res Ther. 2011;13:247. doi: 10.1186/ar3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi D, Felson DT, Niu J, Hunter DJ, Roemer FW, Aliabadi P, et al. Pre-radiographic osteoarthritic changes are highly prevalent in the medial patella and medial posterior femur in older persons: Framingham OA Study. Osteoarthritis Cartilage. 2014;22:76–83. doi: 10.1016/j.joca.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Dis. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study) BMJ. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oei EH. Magnetic resonance imaging for traumatic knee injury. Rotterdam: Erasmus University; 2009. [Google Scholar]
- 20.Koster IM, Oei EH, Hensen JH, Boks SS, Koes BW, Vroegindeweij D, et al. Predictive factors for new onset or progression of knee osteoarthritis one year after trauma: MRI follow-up in general practice. Eur Radiol. 2011;21:1509–16. doi: 10.1007/s00330-011-2089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kornaat PR, Ceulemans RY, Kroon HM, Riyazi N, Kloppenburg M, Carter WO, et al. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS). Inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol. 2005;34:95–102. doi: 10.1007/s00256-004-0828-0. [DOI] [PubMed] [Google Scholar]
- 22.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67:206–11. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 24.Guermazi A, Roemer FW, Haugen IK, Crema MD, Hayashi D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol. 2013;9:236–51. doi: 10.1038/nrrheum.2012.223. [DOI] [PubMed] [Google Scholar]
- 25.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buck RJ, Wyman BT, Le Graverand MP, Hunter D, Vignon E, Wirth W, et al. Using ordered values of subregional cartilage thickness change increases sensitivity in detecting risk factors for osteoarthritis progression. Osteoarthritis Cartilage. 2011;19:302–8. doi: 10.1016/j.joca.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Buck RJ, Wyman BT, Le Graverand MP, Wirth W, Eckstein F. An efficient subset of morphological measures for articular cartilage in the healthy and diseased human knee. Magn Reson Med. 2010;63:680–90. doi: 10.1002/mrm.22207. [DOI] [PubMed] [Google Scholar]
- 28.Sowers M, Karvonen-Gutierrez CA, Jacobson JA, Jiang YB, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am. 2011;93A:241–51. doi: 10.2106/JBJS.I.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis. 2011;70:60–7. doi: 10.1136/ard.2010.131904. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Min ZG, Rana N, Liu HJ. Accuracy of magnetic resonance imaging in grading knee chondral defects. Arthroscopy. 2013;29:349–56. doi: 10.1016/j.arthro.2012.04.138. [DOI] [PubMed] [Google Scholar]
- 31.Burstein D, Gray M, Mosher T, Dardzinski B. Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am. 2009;47:675–86. doi: 10.1016/j.rcl.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31:37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012;64:248–55. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siebelt M, van Tiel J, Waarsing JH, Piscaer TM, Straten M, Booij R, et al. Clinically applied CT arthrography to measure the sulphated glycosaminoglycan content of cartilage. Osteoarthritis Cartilage. 2011;19:1183–9. doi: 10.1016/j.joca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan N, Shetty SK, Williams A, Mikulis B, McKenzie C, Burstein D. Delayed gadolinium-enhanced magnetic resonance imaging of the meniscus: an index of meniscal tissue degeneration? Arthritis Rheum. 2007;56:1507–11. doi: 10.1002/art.22592. [DOI] [PubMed] [Google Scholar]
- 36.Williams A, Qian Y, Golla S, Chu CR. UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage. 2012;20:486–94. doi: 10.1016/j.joca.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stehling C, Luke A, Stahl R, Baum T, Joseph G, Pan JD, et al. Meniscal T1rho and T2 measured with 3. 0T MRI increases directly after running a marathon. Skeletal Radiol. 2011;40:725–35. doi: 10.1007/s00256-010-1058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lotz JC, Haughton V, Boden SD, An HS, Kang JD, Masuda K, et al. New treatments and imaging strategies in degenerative disease of the intervertebral disks. Radiology. 2012;264:6–19. doi: 10.1148/radiol.12110339. [DOI] [PubMed] [Google Scholar]
- 39.Haneder S, Apprich SR, Schmitt B, Michaely HJ, Schoenberg SO, Friedrich KM, et al. Assessment of glycosaminoglycan content in intervertebral discs using chemical exchange saturation transfer at 3. 0 Tesla: preliminary results in patients with low-back pain. Eur Radiol. 2013;23:861–8. doi: 10.1007/s00330-012-2660-6. [DOI] [PubMed] [Google Scholar]
- 40.Maroudas A, Bayliss MT, Venn MF. Further studies on the composition of human femoral-head cartilage. Ann Rheum Dis. 1980;39:514–23. doi: 10.1136/ard.39.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977;36:121–9. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 43.Mosher TJ. Functional anatomy and structure of the “osteochondral unit”. In: Bruno MA, Mosher TJ, Gold GE, editors. Arthritis in color: advanced imaging of arthritis. Philadelphia: Elsevier Saunders; 2009. pp. 23–32. [Google Scholar]
- 44.Franzen A, Inerot S, Hejderup SO, Heinegard D. Variations in the composition of bovine hip articular-cartilage with distance from the articular surface. Biochem J. 1981;195:535–43. doi: 10.1042/bj1950535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez S, Toffanin R, Bernstorff S, Romanello M, Amenitsch H, Rappolt M, et al. Collagen fibrils are differently organized in weight-bearing and not-weight-bearing regions of pig articular cartilage. J Exp Zool. 2000;287:346–52. [PubMed] [Google Scholar]
- 46.Maroudas A, Ziv I, Weisman N, Venn M. Studies of hydration and swelling pressure in normal and osteoarthritic cartilage. Biorheology. 1985;22:159–69. doi: 10.3233/bir-1985-22206. [DOI] [PubMed] [Google Scholar]
- 47.Bron EE, van Tiel J, Smit H, Poot DH, Niessen WJ, Krestin GP, et al. Image registration improves human knee cartilage T1 mapping with delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) Eur Radiol. 2013;23:246–52. doi: 10.1007/s00330-012-2590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miese F, Kropil P, Ostendorf B, Scherer A, Buchbender C, Quentin M, et al. Motion correction improves image quality of dGEMRIC in finger joints. Eur J Radiol. 2011;80:E427–31. doi: 10.1016/j.ejrad.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Bashir A, Gray ML, Burstein D. Gd-DTPA2 as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–73. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 50.Tiderius CJ, Olsson LE, de Verdier H, Leander P, Ekberg O, Dahlberg L. Gd-DTPA2-enhanced MRI of femoral knee cartilage: a dose-response study in healthy volunteers. Magn Reson Med. 2001;46:1067–71. doi: 10.1002/mrm.1300. [DOI] [PubMed] [Google Scholar]
- 51.Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49:488–92. doi: 10.1002/mrm.10389. [DOI] [PubMed] [Google Scholar]
- 52.Trattnig S, Burstein D, Szomolanyi P, Pinker K, Welsch GH, Mamisch TC. T1 (Gd) gives comparable information as ΔT1 relaxation rate in dGEMRIC: evaluation of cartilage repair tissue. Invest Radiol. 2009;44:598–602. doi: 10.1097/RLI.0b013e3181b4c236. [DOI] [PubMed] [Google Scholar]
- 53.Bittersohl B, Hosalkar HS, Kim YJ, Werlen S, Siebenrock KA, Mamisch TC. Delayed gadolinium-enhanced magnetic resonance imaging (dGEMRIC) of hip joint cartilage in femoroacetabular impingement (FAI): are pre- and postcontrast imaging both necessary? Magn Reson Med. 2009;62:1362–7. doi: 10.1002/mrm.22166. [DOI] [PubMed] [Google Scholar]
- 54.Bittersohl B, Hosalkar HS, Werlen S, Trattnig S, Siebenrock KA, Mamisch TC. Intravenous versus intra-articular delayed gadolinium-enhanced magnetic resonance imaging in the hip joint: a comparative analysis. Invest Radiol. 2010;45:538–42. doi: 10.1097/RLI.0b013e3181ea5bb5. [DOI] [PubMed] [Google Scholar]
- 55.Boesen M, Jensen KE, Qvistgaard E, Danneskiold-Samsoe B, Thomsen C, Ostergaard M, et al. Delayed gadolinium-enhanced magnetic resonance imaging (dGEMRIC) of hip joint cartilage: better cartilage delineation after intra-articular than intravenous gadolinium injection. Acta Radiol. 2006;47:391–6. doi: 10.1080/02841850600596792. [DOI] [PubMed] [Google Scholar]
- 56.Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI: (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 57.McKenzie CA, Williams A, Prasad PV, Burstein D. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1.5T and 3. 0T. J Magn Reson Imaging. 2006;24:928–33. doi: 10.1002/jmri.20689. [DOI] [PubMed] [Google Scholar]
- 58.Tiderius CJ, Jessel R, Kim YJ, Burstein D. Hip dGEMRIC in asymptomatic volunteers and patients with early osteoarthritis: the influence of timing after contrast injection. Magn Reson Med. 2007;57:803–5. doi: 10.1002/mrm.21190. [DOI] [PubMed] [Google Scholar]
- 59.Kimelman T, Vu A, Storey P, McKenzie C, Burstein D, Prasad P. Three-dimensional T1 mapping for dGEMRIC at 3. 0T using the look locker method. Invest Radiol. 2006;41:198–203. doi: 10.1097/01.rli.0000195842.49255.ea. [DOI] [PubMed] [Google Scholar]
- 60.Trattnig S, Marlovits S, Gebetsroither S, Szomolanyi P, Welsch GH, Salomonowitz E, et al. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) for in vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3. 0T: preliminary results. J Magn Reson Imaging. 2007;26:974–82. doi: 10.1002/jmri.21091. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe A, Wada Y, Obata T, Ueda T, Tamura M, Ikehira H, et al. Delayed gadolinium-enhanced MR to determine glycosaminoglycan concentration in reparative cartilage after autologous chondrocyte implantation: preliminary results. Radiology. 2006;239:201–8. doi: 10.1148/radiol.2383050173. [DOI] [PubMed] [Google Scholar]
- 62.Multanen J, Rauvala E, Lammentausta E, Ojala R, Kiviranta I, Hakkinen A, et al. Reproducibility of imaging human knee cartilage by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1. 5 Tesla. Osteoarthritis Cartilage. 2009;17:559–64. doi: 10.1016/j.joca.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Siversson C, Tiderius CJ, Neuman P, Dahlberg L, Svensson J. Repeatability of T1-quantification in dGEMRIC for three different acquisition techniques: two-dimensional inversion recovery, three-dimensional look locker, and three-dimensional variable flip angle. J Magn Reson Imaging. 2010;31:1203–9. doi: 10.1002/jmri.22159. [DOI] [PubMed] [Google Scholar]
- 64.Van Tiel J, Bron EE, Tiderius CJ, Bos PK, Reijman M, Klein S, et al. Reproducibility of 3D delayed gadolinium enhanced MRI of cartilage (dGEMRIC) of the knee at 3. 0 T in patients with early stage osteoarthritis. Eur Radiol. 2013;23:496–504. doi: 10.1007/s00330-012-2616-x. [DOI] [PubMed] [Google Scholar]
- 65.Crema MD, Hunter DJ, Burstein D, Roemer FW, Li L, Eckstein F, et al. Association of changes in delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) with changes in cartilage thickness in the medial tibiofemoral compartment of the knee: a 2 year follow-up study using 3.0 T MRI. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-203083. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Neuman P, Tjornstrand J, Svensson J, Ragnarsson C, Roos H, Englund M, et al. Longitudinal assessment of femoral knee cartilage quality using contrast enhanced MRI (dGEMRIC) in patients with anterior cruciate ligament injury: comparison with asymptomatic volunteers. Osteoarthritis Cartilage. 2011;19:977–83. doi: 10.1016/j.joca.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Klocke NF, Amendola A, Thedens DR, Williams GN, Luty CM, Martin JA, et al. Comparison of T1rho, dGEMRIC, and quantitative T2 MRI in preoperative ACL rupture patients. Acad Radiol. 2013;20:99–107. doi: 10.1016/j.acra.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mamisch TC, Kain MS, Bittersohl B, Apprich S, Werlen S, Beck M, et al. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) in femoacetabular impingement. J Orthop Res. 2011;29:1305–11. doi: 10.1002/jor.21371. [DOI] [PubMed] [Google Scholar]
- 69.Stelzeneder D, Mamisch TC, Kress I, Domayer SE, Werlen S, Bixby SD, et al. Patterns of joint damage seen on MRI in early hip osteoarthritis due to structural hip deformities. Osteoarthritis Cartilage. 2012;20:661–9. doi: 10.1016/j.joca.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Xu L, Su Y, Kienle KP, Hayashi D, Guermazi A, Zhang J, et al. Evaluation of radial distribution of cartilage degeneration and necessity of pre-contrast measurements using radial dGEMRIC in adults with acetabular dysplasia. BMC Musculoskelet Disord. 2012;13:212. doi: 10.1186/1471-2474-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McAlindon TE, Nuite M, Krishnan N, Ruthazer R, Price LL, Burstein D, et al. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: a pilot randomized controlled trial. Osteoarthritis Cartilage. 2011;19:399–405. doi: 10.1016/j.joca.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Van Tiel J, Reijman M, Bos PK, Hermans J, van Buul GM, Bron EE, et al. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) shows no change in cartilage structural composition after viscosupplementation in patients with early-stage knee osteoarthritis. PLoS One. 2013;8:e79785. doi: 10.1371/journal.pone.0079785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rutgers M, Bartels LW, Tsuchida AI, Castelein RM, Dhert WJ, Vincken KL, et al. dGEMRIC as a tool for measuring changes in cartilage quality following high tibial osteotomy: a feasibility study. Osteoarthritis Cartilage. 2012;20:1134–41. doi: 10.1016/j.joca.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Anandacoomarasamy A, Leibman S, Smith G, Caterson I, Giuffre B, Fransen M, et al. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Ann Rheum Dis. 2012;71:26–32. doi: 10.1136/ard.2010.144725. [DOI] [PubMed] [Google Scholar]
- 75.Vasiliadis HS, Danielson B, Ljunberg M, McKeon B, Lindahl A, Peterson L. Autologous chondrocyte implantation in cartilage lesions of the knee long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38:943–9. doi: 10.1177/0363546509358266. [DOI] [PubMed] [Google Scholar]
- 76.Domayer SE, Trattnig S, Stelzeneder D, Hirschfeld C, Quirbach S, Dorotka R, et al. Delayed gadolinium-enhanced MRI of cartilage in the ankle at 3T: feasibility and preliminary results after matrix-associated autologous chondrocyte implantation. J Magn Reson Imaging. 2010;31:732–9. doi: 10.1002/jmri.22093. [DOI] [PubMed] [Google Scholar]
- 77.Miese F, Buchbender C, Scherer A, Wittsack HJ, Specker C, Schneider M, et al. Molecular imaging of cartilage damage of finger joints in early rheumatoid arthritis with delayed gadolinium-enhanced magnetic resonance imaging. Arthritis Rheum. 2012;64:394–9. doi: 10.1002/art.33352. [DOI] [PubMed] [Google Scholar]
- 78.Tiderius CJ, Sandin J, Svensson J, Dahlberg LE, Jacobsson L. Knee cartilage quality assessed with dGEMRIC in rheumatoid arthritis patients before and after treatment with a TNF inhibitor. Acta Radiol. 2010;51:1034–7. doi: 10.3109/02841851.2010.510482. [DOI] [PubMed] [Google Scholar]
- 79.Salo EN, Nissi MJ, Kulmala KA, Tiitu V, Toyras J, Nieminen MT. Diffusion of Gd-DTPA(−) into articular cartilage. Osteoarthritis Cartilage. 2012;20:117–26. doi: 10.1016/j.joca.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 80.Wiener E, Settles M, Weirich G, Schmidt C, Diederichs G. The influence of collagen network integrity on the accumulation of gadolinium-based MR contrast agents in articular cartilage. Rofo-Fortschr Rontg. 2011;183:226–32. doi: 10.1055/s-0029-1245739. [DOI] [PubMed] [Google Scholar]
- 81.Tiderius C, Hori M, Williams A, Sharma L, Prasad PV, Finnell M, et al. dGEMRIC as a function of BMI. Osteoarthritis Cartilage. 2006;14:1091–7. doi: 10.1016/j.joca.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Winalski CS, Aliabadi P, Wright RJ, Shortkroff S, Sledge CB, Weissman BN. Enhancement of joint fluid with intravenously administered gadopentetate dimeglumine: technique, rationale, and implications. Radiology. 1993;187:179–85. doi: 10.1148/radiology.187.1.8451409. [DOI] [PubMed] [Google Scholar]
- 83.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–68. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 84.David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22:673–82. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 85.Dardzinski BJ, Mosher TJ, Li SZ, van Slyke MA, Smith MB. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205:546–50. doi: 10.1148/radiology.205.2.9356643. [DOI] [PubMed] [Google Scholar]
- 86.Quaia E, Toffanin R, Guglielmi G, Ukmar M, Rossi A, Martinelli B, et al. Fast T2 mapping of the patellar articular cartilage with gradient and spin-echo magnetic resonance imaging at 1. 5 T: validation and initial clinical experience in patients with osteoarthritis. Skeletal Radiol. 2008;37:511–7. doi: 10.1007/s00256-008-0478-8. [DOI] [PubMed] [Google Scholar]
- 87.Van Breuseghem I, Bosmans HT, Elst LV, Maes F, Pans SD, Brys PP, et al. T2 mapping of human femorotibial cartilage with turbo mixed MR imaging at 1. 5 T: feasibility. Radiology. 2004;233:609–14. doi: 10.1148/radiol.2332030891. [DOI] [PubMed] [Google Scholar]
- 88.Chen W, Takahashi A, Han ET. 3D quantitative imaging of T1rho and T2. International Society for Magnetic Resonance in Medicine Annual Meeting & Exhibition; 2011 May 7–13; Montreal, Quebec, Canada. [Google Scholar]
- 89.Chen W, Zhang T, Han ET, Gold GE. Joint anatomical and biochemical imaging using 3D FSE. International Society for Magnetic Resonance in Medicine Annual Meeting & Exhibition; 2011 May 7–13; Montreal, Quebec, Canada. [Google Scholar]
- 90.Staroswiecki E, Granlund KL, Alley MT, Gold GE, Hargreaves BA. Simultaneous estimation of T-2 and apparent diffusion coefficient in human articular cartilage in vivo with a modified three-dimensional double echo steady state (DESS) sequence at 3T. Magn Reson Med. 2012;67:1086–96. doi: 10.1002/mrm.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Welsch GH, Mamisch TC, Zak L, Mauerer A, Apprich S, Stelzeneder D, et al. Morphological and biochemical T2 evaluation of cartilage repair tissue based on a hybrid double echo at steady state (DESS-T2d) approach. J Magn Reson Imaging. 2011;34:895–903. doi: 10.1002/jmri.22677. [DOI] [PubMed] [Google Scholar]
- 92.Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, et al. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology. 2011;258:832–42. doi: 10.1148/radiol.10101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–8. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nissi MJ, Toyras J, Laasanen MS, Rieppo J, Saarakkala S, Lappalainen R, et al. Proteoglycan and collagen sensitive MRI evaluation of normal and degenerated articular cartilage. J Orthop Res. 2004;22:557–64. doi: 10.1016/j.orthres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 95.Yao WW, Qu N, Lu ZH, Yang SX. The application of T1 and T2 relaxation time and magnetization transfer ratios to the early diagnosis of patellar cartilage osteoarthritis. Skeletal Radiol. 2009;38:1055–62. doi: 10.1007/s00256-009-0769-8. [DOI] [PubMed] [Google Scholar]
- 96.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50–5. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 97.Keenan KE, Besier TF, Pauly JM, Han E, Rosenberg J, Smith RL, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage. 2011;19:171–9. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishioka H, Hirose J, Nakamura E, Oniki Y, Takada K, Yamashita Y, et al. T1rho and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2012;35:147–55. doi: 10.1002/jmri.22811. [DOI] [PubMed] [Google Scholar]
- 99.Wong CS, Yan CH, Gong NJ, Li T, Chan Q, Chu YC. Imaging biomarker with T1rho and T2 mappings in osteoarthritis: in vivo human articular cartilage study. Eur J Radiol. 2013;82:647–50. doi: 10.1016/j.ejrad.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 100.Van Ginckel A, Verdonk P, Victor J, Witvrouw E. Cartilage status in relation to return to sports after anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41:550–9. doi: 10.1177/0363546512473568. [DOI] [PubMed] [Google Scholar]
- 101.Kurkijarvi JE, Mattila L, Ojala RO, Vasara AI, Jurvelin JS, Kiviranta I, et al. Evaluation of cartilage repair in the distal femur after autologous chondrocyte transplantation using T2 relaxation time and dGEMRIC. Osteoarthritis Cartilage. 2007;15:372–8. doi: 10.1016/j.joca.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 102.Theologis AA, Schairer WW, Carballido-Gamio J, Majumdar S, Li X, Ma CB. Longitudinal analysis of T1rho and T2 quantitative MRI of knee cartilage laminar organization following microfracture surgery. Knee. 2012;19:652–7. doi: 10.1016/j.knee.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marik W, Apprich S, Welsch GH, Mamisch TC, Trattnig S. Biochemical evaluation of articular cartilage in patients with osteochondrosis dissecans by means of quantitative T2-and T2-mapping at 3T MRI: a feasibility study. Eur J Radiol. 2012;81:923–7. doi: 10.1016/j.ejrad.2011.01.124. [DOI] [PubMed] [Google Scholar]
- 104.Friedrich KM, Shepard T, Chang G, Wang L, Babb JS, Schweitzer M, et al. Does joint alignment affect the T2 values of cartilage in patients with knee osteoarthritis? Eur Radiol. 2010;20:1532–8. doi: 10.1007/s00330-009-1689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Friedrich KM, Shepard T, de Oliveira VS, Wang LG, Babb JS, Schweitzer M, et al. T2 measurements of cartilage in osteoarthritis patients with meniscal tears. Am J Roentgenol. 2009;193:W411–5. doi: 10.2214/AJR.08.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T-1 rho and T-2 relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:69–76. doi: 10.1016/j.joca.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nishii T, Tanaka H, Sugano N, Sakai T, Hananouchi T, Yoshikawa H. Evaluation of cartilage matrix disorders by T2 relaxation time in patients with hip dysplasia. Osteoarthritis Cartilage. 2008;16:227–33. doi: 10.1016/j.joca.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 108.Miese FR, Zilkens C, Holstein A, Bittersohl B, Kropil P, Mamisch TC, et al. Assessment of early cartilage degeneration after slipped capital femoral epiphysis using T2 and T2* mapping. Acta Radiol. 2011;52:106–10. doi: 10.3109/02841851.2010.516015. [DOI] [PubMed] [Google Scholar]
- 109.Quirbach S, Trattnig S, Marlovits S, Zimmermann V, Domayer S, Dorotka R, et al. Initial results of in vivo high-resolution morphological and biochemical cartilage imaging of patients after matrix-associated autologous chondrocyte transplantation (MACT) of the ankle. Skeletal Radiol. 2009;38:751–60. doi: 10.1007/s00256-009-0682-1. [DOI] [PubMed] [Google Scholar]
- 110.Battaglia M, Vannini F, Buda R, Cavallo M, Ruffilli A, Monti C, et al. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc. 2011;19:1376–84. doi: 10.1007/s00167-011-1509-x. [DOI] [PubMed] [Google Scholar]
- 111.Giannini S, Buda R, Battaglia M, Cavallo M, Ruffilli A, Ramponi L, et al. One-step repair in talar osteochondral lesions: 4-year clinical results and T2-mapping capability in outcome prediction. Am J Sports Med. 2013;41:511–8. doi: 10.1177/0363546512467622. [DOI] [PubMed] [Google Scholar]
- 112.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012;20:727–35. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the Osteoarthritis Initiative. J Magn Reson Imaging. 2012;35:370–8. doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haverkamp DJ, Schiphof D, Bierma-Zeinstra SM, Weinans H, Waarsing JH. Variation in joint shape of osteoarthritic knees. Arthritis Rheum. 2011;63:3401–7. doi: 10.1002/art.30575. [DOI] [PubMed] [Google Scholar]
- 115.Schiphof D, de Klerk BM, Waarsing JH, Koes BW, Ginai AZ, Pols HA, et al. Identification of pre-clinical osteoarthritis: a subcohort in the Rotterdam study. Osteoarthritis Cartilage. 2008;16 (Suppl):S166. [Google Scholar]
- 116.Levitt M. Spin dynamics: basics of nuclear magnetic resonance. New York: Wiley; 2008. [Google Scholar]
- 117.Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol. 2001;177:665–9. doi: 10.2214/ajr.177.3.1770665. [DOI] [PubMed] [Google Scholar]
- 118.Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24:1–12. doi: 10.1016/j.csm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 119.McDevitt CA. Biochemistry of articular cartilage: nature of proteoglycans and collagen of articular cartilage and their role in ageing and in osteoarthrosis. Ann Rheum Dis. 1973;32:364–78. doi: 10.1136/ard.32.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matthews BF. Composition of articular cartilage in osteoarthritis: changes in collagen/chondroitin-sulphate ratio. Brit Med J. 1953;2:660–1. doi: 10.1136/bmj.2.4837.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–7. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 122.Pakin SK, Schweitzer ME, Regatte RR. 3D-T1rho quantitation of patellar cartilage at 3. 0T. J Magn Reson Imaging. 2006;24:1357–63. doi: 10.1002/jmri.20733. [DOI] [PubMed] [Google Scholar]
- 123.Duvvuri U, Kudchodkar S, Reddy R, Leigh JS. T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis Cartilage. 2002;10:838–44. doi: 10.1053/joca.2002.0826. [DOI] [PubMed] [Google Scholar]
- 124.Regatte RR, Akella SV, Borthakur A, Reddy R. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J Magn Reson Imaging. 2003;17:114–21. doi: 10.1002/jmri.10228. [DOI] [PubMed] [Google Scholar]
- 125.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, et al. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29:324–34. doi: 10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol. 2002;9:1388–94. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 127.Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance. Imaging Magn Reson Med. 2005;54:1087–93. doi: 10.1002/mrm.20678. [DOI] [PubMed] [Google Scholar]
- 128.Tsushima H, Okazaki K, Takayama Y, Hatakenaka M, Honda H, Izawa T, et al. Evaluation of cartilage degradation in arthritis using T1rho magnetic resonance imaging mapping. Rheumatol Int. 2012;32:2867–75. doi: 10.1007/s00296-011-2140-3. [DOI] [PubMed] [Google Scholar]
- 129.Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51:503–9. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 130.Goto H, Iwama Y, Fujii M, Aoyama N, Kubo S, Kuroda R, et al. A preliminary study of the T1rho values of normal knee cartilage using 3T-MRI. Eur J Radiol. 2012;81:e796–803. doi: 10.1016/j.ejrad.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 131.Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage. 2010;18:1408–16. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bolbos RI, Ma CB, Link TM, Majumdar S, Li X. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance. Imaging Invest Radiol. 2008;43:782–8. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Theologis AA, Haughom B, Liang F, Zhang Y, Majumdar S, Link TM, et al. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc. 2014;22:298–307. doi: 10.1007/s00167-013-2397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Holtzman DJ, Theologis AA, Carballido-Gamio J, Majumdar S, Li X, Benjamin C. T(1rho) and T quantitative magnetic resonance imaging analysis of cartilage regeneration following microfracture and mosaicplasty cartilage resurfacing procedures. J Magn Reson Imaging. 2010;32:914–23. doi: 10.1002/jmri.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Souza RB, Fang CL, Luke A, Wu S, Li XJ, Majumdar S. Relationship between knee kinetics during jumping tasks and knee articular cartilage MRI T1rho and T2 relaxation times. Clin Biotech. 2012;27:403–8. doi: 10.1016/j.clinbiomech.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Souza RB, Stehling C, Wyman BT, Le Graverand MP, Li X, Link TM, et al. The effects of acute loading on T1rho and T2 relaxation times of tibiofemoral articular cartilage. Osteoarthritis Cartilage. 2010;18:1557–63. doi: 10.1016/j.joca.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 137.Souza RB, Baum T, Wu S, Feeley BT, Kadel N, Li XJ, et al. Effects of unloading on knee articular cartilage T1rho and T2 magnetic resonance imaging relaxation times: a case series. J Orthop Sports Phys. 2012;42:511–20. doi: 10.2519/jospt.2012.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Subburaj K, Kumar D, Souza RB, Alizai H, Li XJ, Link TM, et al. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med. 2012;40:2134–41. doi: 10.1177/0363546512449816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luke AC, Stehling C, Stahl R, Li X, Kay T, Takamoto S, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: does long-distance running lead to cartilage damage? Am J Sports Med. 2010;38:2273–80. doi: 10.1177/0363546510372799. [DOI] [PubMed] [Google Scholar]
- 140.Rakhra KS, Lattanzio PJ, Cardenas-Blanco A, Cameron IG, Beaule PE. Can T1-rho MRI detect acetabular cartilage degeneration in femoroacetabular impingement? A pilot study. J Bone Joint Surg Br. 2012;94:1187–92. doi: 10.1302/0301-620X.94B9.29981. [DOI] [PubMed] [Google Scholar]
- 141.Pedersen DR, Klocke NF, Thedens DR, Martin JA, Williams GN, Amendola A. Integrating carthage-specific T1rho MRI into knee clinic diagnostic imaging. Iowa Orthop J. 2011;31:99–109. [PMC free article] [PubMed] [Google Scholar]
- 142.Gold GE, Pauly JM, Macovski A, Herfkens RJ. MR spectroscopic imaging of collagen: tendons and knee menisci. Magn Reson Med. 1995;34:647–54. doi: 10.1002/mrm.1910340502. [DOI] [PubMed] [Google Scholar]
- 143.Du J, Diaz E, Carl M, Bae W, Chung CB, Bydder GM. Ultra-short echo time imaging with bicomponent analysis. Magn Reson Med. 2012;67:645–9. doi: 10.1002/mrm.23047. [DOI] [PubMed] [Google Scholar]
- 144.Du J, Takahashi AM, Chung CB. Ultrashort TE spectroscopic imaging (UTESI): application to the imaging of short T2 relaxation tissues in the musculoskeletal system. J Magn Reson Imaging. 2009;29:412–21. doi: 10.1002/jmri.21465. [DOI] [PubMed] [Google Scholar]
- 145.Bae WC, Chen PC, Chung CB, Masuda K, D’Lima D, Du J. Quantitative ultrashort echo time (UTE) MRI of human cortical bone: correlation with porosity and biomechanical properties. J Bone Miner Res. 2012;27:848–57. doi: 10.1002/jbmr.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Du J, Carl M, Bae WC, Statum S, Chang EY, Bydder GM, et al. Dual inversion recovery ultrashort echo time (DIR-UTE) imaging and quantification of the zone of calcified cartilage (ZCC) Osteoarthritis Cartilage. 2013;21:77–85. doi: 10.1016/j.joca.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rautiainen J, Lehto LJ, Tiitu V, Kiekara O, Pulkkinen H, Brunott A, et al. Osteochondral repair: evaluation with sweep imaging with fourier transform in an equine model. Radiology. 2013;269:113–21. doi: 10.1148/radiol.13121433. [DOI] [PubMed] [Google Scholar]
- 148.Williams A, Qian Y, Chu CR. UTE-T2* mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthritis Cartilage. 2011;19:84–8. doi: 10.1016/j.joca.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tyler DJ, Robson MD, Henkelman RM, Young IR, Bydder GM. Magnetic resonance imaging with ultrashort TE (UTE) PULSE sequences: technical considerations. J Magn Reson Imaging. 2007;25:279–89. doi: 10.1002/jmri.20851. [DOI] [PubMed] [Google Scholar]
- 150.Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10:135–44. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- 151.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) P Natl Acad Sci USA. 2008;105:2266–70. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schmitt B, Zbyn S, Stelzeneder D, Jellus V, Paul D, Lauer L, et al. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and Na-23 MR imaging at 7T. Radiology. 2011;260:257–64. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 153.Singh A, Haris M, Cai KJ, Kassey VB, Kogan F, Reddy D, et al. Chemical exchange saturation transfer magnetic resonance imaging of human knee cartilage at 3T and 7T. Magn Reson Med. 2012;68:588–94. doi: 10.1002/mrm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krusche-Mandl I, Schmitt B, Zak L, Apprich S, Aldrian S, Juras V, et al. Long-term results 8 years after autologous osteochondral transplantation: 7 T gagCEST and sodium magnetic resonance imaging with morphological and clinical correlation. Osteoarthritis Cartilage. 2012;20:357–63. doi: 10.1016/j.joca.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 155.Schmitt B, Trattnig S, Sallinger C, Hofstatter J, Windhager R, Domayer S. Evaluation of the dependency of glycosaminoglycan (GAG) chemical exchange saturation transfer (gagCEST) imaging on cartilage GAG content in the ankle at 3T. Chicago: Radiological Society of North America; 2013. [Google Scholar]
- 156.Granot J. Sodium imaging of human body organs and extremities in vivo. Radiology. 1988;167:547–50. doi: 10.1148/radiology.167.2.3357970. [DOI] [PubMed] [Google Scholar]
- 157.Insko EK, Kaufman TH, Leigh JS, Reddy R. Sodium NMR evaluation of articular cartilage degradation. Magn Reson Med. 1999;41:30–4. doi: 10.1002/(sici)1522-2594(199901)41:1<30::aid-mrm6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 158.Shapiro EM, Borthakur A, Gougoutas A, Reddy R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47:284–91. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Borthakur A, Shapiro EM, Beers J, Kudchodkar S, Kneeland JB, Reddy R. Sensitivity of MRI to proteoglycan depletion in cartilage: comparison of sodium and proton MRI. Osteoarthritis Cartilage. 2000;8:288–93. doi: 10.1053/joca.1999.0303. [DOI] [PubMed] [Google Scholar]
- 160.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857–65. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 161.Newbould RD, Miller SR, Tielbeek JA, Toms LD, Rao AW, Gold GE, et al. Reproducibility of sodium MRI measures of articular cartilage of the knee in osteoarthritis. Osteoarthritis Cartilage. 2012;20:29–35. doi: 10.1016/j.joca.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Madelin G, Babb JS, Xia D, Chang G, Jerschow A, Regatte RR. Reproducibility and repeatability of quantitative sodium magnetic resonance imaging in vivo in articular cartilage at 3T and 7T. Magn Reson Med. 2012;68:841–9. doi: 10.1002/mrm.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gold GE, Hargreaves BA, Stevens KJ, Beaulieu CF. Advanced magnetic resonance imaging of articular cartilage. Orthop Clin N Am. 2006;37:331–47. doi: 10.1016/j.ocl.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 164.Zbyn S, Stelzeneder D, Welsch GH, Negrin LL, Juras V, Mayerhoefer ME, et al. Evaluation of native hyaline cartilage and repair tissue after two cartilage repair surgery techniques with 23Na MR imaging at 7T: initial experience. Osteoarthritis Cartilage. 2012;20:837–45. doi: 10.1016/j.joca.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 165.Chang G, Madelin G, Sherman OH, Strauss EJ, Xia D, Recht MP, et al. Improved assessment of cartilage repair tissue using fluid-suppressed Na inversion recovery MRI at 7 Tesla: preliminary results. Eur Radiol. 2012;22:1341–9. doi: 10.1007/s00330-012-2383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Madelin G, Babb J, Xia D, Chang G, Krasnokutsky S, Abramson SB, et al. Articular cartilage: evaluation with fluid-suppressed 7. 0-T sodium MR imaging in subjects with and subjects without osteoarthritis. Radiology. 2013;268:481–91. doi: 10.1148/radiol.13121511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Siebelt M, Waarsing JH, Kops N, Piscaer TM, Verhaar JA, Oei EH, et al. Quantifying osteoarthritic cartilage changes accurately using in vivo microCT arthrography in three etiologically distinct rat models. J Orthop Res. 2011;29:1788–94. doi: 10.1002/jor.21444. [DOI] [PubMed] [Google Scholar]
- 168.Piscaer TM, Waarsing JH, Kops N, Pavljasevic P, Verhaar JA, van Osch GJ, et al. In vivo imaging of cartilage degeneration using microCT-arthrography. Osteoarthritis Cartilage. 2008;16:1011–7. doi: 10.1016/j.joca.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 169.Hirvasniemi J, Kulmala KA, Lammentausta E, Ojala R, Lehenkari P, Kamel A, et al. In vivo comparison of delayed gadolinium: enhanced MRI of cartilage and delayed quantitative CT arthrography in imaging of articular cartilage. Osteoarthritis Cartilage. 2013;21:434–42. doi: 10.1016/j.joca.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 170.Van Tiel J, Siebelt M, Waarsing JH, Piscaer TM, van Straten M, Booij R, et al. CT arthrography of the human knee to measure cartilage quality with low radiation dose. Osteoarthritis Cartilage. 2012;20:678–85. doi: 10.1016/j.joca.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 171.Aula AS, Jurvelin JS, Toyras J. Simultaneous computed tomography of articular cartilage and subchondral bone. Osteoarthritis Cartilage. 2009;17:1583–8. doi: 10.1016/j.joca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 172.Williams A, Mikulis B, Krishnan N, Gray M, McKenzie C, Burstein D. Suitability of T-1Gd as the “dGEMRIC Index” at 1.5T and 3. 0T. Magn Reson Med. 2007;58:830–4. doi: 10.1002/mrm.21376. [DOI] [PubMed] [Google Scholar]
- 173.Shiomi T, Nishii T, Nakata K, Tamura S, Tanaka H, Yamazaki Y, et al. Three-dimensional topographical variation of femoral cartilage T2 in healthy volunteer knees. Skeletal Radiol. 2013;42:363–70. doi: 10.1007/s00256-012-1522-2. [DOI] [PubMed] [Google Scholar]
- 174.Hirose J, Nishioka H, Nakamura E, Oniki Y, Yamashita Y, Mizuta H. T1rho and T2 mapping of the proximal tibiofibular joint in relation to aging and cartilage degeneration. Eur J Radiol. 2012;81:2776–82. doi: 10.1016/j.ejrad.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 175.Pan JD, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011;261:507–15. doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Souza RB, Feeley BT, Zarins ZA, Link TM, Li XJ, Majumdar S. T1rho MRI relaxation in knee OA subjects with varying sizes of cartilage lesions. Knee. 2013;20:113–9. doi: 10.1016/j.knee.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mamisch TC, Hughes T, Mosher TJ, Mueller C, Trattnig S, Boesch C, et al. T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skeletal Radiol. 2012;41:287–92. doi: 10.1007/s00256-011-1171-x. [DOI] [PubMed] [Google Scholar]
- 178.Staroswiecki E, Bangerter NK, Gurney PT, Grafendorfer T, Gold GE, Hargreaves BA. In vivo sodium imaging of human patellar cartilage with a 3D cones sequence at 3T and 7T. J Magn Reson Imaging. 2010;32:446–51. doi: 10.1002/jmri.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]