Abstract
Background
Management of hepatocellular carcinoma (HCC) in the Model for End-Stage Liver Disease (MELD) exception era remains regionally variable. Outcomes were compared for patients undergoing transplant versus resection at a single institution in a UNOS region with short wait times for organ availability.
Methods
All patients who underwent resection of HCC from January 2000 to August 2012 and patients who underwent transplant post-January 2006, during the Milan Criteria (MC)-based MELD exception policy for HCC, were identified. Primary outcomes were overall survival (OS) and recurrence-free survival (RFS).
Results
Two hundred fifty-seven patients were analyzed, of whom 131 underwent transplant and 126 underwent resection. All transplant patients met MC; 45 (36%) resection patients met MC. Median follow-up time was 30 months. Median wait time to transplant was 55 days; no patients dropped off the waitlist while awaiting an organ.
Among patients meeting MC, transplant demonstrated significantly greater 5-year OS (65.7% vs. 43.8%; P = 0.005) and RFS (85.3% vs. 22.7%; P < 0.001) versus resection. For patients with hepatitis C, transplant (n = 87) demonstrated significantly improved 5-year outcomes compared to patients meeting MC who underwent resection (n = 21; OS: 63.5% vs. 23.3%; P = 0.001; RFS: 83.5% vs. 23.7%; P < 0.001).
Conclusion
In a region with short waitlist times for organ availability, liver transplant is associated with improved survival compared to resection for HCC within MC and should be considered for all patients meeting MC, particularly those with hepatitis C.
Keywords: hepatocellular carcinoma, hepatic resection, liver transplant, Milan Criteria, waitlist time
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third-leading cause of cancer mortality worldwide, and the annual incidence of both new diagnoses and deaths attributed to HCC continues to increase [1,2]. Despite advances in nonsurgical interventional therapies, the best potential curative treatment options for HCC remain liver transplantation or hepatic resection [3–5]. Optimal surgical management of HCC patients remains a point of debate, with practices varying between institutions globally and regionally within the United States. In 1996, Mazzaferro et al. [6] first reported their improved results after transplantation for patients with HCC meeting their Milan Criteria (MC), defined as a single lesion <5 cm or ≤3 lesions each < 3 cm in size, with no evidence of macrovascular invasion or extrahepatic disease on imaging. Numerous studies worldwide, many included in a comprehensive 2011 meta-analysis by the Milan group, have confirmed the favorable outcomes that can be achieved with transplantation for patients meeting these criteria [7]. In light of these results, United Network for Organ Sharing (UNOS) guidelines, universally implemented since 2006, have allocated Model for End-Stage Liver Disease (MELD) exception points to patients on the liver transplant waitlist with a diagnosis of HCC meeting MC.
Liver transplantation for HCC provides the additional benefit of removing the underlying diseased liver, however, it is limited by donor organ availability. Hepatic resection is potentially more widely applicable, but is limited by the substantial morbidity and mortality associated with hepatectomy in patients with significant underlying liver dysfunction [8]. Given the limited number of organs available for transplant and the risk of disease progression while awaiting an organ, resection has historically been advocated for most patients with HCC, especially those with no underlying liver disease or with well-compensated cirrhosis. Given the lack of randomized, prospective data and the difficulties in comparing retrospective studies with heterogeneous cohorts and different inclusion criteria, the question of whether transplantation or resection provides superior overall survival (OS) and recurrence-free survival (RFS) for early HCC remains unclear [4].
Wait times for transplant vary widely among reported studies, significantly affecting outcomes and influencing comparisons with resection, as a function of patient dropout from the waitlist due to disease progression or death. In countries and UNOS regions with comparatively short transplant wait times and greater organ availability, transplantation may be more feasible and associated with better outcomes. While other standards for transplantation such as the UCSF criteria have been validated, the most widely utilized guidelines for patient selection worldwide are based on the MC. The variance in median waitlist times for liver transplantation across UNOS regions is considerable, ranging from 27 to 490 days for patients with a MELD score of 19–24 in 2004, prior to the MELD exception era for HCC [9]. Further underscoring this disparity in wait times, the percentage of all patients underdoing liver transplantation within a wait period of 90 days or less from 1999 to 2008 ranged from 70.9% in Region 3, where the current study was conducted, to only 42.8% of patients in Region 5 (Fig. 1) [10]. We hypothesized that in a region with short waitlist times and greater organ availability, transplantation for the treatment of HCC in the MELD exception era would be associated with improved OS and RFS as compared to hepatic resection.
Fig. 1.
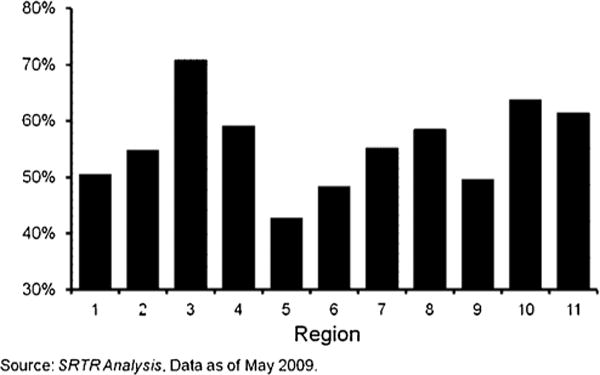
Proportion of liver transplant recipients with a waiting time of 90 days or less by UNOS region, 1999–2008, per Organ Procurement and Transplantation Network (OPTN)/Scientific Registry of Transplant Recipients (SRTR) 2009 Annual Report.
MATERIALS AND METHODS
This study was conducted with the approval of the Institutional Review Board and all research activities were performed in compliance with the Health Insurance Portability and Accountability Act of 1996. All patients undergoing curative intent hepatic resection for a diagnosis of HCC at a single academic institution between January 2000 and June 2012 were identified from a prospectively maintained surgical database. All patients placed on the wait list for orthotopic liver transplantation (OLT) at our institution who received MELD exception points for a known diagnosis of HCC from January 2006 to June 2012 were identified, as the allocation policy of awarding MELD exception points for HCC within MC was universally implemented by January 2006. All transplant patients met MC based on preoperative cross-sectional imaging. Patients with HCC diagnosed incidentally after transplant on examination of the explant specimen were excluded from analysis. Waitlist time was calculated as the period of time from the date of active listing with a diagnosis of HCC for which the patient was awarded MELD exception points, to the date of transplantation. For patients who were placed on the organ allocation waitlist prior to the MELD exception era implementation in January 2006, wait time was calculated from January 1, 2006, as this was the date on which they received their MELD exception points that facilitated their receipt of a liver transplant.
A comprehensive retrospective chart review was conducted to identify patient demographics, clinicopathologic features, and preoperative laboratory values. Preoperative cross-sectional imaging was reviewed to determine whether patients who underwent resection met MC based on tumor size and focality. Raw MELD scores and Child-Pugh scores were calculated retrospectively for each patient, and for patients in the transplant cohort, these scores were validated with the pretransplant evaluations from the medical record. Tumor size, presence of macroscopic or microscopic vascular invasion, tumor differentiation, and the presence of cirrhosis were confirmed from the surgical pathology report.
Statistical Analysis
Comparison of perioperative clinicopathologic features between the transplant and resection cohorts was conducted using chi-square analysis for categorical variables and the Student’s t-test for continuous variables. Kaplan-Meier survival plots and log-rank analysis were conducted to compare outcomes for transplantation versus resection for the primary endpoints of OS and RFS. Survival was calculated from the operative date to the date of confirmed death or the date of last follow-up, with those patients lost to follow-up censored at the date of last contact noted in the medical record or date of confirmed death. The Social Security Death Index was used to verify survival data. RFS was calculated from the operative date to the time of confirmed recurrence on cross-sectional imaging; per Kaplan-Meier methodology, patients without recurrence were censored at their date of death or date of last follow-up. To account for potential differences in clinicopathologic variables between the transplant and resection cohorts, univariate and multivariate Cox regression analyses were performed for OS and RFS. All variables with a P-value ≤0.05 on univariate analysis were included into the multivariate model for each endpoint. Statistical significance was defined as a P-value ≤0.05. All statistical analysis was performed with Statistical Package for the Social Sciences 19.0 software (IBM Corporation, Armonk, NY).
RESULTS
A total of 261 patients were identified. Of the 133 patients with HCC who underwent transplantation during the MELD exception era, 2 patients were excluded from analysis because HCC was only incidentally diagnosed on explants, leaving 131 patients for analysis. Hepatic resection was performed in 128 patients for a diagnosis of HCC. In order to clearly determine the outcomes of each procedure independently, two patients who initially underwent resection and subsequently underwent salvage transplantation for recurrence of HCC were excluded from analysis, leaving 126 patients in the resection cohort (Fig. 2). Demographics and clinicopathologic features for the qualifying 257 patients, classified by operation type, are presented in Table I. Median wait time to transplant was 55 days, and no patients listed with a diagnosis of HCC dropped off the waitlist while awaiting an organ during the study period. Ninety patients (68.7%) listed for transplant underwent bridging therapy while on the wait list; 68 patients received transarterial chemoembolization (TACE) and 22 patients underwent radiofrequency ablation (RFA). The median wait time for the 41 patients who did not receive bridging therapy prior to transplant was 34 days. Median overall follow-up time was 30 months.
Fig. 2.
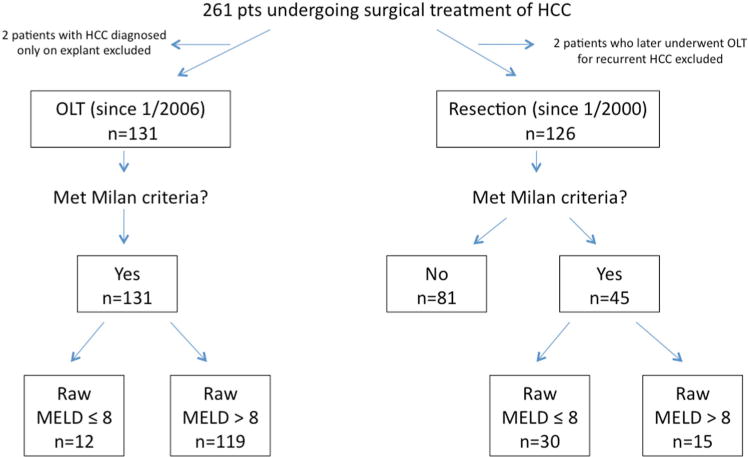
Stratification of patients undergoing surgical treatment of HCC by surgical modality, Milan Criteria, and raw MELD score. HCC, hepatocellular carcinoma; OLT, orthotopic liver transplantation; MELD, Model for End-Stage Liver Disease.
TABLE I.
Demographics and Clinicopathologic Features of All Patients Undergoing Transplantation versus Resection for HCC
| Total (n = 257), n (% total) |
OLT pts (n = 131), n (% group) |
Resection pts (n = 126), n (% group) |
P-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Gender | ||||
| Male | 183 (71.2%) | 102 (77.9%) | 81 (64.3%) | 0.02 |
| Race | ||||
| White | 166 (64.6%) | 100 (76.3%) | 66 (52.4%) | <0.001 |
| Black | 47 (18.3) | 17 (13.0) | 30 (23.8) | |
| Other | 44 (17.1) | 14 (10.7) | 30 (23.8) | |
| Age, median [range], years | 59.0 [20.3–89.8] | 57.0 [38.9–73.3] | 61.5 [20.3–89.8] | 0.02 |
| ASA class | ||||
| 2 | 26 (10.1%) | 26 (20.6%) | <0.001 | |
| 3 | 86 (33.5) | 86 (68.2) | ||
| 4 | 145 (56.4) | 131 (100%) | 14 (11.1) | |
| Hepatitis B | 66 (25.7) | 45 (34.4) | 21 (16.7) | 0.002 |
| Hepatitis C | 123 (47.9) | 87 (66.4) | 36 (28.6) | <0.001 |
| Alcoholic cirrhosis | 55 (21.4) | 36 (27.5) | 19 (15.1) | 0.03 |
| Preoperative labs, median [range] | ||||
| Platelet (×103/μl) | 107 [11–747] | 54 [11–171] | 223 [54–747] | <0.001 |
| Albumin (gm/dl) | 3.2 [1.4–4.8] | 2.9 [1.4–4.1] | 3.5 [1.6–4.8] | <0.001 |
| Total bilirubin (mg/dl) | 1.1 [0.1–16.9] | 2.8 [0.5–16.9] | 0.7 [0.1–8.4] | <0.001 |
| INR | 1.16 [0.86–5.13] | 1.41 [0.93–5.13] | 1.03 [0.86–1.50] | <0.001 |
| Creatinine (mg/dl) | 0.90 [0.40–5.50] | 1.00 [0.46–5.50] | 0.90 [0.40–4.95] | 0.03 |
| AFP | 19 [0–38000] | 13.5 [0–2400] | 34 [1–38000] | 0.02 |
| Raw MELD score | 10 [6–39] | 15 [6–39] | 8 [6–23] | <0.001 |
| Patients within Milan Criteria | 176 (68.5%) | 131 (100%) | 45 (35.7%) | <0.001 |
| Child-Pugh Class | ||||
| Class A | 145 (56.4%) | 37 (28.2%) | 108 (85.7%) | <0.001 |
| Class B | 93 (36.2) | 75 (57.3) | 18 (14.3) | |
| Class C | 19 (7.4) | 19 (14.5) | 0 | |
| Pathology | ||||
| Number of lesions | ||||
| Unifocal | 176 (68.5%) | 73 (55.7%) | 103 (81.7%) | <0.001 |
| Multifocal | 81 (31.5) | 58 (44.3) | 23 (18.3) | |
| Largest tumor size (cm) | 3.7 [0.8–29.0] | 2.5 [0.8–6.0] | 7.0 [1.5–29.0] | <0.001 |
| Differentiation | ||||
| Well/moderate | 227 (87.5%) | 122 (93.1%) | 103 (81.7%) | 0.01 |
| Poor | 32 (12.5) | 9 (6.9) | 23 (18.3) | |
| Macrovascular invasion | 20 (7.8) | 7 (5.3) | 13 (10.3) | 0.21 |
| Microvascular invasion | 78 (30.4) | 23 (17.6) | 55 (43.7) | <0.001 |
| Cirrhosis/fibrosis present | 204 (79.4) | 131 (100) | 73 (57.9) | <0.001 |
| Outcomes | ||||
| 30 day mortality | 12 (4.6%) | 2 (1.5%) | 10 (7.9%) | 0.02 |
| Recurrence, any | 80 (30.9) | 14 (10.7) | 66 (52.4) | <0.001 |
| Intrahepatic recurrence | 62 (23.9) | 11 (8.4) | 51 (41.8) | <0.001 |
| Extrahepatic recurrence | 30 (11.6) | 6 (4.6) | 24 (19.0) | <0.001 |
HCC, hepatocellular carcinoma; OLT, orthotopic liver transplantation; ASA, American Society of Anesthesiology; INR, International Normalized Ratio; AFP, alpha fetoprotein; MELD, Model for End-Stage Liver Disease.
Overall, median age was 59.0 years, and 71% of patients were male. Transplant patients were more likely than patients who underwent resection to have hepatitis B (34.4% vs. 16.7%, P = 0.002) or hepatitis C (66.4% vs. 28.6%, P < 0.001). The mean raw MELD score for the transplant cohort was significantly higher than the resection cohort (15 vs. 8, P< 0.001). Based on preoperative imaging, all 131 of the transplant patients met MC, whereas 45 patients (36%) within the resection cohort met MC. Of the transplant cohort, only 1 patient who initially presented outside of MC was down-staged with locoregional therapy before being placed on the waitlist and subsequently transplanted. Among the resection patients meeting MC, 30 patients had relatively preserved hepatic function, defined as a raw MELD ≤ 8, versus 12 such patients among the transplant cohort (Fig. 2). The majority of patients within the transplant cohort were Child-Pugh Class B or C (71.8%), as opposed to the resection cohort, where most patients were Child-Pugh Class A (85.7%).
The comparison of transplant patients to the 45 patients who underwent resection and met MC is detailed in Table II. Unifocal tumors were present in 73 patients (55.7%) among the transplant cohort, as compared to 39 (86.7%, P < 0.001) patients in the Milan-meeting resection cohort. Patients undergoing resection had significantly larger maximum tumor size (3.9 vs. 2.5 cm, P< 0.001), and had a greater proportion of poorly differentiated tumors (22.2% vs. 6.9%, P = 0.01). Rates of tumor macrovascular and microvascular invasion were not significantly different for patients undergoing transplant versus resection (P = 0.68 and 0.13, respectively). Three patients (6.7%) had microscopically positive margins following resection. Pathologic evidence of cirrhosis or significant fibrosis was present in all 131 patients who underwent transplantation and 39 patients (86.7%) within the resection cohort. No patients undergoing transplantation had fibrolamellar HCC on pathology, versus 1 such patient in the resection cohort.
TABLE II.
Clinicopathologic Features of Patients Undergoing Transplant (n = 131) vs. Resection (n = 45) for HCC Within Milan Criteria
| OLT patients (n = 131), n (% group) | Resection patients within MC (n = 45), n (% group) | P-Value | |
|---|---|---|---|
| Clinical features | |||
| Hepatitis B | 45 (34.4) | 8 (17.8) | 0.04 |
| Hepatitis C | 87 (66.4) | 21 (46.7) | 0.02 |
| Alcoholic cirrhosis | 36 (27.5) | 9 (20.0) | 0.43 |
| Raw MELD, median [range] | 15 [6–39] | 8 [6–23] | <0.001 |
| Child-Pugh Class | |||
| Class A | 37 (28.2%) | 39 (86.7%) | <0.001 |
| Class B | 75 (57.3) | 6 (13.3) | |
| Class C | 19 (14.5) | 0 | |
| Pathologic features | |||
| Number of lesions | |||
| Unifocal | 73 (55.7%) | 39 (86.7%) | <0.001 |
| Multifocal | 58 (44.3) | 6 (13.3) | |
| Largest tumor size (cm) | 2.5 [0.8–6.0] | 3.9 [1.5–7.0] | <0.001 |
| Differentiation | |||
| Well/moderate | 122 (93.1%) | 35 (77.8%) | 0.01 |
| Poor | 9 (6.9) | 10 (22.2) | |
| Macroscopic vascular invasion | 7 (5.3) | 1 (2.2) | 0.68 |
| Microscopic vascular invasion | 23 (17.6) | 13 (28.9) | 0.13 |
| Margin positivity | 0 | 3 (6.7) | 0.02 |
| Cirrhosis/fibrosis present | 131 (100) | 39 (86.7) | <0.001 |
| Outcomes | |||
| 30 day mortality | 2 (1.5%) | 3 (6.7%) | 0.11 |
| Recurrence, any | 14 (10.7) | 22 (48.9) | <0.001 |
| Intrahepatic recurrence | 11 (8.4) | 19 (42.2) | <0.001 |
| Extrahepatic recurrence | 6 (4.6) | 4 (8.9) | 0.28 |
MC, Milan Criteria; HCC, hepatocellular carcinoma; OLT, orthotopic liver transplantation; MELD, Model for End-Stage Liver Disease.
Recurrence and Survival
Among the entire cohort, 80 patients (30.9%) had recurrence of HCC, with significantly greater rates of recurrence among patients undergoing resection as compared to transplant (52.4% vs. 10.7%, P < 0.001). The majority of recurrences in both groups occurred within the liver (Table I). Of the 45 patients within MC who underwent resection, 22 (48.9%) experienced recurrence of their HCC (Table II). Only 4 of these 22 patients had a recurrence within MC following resection. Of these 22 patients, 6 subsequently underwent repeat hepatic resection, 5 were treated with TACE or Yttrium (Y-90) radio-embolization, 3 with RFA, and 6 were started on sorafenib therapy. Among the 131 transplant patients, 14 (10.7%; P< 0.001) experienced HCC recurrence; 2 underwent surgical resection, 4 were treated with TACE or Y-90 radio-embolization, 2 with RFA, 1 with systemic chemotherapy, and 3 were started on sorafenib.
Among all patients, transplant was associated with significantly greater 5-year OS and 5-year RFS as compared to resection (OS: 65.7% vs. 33.2%, P< 0.001; RFS: 85.3% vs. 22.9%, P< 0.001). When comparing all transplant patients to those undergoing resection who met MC (n = 45), transplantation was associated with significantly improved 5-year OS (65.7% vs. 43.8%, P = 0.005; Fig. 3) and RFS (85.3% vs. 22.7%, P < 0.001; Fig. 4). On subset analysis of patients meeting MC with hepatitis C, patients undergoing transplantation (n = 87) demonstrated significantly greater 5-year OS (63.5% vs. 23.3%, P = 0.001; Fig. 5) and RFS (83.5% vs. 23.7%, P < 0.001; Fig. 6) as compared to those patients undergoing resection (n = 21).
Fig. 3.
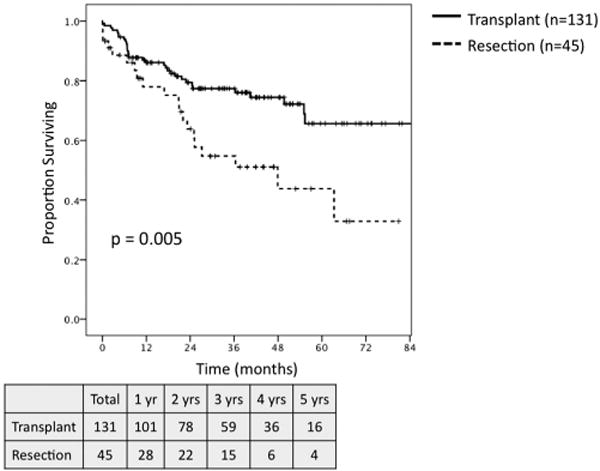
Overall survival for all patients meeting Milan Criteria based on preoperative imaging undergoing transplant (n = 131) versus resection (n = 45).
Fig. 4.
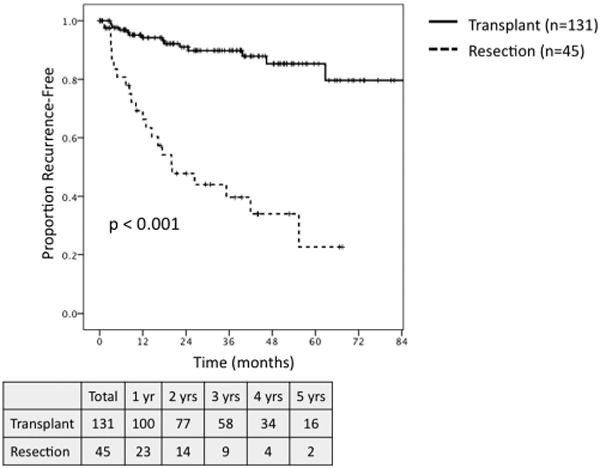
Recurrence-free survival for all patients meeting Milan Criteria based on preoperative imaging undergoing transplant (n = 131) versus resection (n = 45).
Fig. 5.
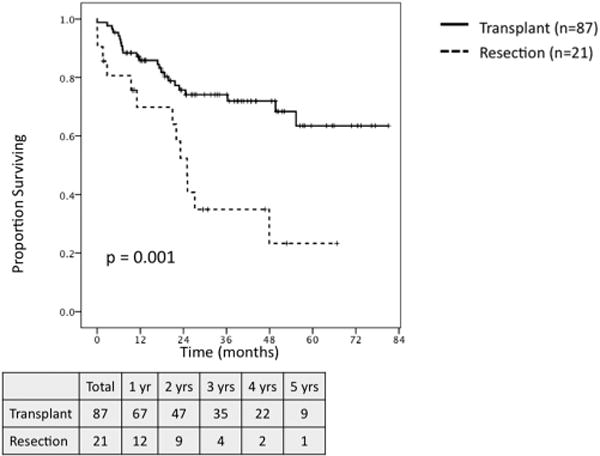
Overall survival for patients meeting Milan Criteria with hepatitis c undergoing transplant (n = 87) versus resection (n = 21).
Fig. 6.
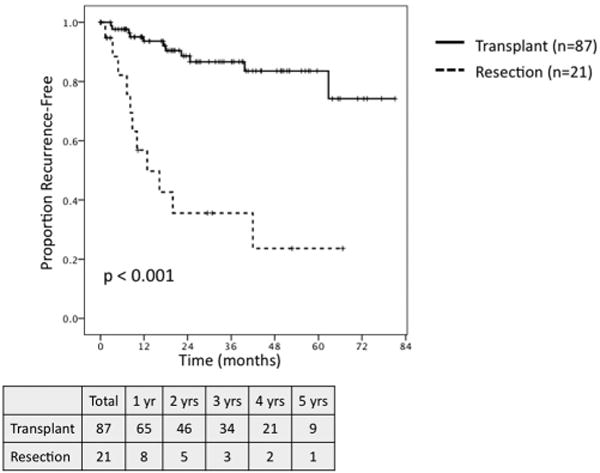
Recurrence-free survival for patients meeting Milan Criteria with hepatic C undergoing transplant (n = 87) versus resection (n = 21).
On survival analysis of patients meeting MC with preserved hepatic function, defined as a raw MELD score ≤ 8, transplantation (n = 12) and resection (n = 30) were associated with similar 5-year OS (62.5% vs. 48.9%, P = NS; Fig. 7), but transplantation demonstrated a trend towards greater RFS (71.6% vs. 30.8%, P = 0.08; Fig. 8). When comparing outcomes for patients meeting MC with well-compensated Child-Pugh Class A cirrhosis, transplantation (n = 37) demonstrated a trend towards greater 5-year OS (56% vs. 35%, P = 0.07; Fig. 9) and was associated with significantly improved RFS (71% vs. 37%, P = 0.04; Fig. 10) as compared to resection (n = 16).
Fig. 7.
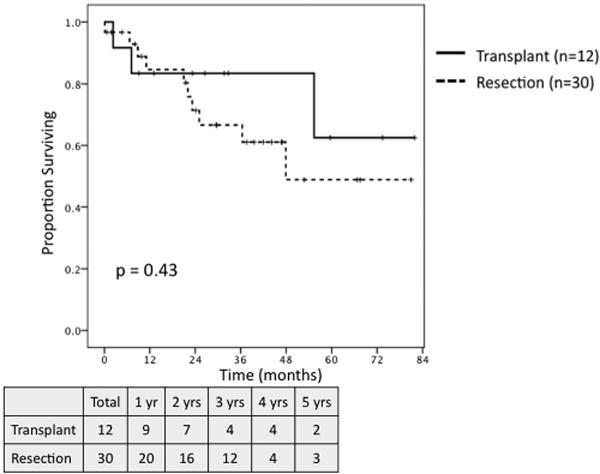
Overall survival for patients meeting Milan Criteria with raw MELD ≤ 8 undergoing transplant (n = 12) versus resection (n = 30).
Fig. 8.
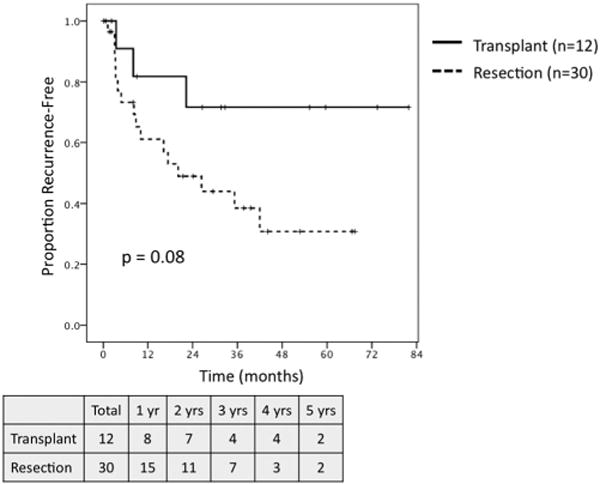
Recurrence-free survival for patients meeting Milan Criteria with raw MELD ≤ 8 undergoing transplant (n = 12) versus resection (n = 30).
Fig. 9.
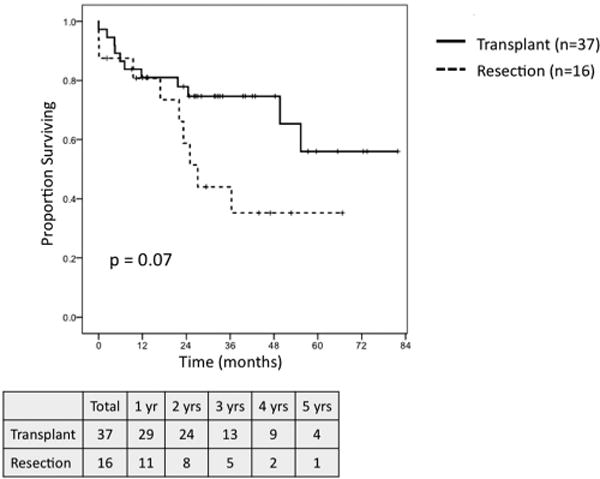
Overall survival for patients meeting Milan Criteria with Child-Pugh Class A cirrhosis undergoing transplant (n = 37) versus resection (n = 16).
Fig. 10.
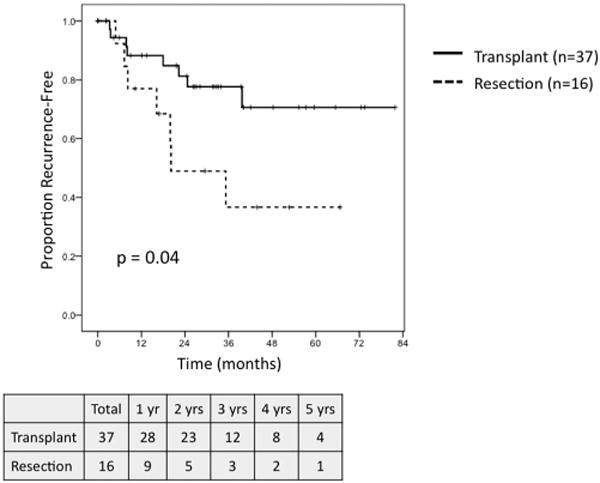
Recurrence-free survival for patients meeting Milan Criteria with Child-Pugh Class A cirrhosis undergoing transplant (n = 37) versus resection (n = 16).
Prognostic Factors for OS and RFS in Patients Meeting Milan Criteria
The univariate and multivariate Cox regression analyses of all patients meeting MC (n = 176) for OS and RFS are presented in Tables III and IV, respectively. After accounting for other adverse pathologic features, resection remained independently associated with decreased OS (HR 2.91; 95% CI: 1.52–5.57; P = 0.001) and decreased RFS (HR 9.98; 95% CI; 2.60–38.39; P = 0.001), as compared to transplant.
TABLE III.
Univariate and Multivariate Analysis of Risk Factors Associated With Overall Survival for Patients With HCC Within Milan Criteria (n = 176)
| Univariate
|
Multivariate
|
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-Value | HR (95% CI) | P-Value |
| Age | 1.02 (0.98–2.05) | 0.36 | ||
| Gender, male | 1.40 (0.70–2.80) | 0.34 | ||
| Race | ||||
| White | Ref | Ref | ||
| Black | 1.97 (1.05–3.69) | 0.03 | 1.57 (0.82–3.00) | 0.18 |
| Other | 0.71 (0.28–1.82) | 0.47 | 0.56 (0.21–1.47) | 0.24 |
| ASA class | ||||
| 2 | Ref | |||
| 3 | 0.55 (0.12–2.39) | 0.42 | ||
| 4 | 0.30 (0.07–1.24) | 0.11 | ||
| Hepatitis B | 1.68 (0.96–2.92) | 0.07 | ||
| Hepatitis C | 1.54 (0.85–2.77) | 0.15 | ||
| Alcohol abuse | 1.64 (0.90–2.99) | 0.11 | ||
| Tumor size | 1.16 (0.96–1.41) | 0.15 | ||
| Macrovascular invasion | 3.40 (1.35–8.61) | 0.01 | 3.56 (1.32–9.58) | 0.01 |
| Microvascular invasion | 1.51 (0.80–2.83) | 0.20 | ||
| Tumor grade, poor | 2.30 (1.12–4.72) | 0.02 | 1.68 (0.78–3.62) | 0.19 |
| Resection | 2.20 (1.26–3.85) | 0.006 | 2.23 (1.20–4.14) | 0.01 |
HCC, hepatocellular carcinoma; ASA, American Society of Anesthesiology; HR, hazard ratio; CI, confidence interval.
The bolded text appearing in Table designates statistical significance (P ≤ 0.05).
TABLE IV.
Univariate and Multivariate Analysis of Risk Factors Associated With Recurrence-Free Survival for Patients With HCC Within Milan Criteria (n = 176)
| Univariate
|
Multivariate
|
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-Value | HR (95% CI) | P-Value |
| Age | 1.03 (0.99–1.08) | 0.17 | ||
| Gender, male | 1.21 (0.55–2.65) | 0.64 | ||
| Race | ||||
| White | Ref | Ref | ||
| Black | 2.47 (1.16–5.25) | 0.02 | 1.38 (0.60–3.20) | 0.45 |
| Other | 1.13 (0.42–3.00) | 0.81 | 0.54 (0.18–1.61) | 0.27 |
| ASA class | 2.29 (1.18–3.46) | <0.001 | 1.54 (0.47–5.02) | 0.48 |
| Hepatitis B | 0.92 (0.44–1.91) | 0.82 | ||
| Hepatitis C | 1.05 (0.54–2.05) | 0.90 | ||
| Alcohol abuse | 1.16 (0.53–2.56) | 0.71 | ||
| Tumor size | 1.55 (1.25–1.94) | <0.001 | 1.24 (0.95–1.62) | 0.12 |
| Macrovascular invasion | 4.14 (1.45–11.78) | 0.01 | 8.51 (1.93–37.57) | 0.005 |
| Microvascular invasion | 2.39 (1.19–4.78) | 0.01 | 1.17 (0.42–3.29) | 0.76 |
| Tumor grade, poor | 4.18 (1.96–8.92) | <0.001 | 2.37 (0.93–6.03) | 0.07 |
| Resection | 7.40 (3.77–14.52) | <0.001 | 9.98 (2.60–38.39) | 0.001 |
HCC, hepatocellular carcinoma; ASA, American Society of Anesthesiology; HR, hazard ratio; CI, confidence interval.
The bolded text appearing in Table designates statistical significance (P ≤ 0.05).
DISCUSSION
This study represents a single-institution comparison of outcomes for transplantation versus hepatic resection for the treatment of HCC in the MELD exception era in a region with short waitlist times for organ availability. Overall, transplantation was associated with greater 5-year OS and RFS versus resection. The more clinically relevant comparison limited to those patients meeting MC demonstrated significantly greater 5-year OS (65.7% vs. 43.8%, P = 0.005) and RFS (85.3% vs. 22.7%, P < 0.001) in favor of transplantation as compared to resection.
The optimal surgical management of early HCC within MC remains controversial and regionally dependent. The results of the present study are consistent with several studies over the past decade that have suggested that transplantation, despite the potential morbidity of the procedure and the burden of life-long immunosuppression, may offer superior survival and substantially less risk of recurrence compared to resection [11–15]. Other studies have suggested that hepatic resection may provide equivalent or superior results to transplantation for select patient subgroups, particularly those with minimal or well-compensated hepatic dysfunction [16–18]. A recent meta-analysis of outcomes for patients undergoing hepatic resection of HCC meeting MC concluded that resection in patients with preserved liver function produced good outcomes, with a 5-year OS of 67% (range, 27–81%), but was associated with a substantial risk of disease recurrence (5-year RFS: 37%; range, 21–57%) [19].
When analyzing patients in the current study with preserved hepatic function and a raw MELD score ≤ 8, similar 5-year OS was observed for transplantation and resection (62.5% vs. 48.9%, P = NS), although transplantation was associated with a trend towards improved RFS (71.6% vs. 30.8%, P = 0.08). When stratified by Child-Pugh score, patients with well-compensated Child-Pugh Class A cirrhosis demonstrated a trend towards improved 5-year OS with transplantation (56% vs. 35%, P = 0.07) and significantly lower rates of recurrence (5-year RFS: 71% vs. 37%, P = 0.04). These results suggest that while patients with minimal liver dysfunction undergoing resection for HCC within MC may achieve comparable survival in some cases, the majority of such patients are living with recurrent disease.
A separate meta-analysis by Dhir et al. [20] comparing outcomes for transplantation versus resection in patients with early HCC found no significant difference in survival, based on an intention-to-treat strategy. When the analysis was limited to only patients with HCC and well-compensated cirrhosis, transplantation was associated with significantly improved 5-year OS, although the authors remarked that only three small studies qualified for this subset meta-analysis [20]. A 2009 study by Cherqui et al. [21] of 67 patients with Child-Pugh Class A cirrhosis and HCC meeting MC reported excellent 5-year OS of 72% following resection, but significant risk of recurrence. Some authors have advocated a strategy of initial resection for patients with HCC within MC with preserved liver function, followed by “salvage transplantation” for recurrent disease [21–24]. Unfortunately a significant number of these patients never reach salvage transplantation due to recurrence outside of MC or extra-hepatic recurrence, questioning the role of such a strategy [21–23]. While the present study was not designed to assess the utility of salvage transplantation for recurrent HCC following initial resection, only 4 of the 22 patients analyzed in this study with recurrent disease following primary resection of HCC within MC had a recurrence within MC that would have afforded them the opportunity for transplantation. Given the substantial number of patients expected to recur following resection and the low likelihood of qualifying for salvage transplantation, primary transplantation appears to confer an oncologic advantage over resection and should be considered for most patients with HCC within MC in regions with short waitlist times.
Another consideration in evaluating the role of these two surgical modalities for patients with HCC is the presence of underlying hepatitis C. Patient with hepatitis C tend to have high rates of recurrence and poor outcomes following resection for HCC [25,26]; even in the setting of relatively preserved hepatic function, some have argued that these patients may derive significant benefit from transplantation [27]. Of particular interest in the present study was the significantly improved OS and RFS associated with transplantation as compared to resection for those patients meeting MC with hepatitis C. Given similar recent findings of poor outcomes for hepatitis C-positive patients undergoing resection for HCC within MC [25–27], these current results suggest that transplantation should be favored over resection for patients with underlying hepatitis C.
The 5-year RFS for patients who underwent transplantation was in fact greater than the 5-year OS; this was not the case for resection patients. The explanation for this finding lies in the fact that, unlike the resection cohort, very few patients within the transplant cohort died due to recurrent HCC (n = 8). Per Kaplan-Meier methodology, an analysis of RFS measures the event of recurrence and the duration of survival until that event. By definition, RFS is terminated at the date of recurrence; all patients who remain recurrence-free at the time of death or time of last follow-up are censored. While transplant patients experienced mortality that related to their liver transplant (sepsis, graft failure, etc.), only 8 of the 32 deceased transplant patients had recurrent HCC at the time of their death. The remaining 24 deceased transplant patients had no evidence of HCC recurrence and thus their RFS duration was appropriately censored at their time of death. This finding is what accounts for the fact that RFS exceeded OS in the transplant cohort.
These results contrasted starkly with the resection cohort. The recurrence rate for HCC was not only significantly greater for patients within MC undergoing resection versus transplantation (49% vs. 11%, P < 0.001), but increased recurrence directly translated into significantly worse survival for patients treated with resection. Of the 20 deceased resection patients, 14 had recurrent HCC at the time of their death. These findings further highlight the significantly greater risk of recurrence and the detrimental effect of recurrence on survival for patients undergoing resection for HCC within MC, compared to transplantation in a UNOS region where organs are readily available and wait times are routinely less than 60 days.
The present study, similarly to most previously published series of HCC patients, is limited by its retrospective design and nonrandomized patient selection. As expected, the clinicopathologic features of the transplantation and resection groups in the present study were significantly different, with patients in the resection cohort having larger and more poorly differentiated tumors than those in the transplant cohort. By limiting the analysis to patients within MC and stratifying patients by MELD score and Child-Pugh score, we sought to minimize confounding discrepancies and optimize the comparisons between the two cohorts. Additionally, Cox regression analyses were performed in order to account for potential differences between the transplant and resection cohorts. Even after accounting for adverse pathologic features such as tumor size, differentiation, and vascular invasion, resection remained independently associated with worse OS and RFS as compared to transplant for patients meeting MC.
Another limitation is the potential for bias in terms of patient follow-up; transplant patients are routinely seen in clinic every 4–6 months posttransplant, while more patients who underwent resection were lost to follow-up over the study period. The perioperative mortality rate of the patients undergoing resection was higher than expected, suggesting that some of these patients may have been borderline resection candidates, and perhaps would have been better served with transplantation or primary therapy with interventional techniques such as RFA, TACE, or Y-90. All patients at our institution placed on the transplant wait list with a diagnosis of HCC are considered for bridging therapy. Ninety patients (68.7%) received pretransplant TACE or RFA, and the median time on the wait list for those patients who did not receive bridging therapy was only 34 days, compared to 55 days for the entire cohort. Studies have not demonstrated a correlation between pretransplant locoregional bridging therapy and improved survival, and given the short waitlist times in the current study, it is unlikely that the improved outcomes associated with transplant are attributable to bridging therapy [28,29].
The results of the current study need to be interpreted in the context of short waitlist times for transplant. They may not be applicable or reproducible in regions with longer waitlist times or lesser organ availability. A study by Shah et al. [30] determined that transplantation was associated with superior survival to resection for patients with HCC within MC and Child-Pugh Class A or B cirrhosis only when wait time to transplant was less than 4 months. A median waitlist time of 55 days in the present study contrasts starkly with median wait times of 6–12 months in many published reports from other regions of the United States and globally [31,32]. In this series, no patients were removed from the waitlist due to disease progression or death while awaiting transplantation; this is certainly not the case in other regions [17,31].
CONCLUSION
In regions with short wait times, transplantation for HCC within MC may provide improved overall and RFS compared to resection. Given the substantial risk of recurrence following primary resection for patients with HCC and underlying hepatitis C and the associated poor outcomes, all patients with hepatitis C should be considered for transplantation. In patients with preserved liver function, whether defined as Child-Pugh Class A or a MELD score ≤ 8, transplantation and resection appear to provide similar OS. The significant recurrence rate associated with resection, however, suggests that transplantation may provide an oncologic advantage in these patients as well. Transplantation should be considered for all patients with HCC meeting MC, including those with minimal liver dysfunction and particularly those with hepatitis C, when being managed in a region with short wait times for organ availability.
Acknowledgments
This study was supported in part by the Katz Foundation. S.B.F. is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Grant sponsor: Katz Foundation; Grant sponsor: National Institutes of Health; Grant number: UL1TR000454.
Abbreviations
- HCC
hepatocellular carcinoma
- MC
Milan Criteria
- MELD
Model for End-Stage Liver Disease
- OLT
orthotopic liver transplantation
- OS
overall survival
- RFA
radiofrequency ablation
- RFS
recurrence-free survival
- TACE
transarterial chemoembolization
- UNOS
United Network for Organ Sharing
Footnotes
Presented at the 2013 Annual Meeting of the Society of Surgery for the Alimentary Tract, Digestive Disease Week, May 2013, Orlando, Florida.
Conflict of interest disclosure: None.
Malcolm H. Squires and Steven I. Hanish shared first authorship.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Jarnagin WR. Management of small hepatocellular carcinoma: A review of transplantation, resection, and ablation. Ann Surg Oncol. 2010;17:1226–1233. doi: 10.1245/s10434-010-0978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: Current management and perspectives for the future. Ann Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: An evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 8.Teh SH, Christein J, Donohue J, et al. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207–1215. doi: 10.1016/j.gassur.2005.09.008. discussion 1215. [DOI] [PubMed] [Google Scholar]
- 9.Organ Procurement and Transplantation Network. Liver Kaplan-Meier Median Waiting Times for Registrations Listed: 1999–2004. As of May 24, 2013. Available at: http://optn.transplant.hrsa.gov.
- 10.2009 OPTN/SRTR Annual Report. Chapter IV: Liver transplantation in the United States 1999–2008. [Available through U.S. Department of Health and Human Services at: http://www.ustransplant.org/annual_reports/current/chapter_iv_AR_cd.htm]
- 11.Lee KK, Kim DG, Moon IS, et al. Liver transplantation versus liver resection for the treatment of hepatocellular carcinoma. J Surg Oncol. 2010;101:47–53. doi: 10.1002/jso.21415. [DOI] [PubMed] [Google Scholar]
- 12.Baccarani U, Isola M, Adani GL, et al. Superiority of transplantation versus resection for the treatment of small hepatocellular carcinoma. Transpl Int. 2008;21:247–254. doi: 10.1111/j.1432-2277.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- 13.Adam R, Bhangui P, Vibert E, et al. Resection or transplantation for early hepatocellular carcinoma in a cirrhotic liver: Does size define the best oncological strategy? Ann Surg. 2012;256:883–891. doi: 10.1097/SLA.0b013e318273bad0. [DOI] [PubMed] [Google Scholar]
- 14.Bellavance EC, Lumpkins KM, Mentha G, et al. Surgical management of early-stage hepatocellular carcinoma: Resection or transplantation? J Gastrointest Surg. 2008;12:1699–1708. doi: 10.1007/s11605-008-0652-2. [DOI] [PubMed] [Google Scholar]
- 15.Kooby DA, Egnatashvili V, Graiser M, et al. Changing management and outcome of hepatocellular carcinoma: Evaluation of 501 patients treated at a single comprehensive center. J Surg Oncol. 2008;98:81–88. doi: 10.1002/jso.21049. [DOI] [PubMed] [Google Scholar]
- 16.Koniaris LG, Levi DM, Pedroso FE, et al. Is surgical resection superior to transplantation in the treatment of hepatocellular carcinoma? Ann Surg. 2011;254:527–537. doi: 10.1097/SLA.0b013e31822ca66f. discussion 537–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facciuto ME, Rochon C, Pandey M, et al. Surgical dilemma: Liver resection or liver transplantation for hepatocellular carcinoma and cirrhosis. Intention-to-treat analysis in patients within and outwith Milan criteria. HPB (Oxford) 2009;11:398–404. doi: 10.1111/j.1477-2574.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margarit C, Escartin A, Castells L, et al. Resection for hepatocellular carcinoma is a good option in Child–Turcotte–Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 2005;11:1242–1251. doi: 10.1002/lt.20398. [DOI] [PubMed] [Google Scholar]
- 19.Lim KC, Chow PK, Allen JC, et al. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622–1629. doi: 10.1002/bjs.8915. [DOI] [PubMed] [Google Scholar]
- 20.Dhir M, Lyden ER, Smith LM, et al. Comparison of outcomes of transplantation and resection in patients with early hepatocellular carcinoma: A meta-analysis. HPB (Oxford) 2012;14:635–645. doi: 10.1111/j.1477-2574.2012.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherqui D, Laurent A, Mocellin N, et al. Liver resection for transplantable hepatocellular carcinoma: Long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250:738–746. doi: 10.1097/SLA.0b013e3181bd582b. [DOI] [PubMed] [Google Scholar]
- 22.Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: An intention-to-treat analysis. Hepatology. 2012;55:132–140. doi: 10.1002/hep.24680. [DOI] [PubMed] [Google Scholar]
- 23.Cucchetti A, Vitale A, Gaudio MD, et al. Harm and benefits of primary liver resection and salvage transplantation for hepatocellular carcinoma. Am J Transplant. 2010;10:619–627. doi: 10.1111/j.1600-6143.2009.02984.x. [DOI] [PubMed] [Google Scholar]
- 24.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: Implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kao WY, Su CW, Chau GY, et al. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg. 2011;35:858–867. doi: 10.1007/s00268-010-0928-z. [DOI] [PubMed] [Google Scholar]
- 26.Huo TI, Wu JC, Hsia CY, et al. Hepatitis C virus infection is a risk factor for tumor recurrence after resection of small hepatocellular carcinomas. World J Surg. 2004;28:787–791. doi: 10.1007/s00268-004-7320-9. [DOI] [PubMed] [Google Scholar]
- 27.Chirica M, Tranchart H, Tan V, et al. Infection with hepatitis C virus is an adverse prognostic factor after liver resection for early-stage hepatocellular carcinoma: Implications for the management of hepatocellular carcinoma eligible for liver transplantation. Ann Surg Oncol. 2013;20:2405–2412. doi: 10.1245/s10434-012-2861-x. [DOI] [PubMed] [Google Scholar]
- 28.Porrett PM, Peterman H, Rosen M, et al. Lack of benefit of pretransplant locoregional hepatic therapy for hepatocellular cancer in the current MELD era. Liver Transpl. 2006;12:665–673. doi: 10.1002/lt.20636. [DOI] [PubMed] [Google Scholar]
- 29.Heckman JT, Devera MB, Marsh JW, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol. 2008;15:3169–3177. doi: 10.1245/s10434-008-0071-3. [DOI] [PubMed] [Google Scholar]
- 30.Shah SA, Cleary SP, Tan JC, et al. An analysis of resection vs transplantation for early hepatocellular carcinoma: Defining the optimal therapy at a single institution. Ann Surg Oncol. 2007;14:2608–2614. doi: 10.1245/s10434-007-9443-3. [DOI] [PubMed] [Google Scholar]
- 31.Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6:1416–1421. doi: 10.1111/j.1600-6143.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 32.Pelletier SJ, Fu S, Thyagarajan V, et al. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl. 2009;15:859–868. doi: 10.1002/lt.21778. [DOI] [PubMed] [Google Scholar]


