Abstract
The migration of cells is a complex process that is dependent on the properties of the surrounding environment. In vivo, the extracellular environment is complex with a wide range of physical features, topographies, and protein compositions. There have been a number of approaches to design substrates that can recapitulate the complex architecture in vivo. Two-dimensional (2D) substrates have been widely used to study the effect of material properties on cell migration. However, such substrates do not capture the intricate structure of the extracellular environment. Recent advances in hydrogel assembly and patterning techniques have enabled the design of new three-dimensional (3D) scaffolds and microenvironments. Investigations conducted on these matrices provide growing evidence that several established migratory trends obtained from studies on 2D substrates could be significantly different when conducted in a 3D environment. Since cell migration is closely linked to a wide range of physiological functions, there is a critical need to examine migratory trends on 3D matrices. In this review, our goal is to highlight recent experimental studies on cell migration within engineered 3D hydrogel environments and how they differ from planar substrates. We provide a detailed examination of the changes in cellular characteristics such as morphology, speed, directionality, and protein expression in 3D hydrogel environments. This growing field of research will have a significant impact on tissue engineering, regenerative medicine, and in the design of biomaterials.
Introduction
Cells migrate in response to changes in their physical and chemical microenvironment.1 Cell migration plays an important role in modulating several physiological processes such as tissue development, wound healing, inflammation, and disease progression.2–4 During embryogenesis, the concentration of morphogens directs cellular migration, which subsequently governs cellular differentiation.5–7 The failure of cells to migrate to appropriate locations can result in altered physiological functions and diseases.2 During wound repair, cells secrete platelet-derived growth factor (PDGF) resulting in the recruitment of fibroblasts.8 Cells that mediate the immune response migrate to the infected tissues to remove invading pathogens and debris.9,10 Cancer cell metastasis occurs due to an altered phenotype caused by changes in the surrounding extracellular matrix (ECM) that guides cells to other tissues.11–15
One of the critical steps that initiate cell migration is the differential localization and concentrations of proteins. This step is known as polarization. This phenomenon occurs upon exposure to a variety of external signals present in the cellular environment. Such cues can be mechanical,16,17 electrical,18 optical,19 or chemical20–22 in nature. Cell polarization is followed by the creation of actin-rich protrusions (e.g., filopodia and lamellipodia).1,23 The actin cytoskeleton combined with proteins and transmembrane integrins leads to the formation of focal adhesions (FAs). FAs in conjunction with GTPases (Rho, Rac, and Cdc42) and myosin proteins enable migration.2,24
There have been significant advances in understanding the processes that guide cell migration on two-dimensional (2D) substrates.2,3,25–29 Despite these major strides, there is growing recognition that 2D substrates cannot capture the complex facets of an in vivo environment.30–36 This limitation constrains our ability to extrapolate biological phenomena observed on planar materials to cellular processes in vivo. The chemical microenvironment through which cells move in vivo is a complex combination of ECM proteins, proteoglycans, polysaccharides, growth factors, and signaling molecules.37 The physical properties of the extracellular environment are dictated by the interactions between these various components. The physical environment that cells experience can be flat or exhibit topographical features. In addition, the cellular environment can be fibrillar, porous, soft, rigid, or viscoelastic. Thus, the migration of cells on 2D substrates and within three-dimensional (3D) matrices is bound to differ underscoring the need to engineer new materials that are suitable to investigate migration in 3D.38–43
In this review, we have focused on highlighting the contrasts between migration on 2D surfaces and in 3D hydrogel matrices. Hydrogel properties can be tuned to recapitulate the structure of the microenvironment found in vivo. Within hydrogels, we have aimed to highlight how changing the landscape from flat to a 3D microenvironment can result in changes in cell morphology, speed, persistence, and protein expression. Although there are trends that are consistently exhibited both on 2D surfaces and in 3D matrices, changes in the chemical structure, cell density, and ECM composition of the microenvironment can alter migratory patterns.44 Throughout this review, we call attention to these distinctions, describe the support in the literature, and summarize the main conclusions. In this article, we have focused our attention on normal cells. Since fibroblasts have been widely used to investigate migration, we have frequently cited reports that elucidate the effects of matrix dimensionality using these cells. There are numerous differences in cellular locomotion between normal and cancerous cells. Describing them in depth is beyond the scope of this review. Instead, we have summarized trends observed with cancerous cells in Table 1.
Table 1.
A Summary of Migratory Behavior of Cancer Cells Cultured Within Hydrogels
| Substrate | Cell type | Results |
|---|---|---|
| Cell-derived matrix | HT1080 fibrosarcoma | Morphologies were a mixture of ameboid and mesenchymal. Lobopodia were absent.38 |
| Collagen (2D and 3D) | U2OS human osteosarcoma | Migration speed goes down from 5 μm/h (2D) to 2 μm/h (3D). The difference was statistically significant.89 |
| Collagen (2D and 3D) | MCF7 breast adenocarcinoma | Cell speed in 3D matrices was extremely low (500 nm/h).89 |
| Collagen (2D and 3D) | HT1080 fibrosarcoma | Well-defined FAs observed only on 2D. In 3D focal adhesions were <0.3 μm and lasted 1 s. In 2D, FAs were ∼15 μm and lasted >15 min.78 |
| PEG | Transformed breast epithelial | Cell speed increased approximately twofold in 3D.90 |
| Matrigel™ | MDA-MB-231 breast cancer | Cells exhibit invadopodia with a high density of paxillin, ARP 2/3 complex, WASP, and cortactin proteins. Some cells also exhibited ameboid morphology.48 |
| Matrigel | MDA-MB-231 breast cancer | Fewer FAs observed on 3D compared with 2D. In 3D, FAs were observed in regions that were in contact with Matrigel.48 |
| Matrigel | MDA-MB-231 breast cancer | Uropods with F-actin and myosin IIa. Cell propulsion was due to uropods and not due to lamellipodia or bleb formation.49 |
| Collagen lattices | Mesenchymal melanoma | Actin-rich filopods mediate migration through extension/retraction cycles that mimic caterpillar-like motion.57 |
| Degradable PEG | HT1080 | Cells were smaller and exhibited elongated morphology within 3D gels.91 |
2D, two-dimensional; 3D, three-dimensional; ARP, actin related protein; FA, focal adhesion; PEG, polyethylene glycol; WASP, Wiskott–Aldrich syndrome protein.
Hydrogels
Hydrogels are widely used for monitoring cellular locomotion since their mechanical and chemical properties can be tuned to match the characteristics of different tissues.45–50 In addition, they are biocompatible and can be functionalized with cell-adhesive ligands.51 Hydrogels can be assembled from a single ECM protein, their mixtures such as Matrigel™,48,52–57 or from synthetic materials such as polyethylene glycol (PEG) and other polymers.58–60
Cell morphology
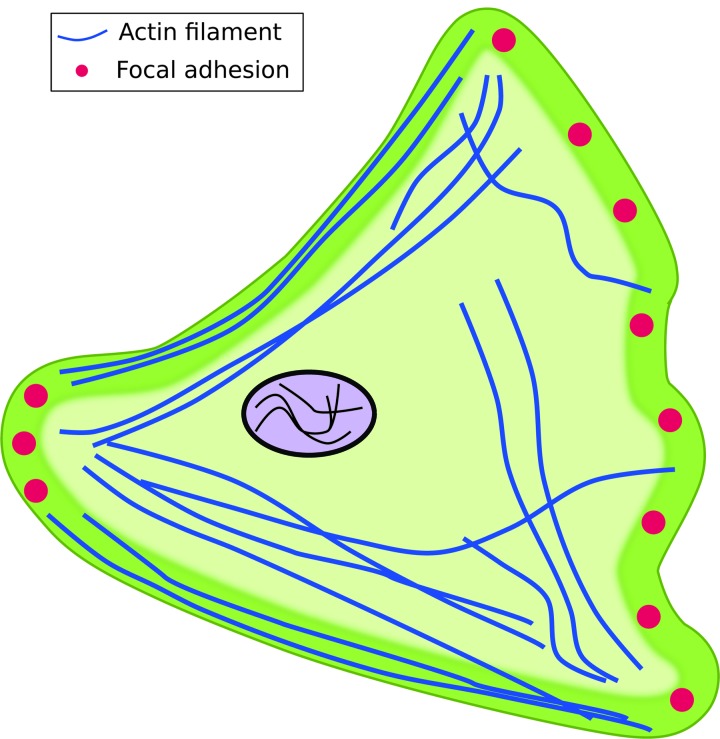
The morphology of cells is often dictated by the protrusions they exhibit. Depending on the actin structure within these protrusions, microvilli, filopodia, lamellipodia, or lobopodia can be exhibited by cells.1,23 Due to differences in the number of lamellipodia and FA distribution, morphology can change between 2D and 3D environments. On 2D substrates, cells such as fibroblasts exhibit a well-spread morphology with multiple lamellipodia and FAs (Fig. 1).2,3,25–29 In contrast, fibroblast morphology in vivo and in 3D can be stellate with fewer lamellipodia and FAs (Fig. 2A) or exhibit blunt lobopodia (Fig. 2B).39,52,61,62
FIG. 1.
Schematic of a cell adherent on a planar two-dimensional (2D) substrate. Cells exhibit a well-spread morphology, lamellipodia, and focal adhesions (FAs). FAs are primarily located in the leading and trailing edges of the cell. Color images available online at www.liebertpub.com/teb
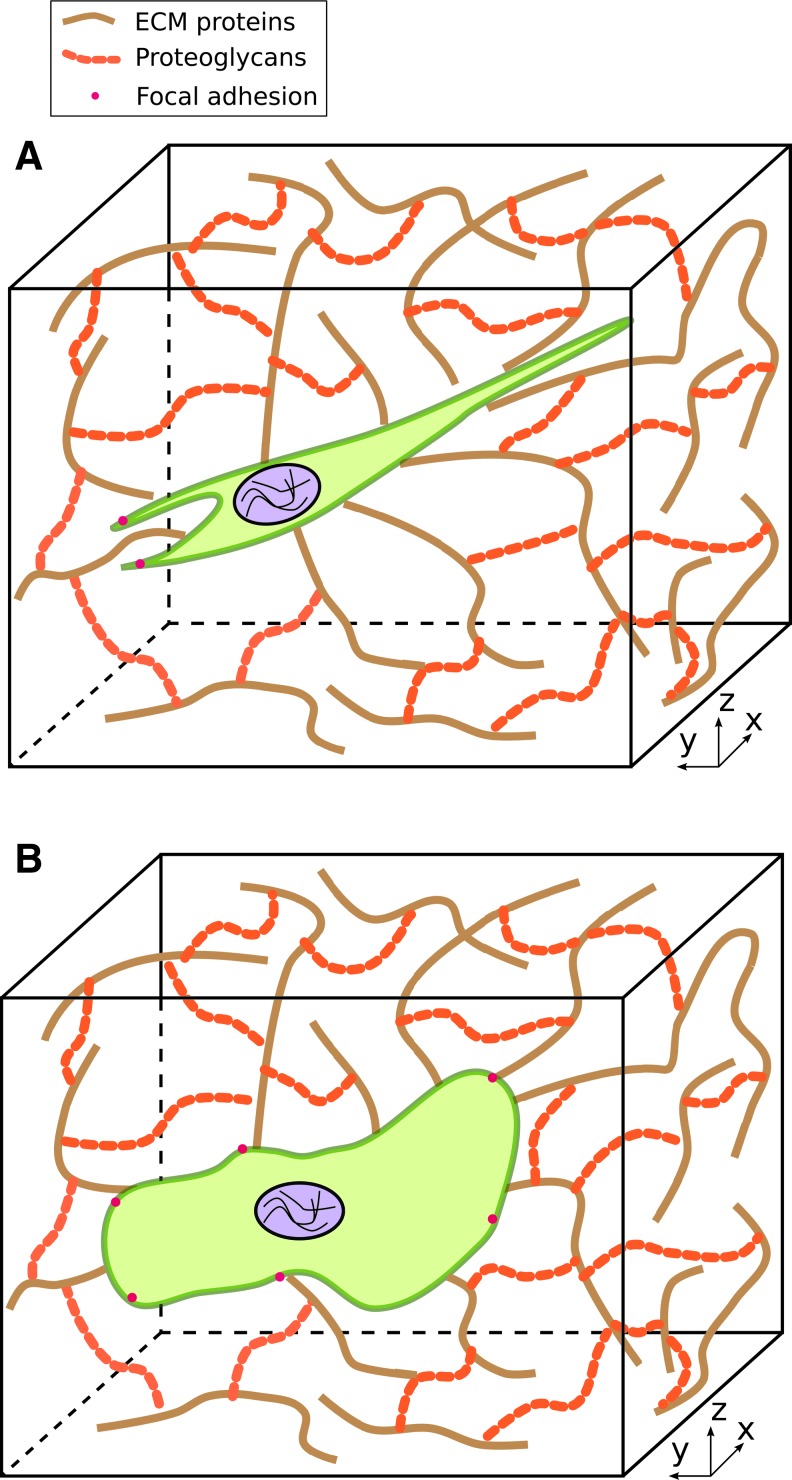
FIG. 2.
Cell morphologies in a three-dimensional (3D) environment. The network of striated fibers represents various components of the extracellular matrix (ECM) (proteins, proteoglycans) through which cells migrate. (A) Schematic of a cell exhibiting a stellate morphology with few lamellipodia. (B) Schematic of a cell exhibiting blunt protrusions known as lobopodia. Color images available online at www.liebertpub.com/teb
When human foreskin fibroblasts (HFFs) were cultured within 3D environments that comprised stiff ECM components (e.g., tissue explants or cell-derived matrices with stiffness ranging from 0.6 to 6.4 kPa), they formed cylindrical protrusions known as lobopodia.38 In addition, such cells formed only lateral blebs. When these cells were cultured within a soft, deformable collagen gel (∼0.015 kPa), they formed several branched protrusions with small lamellipodia. In contrast, when HFFs were cultured in a 2D substrate that comprised cell-derived matrix components, ruffled lamellipodia were observed.38
Fibroblasts encapsulated within a relaxed collagen matrix exhibited microtubule-dependent spreading and a dendritic morphology in contrast to the lamellipodia observed on 2D collagen-coated substrates.63 However, when the 3D collagen matrices were precontracted to enable tight packing of the protein fibrils, fibroblasts began to exhibit more flat and spread morphologies with distinct lamellipodia similar to what was observed on 2D collagen-coated coverslips. When bovine aortic endothelial cells (BAECs) were cultured within (3D) and upon collagen gels (2D), similar trends were observed.64 BAECs formed flat lamellar structures and branched pseudopodia on 2D and within 3D matrices, respectively.
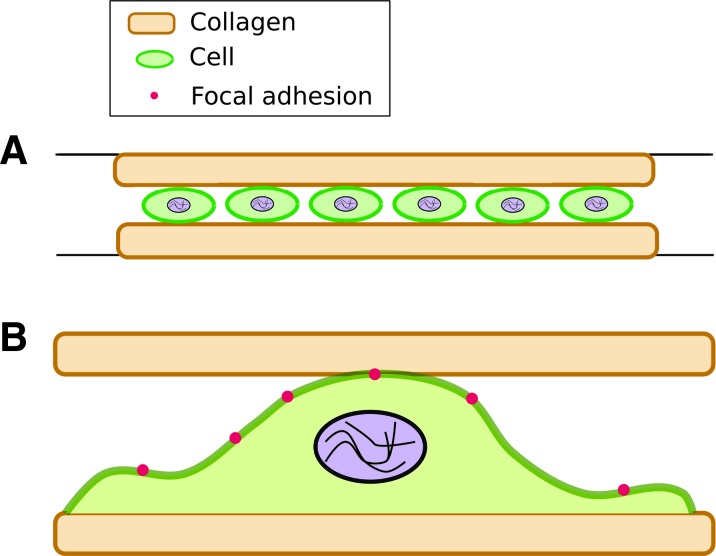
Another method to introduce a 3D environment has been to sandwich cells between hydrogels. Cells are first cultured on the surface of a hydrogel (2D), followed by placing a second gel above, thereby forming a sandwich (Fig. 3A, B).52 Using this approach, changes in NIH 3T3 fibroblasts were investigated when they were adherent on a planar substrate or sandwiched between two polyacrylamide gels. The polyacrylamide gels were coated with either collagen or fibronectin. In 3D matrices, stellate morphologies were visible only on collagen-coated and not on fibronectin-coated sandwiches. The authors state that the stellate morphology observed in sandwiched fibroblasts is representative of a cell shape found in vivo. This transformation in cell shape was attributed to differences in binding to ECM receptors. Another interesting finding was the dramatic change in the aspect ratio of cells soon after the sandwich was formed. The authors reported a 10-fold increase in the aspect ratio of sandwiched NIH 3T3 fibroblasts (3D) that lasted more than 24 h. These changes were observed for cells sandwiched between polyacrylamide gels coated with either collagen or fibronectin.
FIG. 3.
(A) Cells cultured within a hydrogel sandwich. (B) The morphology and localization of FAs change for sandwiched cells. Color images available online at www.liebertpub.com/teb
These reports suggest that despite different approaches utilized to design 3D matrices or the types of cells used in such investigations, distinct changes in cell shape are found. The reduction in the number of lamellipodia occurs in a 3D microenvironment and is representative of cells in vivo.
Speed and directed migration
The speed at which cells move and their directed migration toward a specific location within a scaffold is critical to the success of engineered tissues and implants. To study these effects, human fibroblasts were cultured in four different matrices that comprised collagen, fibrin, cell-derived matrix, or basement membrane extract.44 Cell speed, persistence, and directed migration were monitored on 2D substrates and within 3D environments. In comparison to their 2D counterparts, cell speed was higher in 3D scaffolds that comprised collagen or cell-derived matrix (p<0.01). Cell speed on a 2D substrate that comprised basement membrane extracts was approximately 10-fold higher than the corresponding 3D equivalent. The authors attributed the lack of fibroblast motility within 3D basement membrane extracts to the fact that in vivo, these cells are found in the connective stroma. Cell speeds were statistically insignificant between planar and 3D matrices composed of fibrin. In this same study, cells exhibited directed migration only on substrates that comprised basement membrane extracts. In another study, NIH 3T3 fibroblasts exhibited an ∼54% reduction in cell speed within fibronectin-coated polyacrylamide sandwiches (3D) in comparison to flat 2D substrates.52 The reduction in speed was attributed to a combination of substrate rigidity that the cells experienced within the sandwiched structure as well as the anchoring of cell receptors. The increase in cell speed on collagen- or cell-derived matrices in contrast to polyacrylamide hydrogels can be attributed to the proteolytic breakdown of ECM proteins.
Human macrophages derived from blood monocytes (MDMs) exhibited different modes of migration and speeds that would appear to depend on the chemical structure of their 3D environment.65 MDMs exhibited ameboid or mesenchymal migration within the fibrillar collagen and Matrigel matrices, respectively. In addition, the speed of MDMs was ∼3.5-fold greater when they exhibited an ameboid migratory behavior. These trends suggest that even within 3D environments, the ECM environment can affect the cell speed.
Directed migration was observed using growth-arrested human dermal fibroblasts (HDFs) that were cultured at a low cell seeding density within a 3D type I collagen environment.45 When presented with a microenvironment that exhibited a mechanical gradient, these cells preferentially accumulated on the stiff regions of the substrate over a 6-day period. Despite differences in morphology, these findings suggest that cells exhibit durotactic migration, a phenomenon that has been widely observed on traditional 2D substrates.16,66 This study suggests that durotaxis occurs on 2D surfaces and in 3D environments.
These trends indicate that it is difficult to obtain a clear correlation between increased cell speeds and 3D environments. Future studies that can clearly identify the ability of cells to degrade natural and synthetic biomaterials could provide more definitive reasons.
FAs and protein composition
Cell adhesion differs when cultured within a 3D structure in comparison to conventional flat substrates. This observation can be related to differences in the FA size, their colocalization with ECM proteins, their distribution, and their lifetime.67–70 Although studies on 2D substrates vastly outnumber those conducted within 3D matrices, there is a growing body of evidence that the chemical composition of the substrate, the cell type, the density of cell seeding, as well as proteins that were selectively activated in certain investigations can affect adhesion.67,70,71
Smooth muscle cells exhibited a greater number of FAs on 2D planar substrates in contrast to cells within a collagen matrix.68 The authors also reported that cells cultured in the 3D environment had fewer actin stress fibers and relied on the scaffold for contact guidance.68 In a separate study, NIH 3T3 fibroblasts cultured between polyacrylamide sheets exhibited fewer prominent stress fibers than cells on flat substrates.52 This difference was further validated by changes in the localization of the Arp2/3 (actin-related protein) unit that is responsible for nucleating actin filaments. This protein complex was concentrated at the tips of lamellipodia (2D) and scattered along the projections for sandwiched cells. In addition, FAs in cells within the 3D matrix were observed to have a lifetime of only ∼30 min.
When HFFs were cultured within a cell-derived matrix, FAs were found to be large, elongated, and they colocalized with fibronectin fibers.53 Upon culturing the same cells in a collagen matrix, FAs varied as a function of initial cell seeding density.72 At a higher cell density, FAs were more prominent and were attributed to the remodeling of the collagen matrix due to contractile forces exerted by HFFs. In addition, at a high seeding density, the morphologies of HFFs were dependent on the composition of the culture medium. They were stellate upon the addition of PDGF and bipolar in the presence of lysophosphatic acid. Similar trends were observed within synthetic hydrogels as well.73
In a study on fibroblast migration on planar and within a 3D environment, a significant finding was the difference in the localization of the α5 and activated β1 integrins.74 In 3D migration, these adhesion molecules spanned the entire length of cells, whereas they were localized to discrete locations in the periphery of cells in 2D substrates. Neural precursor cell expressed developmentally downregulated 9 (NEDD9), which is a scaffolding protein used for stabilizing FAs, was shown to influence mouse embryonic fibroblast (MEF) migration.70 In NEDD9-deficient MEFs, migration speeds were higher on 2D collagen gels than in 3D environments.
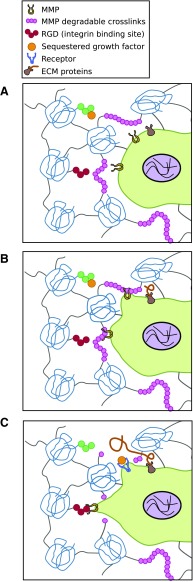
In 3D environments, cells often have to cleave bonds and protein networks to infiltrate and move within the matrix (Fig. 4).75,76 Cells secrete matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) when remodeling a matrix. When mRNA levels from human microvascular endothelial cells seeded on 2D and inside 3D collagen gels were measured, nearly identical expressions of MMP-1, -2, -13, membrane type-1 MMP (MT1-MMP), and TIMP-1 and TIMP-2 were observed.77 Active forms of proMMP2 and proMT1-MMP were increased and TIMP-2 was decreased in the 3D compared with the 2D substrate. Within 3D hydrogel matrices, the density of the network can elicit differential MMP secretion.58 Fibroblasts cultured within a dense PEG gel were found to secrete MMPs in comparison to those cultured within a microporous matrix. These differences could be related to physical resistance by the dense network and the microarchitecture of the polymers.
FIG. 4.
(A) Cells in a 3D matrix secrete matrix metalloproteinases (MMPs) that target specific peptide sequences on proteins. (B) The secreted MMPs cleave protein molecules. (C) The cell breaks its adhesion to old protein molecules and creates links with new proteins to move forward. Color images available online at www.liebertpub.com/teb
Taken together, it is clear that protein expression differs as a function of the dimensionality of the environment. With respect to FAs, differing trends have been reported. Fraley et al. reported that in a 3D collagen gel, fibroblasts did not exhibit discrete FA complexes. Instead, proteins such as zyxin, paxillin, and vinculin were distributed throughout the cell body.78 In contrast, using a truncated promoter, another study reported the presence of well-defined FA complexes in cells located up to 350 μm from the underlying glass substrate.79 Based on the differences reported in FAs upon changing dimensionality, it would be circumspect to state that well-defined adhesion complexes can be observed in 3D. However, issues such as background fluorescence, experimental protocols (e.g., live cell imaging vs. fixed samples), as well as the presence of thicker cellular protrusions in 3D substrates can alter observations. These differences underscore the need for more advanced imaging techniques and unified experimental procedures. In the future, studies that can quantify the temporal dynamics of FA complexes as well as unveil the reasons for their short lifetimes in 3D matrices would fill a significant gap in our current understanding on tying together FA protein expression, MMP and TIMP secretions, and cytoskeletal organization.
3D Patterned Hydrogels
Lithographic patterning can lead to domains of very specific dimensions and precisely positioned biomolecules. Together, these features can exert significant control over cellular adhesion and subsequently motility.80 In this section, we focus on matrices created by lithography that also provide a classical 3D microenvironment. Previous studies have shown that cellular migratory features on patterned environments are similar to what is observed in 3D ECM environments.81–84 These substrates can be assembled with soft gels that contain a photolabile moiety that degrades upon irradiation.85,86 Although the substrate used in many studies is usually a hydrogel, such matrices differ from conventional gel-encapsulated cells. For this reason, we have described these investigations in a separate section. In addition, to the best of our knowledge, there are no studies that demonstrate the presence of FAs and proteins related to cell migration within these environments. For this reason, we did not include discussions on this topic.
Cells from dorsal root ganglia adhered and extended neurites into the patterned channels within an agarose gel that was modified with the arginine–glycine–aspartic acid (RGD) peptide sequence.80 Similar trends were observed with HDFs cultured within a proteolytically degradable PEG gel patterned with RGD. The patterns were generated using two-photon laser scanning lithography.87 In addition, the encapsulated fibroblasts extended sprouts into the hydrogel up to day 10 in culture. When dendritic cells were encapsulated in a patterned, interconnected collagen gel, their migration speed was dependent on pore diameters.88 The authors incorporated pores ranging from 25 to 75 μm in diameter by introducing porous scaffolds within the gel. Cell speed was governed by pore density, wherein smaller pores resulted in lower cell speed. These trends prevailed in the presence of a chemokine gradient.88 Based on the findings thus far, it has been demonstrated that patterned 3D environments induce lower cell velocities and migration is affected by pore sizes. Since lithographical modification of gels is relatively recent, there are fewer investigations to date that reveal differences between planar and patterned matrices. With the advent of more sophisticated patterning methods leading to controlled architectures, additional differences can be unearthed.
Discussion and Conclusions
Migration is a complex physiological process that involves a series of intricate, spatiotemporal signaling pathways. To obtain a comprehensive view of how migration occurs in vivo, investigations must be conducted on materials that can emulate the extracellular microenvironment. The design of hydrogels that can provide a 3D environment to cells may lead to deeper insights into cell locomotion. Reports in the literature have already identified distinct differences in migratory behavior on planar and in 3D matrices. An observation that has been consistently reported is the difference in cell morphology. Changes in morphology appear to be independent of the methodologies used in assembling a 3D environment as well as its chemical composition. In general, cell speed in 3D environments is lower than on planar substrates and appears to be dependent on whether the matrix is composed of naturally occurring or synthetic materials. There are diverging trends that have been reported, specifically with FAs. The identification of FAs within 3D matrices appears to be dependent on imaging capabilities and experimental protocols. With the advent of more sophisticated imaging and analytical capabilities, it is likely that a unifying theme in adhesion complexes between 2D and 3D will emerge.
The majority of investigations conducted so far in identifying changes in migration on 2D and within 3D environments have focused upon normal and cancerous cells. While acknowledging the new insights and research directions these studies have provided the scientific community, it would be very beneficial to investigate changes in stem cell behavior. Such studies would be of critical significance to the burgeoning fields of developmental biology and regenerative medicine. Studies on the migratory behavior in 3D exhibited by cell sheets would enhance our understanding of wound healing. In addition, by harnessing the potential of current genomics and proteomics advances, future investigations into the spatiotemporal signaling events activated during migration in 3D could provide comprehensive information on the up- and downregulation of pathways resulting from a changing landscape.
Acknowledgments
The authors apologize in advance to all researchers whose work they could not cite due to space limitations. They gratefully acknowledge financial support from the NSF grants CAREER 0955873, CBET-0933225, DBI-1062380, and DMR-090750. They also acknowledge support from the Institute of Critical Technology and Applied Science Center for Systems Biology of Engineered Tissues at Virginia Tech.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lauffenburger D.A., and Horwitz A.F.Cell migration: a physically integrated molecular process. Cell 84,359, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Ridley A.J., Schwartz M.A., Burridge K., Firtel R.A., Ginsberg M.H., Borisy G., et al. . Cell migration: integrating signals from front to back. Science 302,1704, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Horwitz A.R., and Parsons J.T.Cell Migration—movin’ on. Science 286,1102, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Horwitz R., and Webb D.Cell migration. Curr Biol 13,R756, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Thiery J.P., Duband J.L., and Tucker G.C.Cell migration in the vertebrate embryo: role of cell adhesion and tissue environment in pattern formation. Annu Rev Cell Biol 1,91, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Little S.C., and Wieschaus E.F.Shifting patterns: merging molecules, morphogens, motility, and methodology. Dev Cell 21,2, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Montell D.J.Morphogenetic cell movements: diversity from modular mechanical properties. Science 322,1502, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Seppä H., Grotendorst G., Seppä S., Schiffmann E., and Martin G.R.Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol 92,584, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin F., Nguyen C.M.-C., Wang S.-J., Saadi W., Gross S.P., and Jeon N.L.Effective neutrophil chemotaxis is strongly influenced by mean IL-8 concentration. Biochem Biophys Res Commun 319,576, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Madrid F., and del Pozo M.A.Leukocyte polarization in cell migration and immune interactions. EMBO J 18,501, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan S., Ferrario C., Saragovi U., Quenneville L., Gaboury L., Baccarelli A., et al. . The influence of tumor-host interactions in the stromal cell-derived factor-1/CXCR4 ligand/receptor axis in determining metastatic risk in breast cancer. Am J Pathol 175,66, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller A., Homey B., Soto H., Ge N., Catron D., Buchanan M.E., et al. . Involvement of chemokine receptors in breast cancer metastasis. Nature 410,50, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Ruppender N.S., Merkel A.R., Martin T.J., Mundy G.R., Sterling J.A., and Guelcher S.A.Matrix rigidity induces osteolytic gene expression of metastatic breast cancer cells. PLoS One 5,e15451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santini D., Perrone G., Roato I., Godio L., Pantano F., Grasso D., et al. . Expression pattern of receptor activator of NF kappa B (RANK) in a series of primary solid tumors and related bone metastases. J Cell Physiol 226,780, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Lu P., Weaver V.M., and Werb Z.The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196,395, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo C.-M., Wang H.-B., Dembo M., and Wang Y.-L.Cell movement is guided by the rigidity of the substrate. Biophys J 79,144, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelham R.J., and Wang Y.L.Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A 94,13661, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M.Electrical fields in wound healing—an overriding signal that directs cell migration. Semin Cell Dev Biol 20,674, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Saranak J., and Foster K.W.Rhodopsin guides fungal phototaxis. Nature 387,465, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Engler A., Bacakova L., Newman C., Hategan A., Griffin M., and Discher D.Substrate compliance versus ligand density in cell on gel responses. Biophys J 86,617, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarvestani A.S., and Jabbari E.Analysis of cell locomotion on ligand gradient substrates. Biotech Bioeng 103,424, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Maheshwari G., Brown G., Lauffenburger D.A., Wells A., and Griffith L.G.Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci 113,1677, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Lodish H.F.Molecular Cell Biology, 7th ed. New York: W.H. Freeman and Co., 2013 [Google Scholar]
- 24.Ridley A.J.Life at the leading edge. Cell 145,1012, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Huttenlocher A., and Horwitz A.R.Integrins in cell migration. Cold Spring Harbor Perspect Biol 3,a005074, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiMilla P., Stone J., Quinn J., Albelda S., and Lauffenburger D.Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol 122,729, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiMilla P.A., Barbee K., and Lauffenburger D.A.Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J 60,15, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hale N.A., Yang Y., and Rajagopalan P.Cell migration at the interface of a dual chemical-mechanical gradient. ACS Appl Mater Interface 2,2317, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Palecek S.P., Loftus J.C., Ginsberg M.H., Lauffenburger D.A., and Horwitz A.F.Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385,537, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Griffith L.G., and Swartz M.A.Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7,211, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Smalley K.S.M., Lioni M., and Herlyn M.Life isn't flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol Anim 42,242, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Yamada K.M., and Cukierman E.Modeling tissue morphogenesis and cancer in 3D. Cell 130,601, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Ghibaudo M., Trichet L., Le Digabel J., Richert A., Hersen P., and Ladoux B.Substrate topography induces a crossover from 2D to 3D behavior in fibroblast migration. Biophys J 97,357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson C.M., VanDuijn M.M., Inman J.L., Fletcher D.A., and Bissell M.J.Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314,298, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaman M.H., Trapani L.M., Siemeski A., MacKellar D., Gong H.Y., Kamm R.D., et al. . Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A 103,10889, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbott A.Cell culture: biology's new dimension. Nature 424,870, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Frantz C., Stewart K.M., and Weaver V.M.The extracellular matrix at a glance. J Cell Sci 123,4195, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrie R.J., Gavara N., Chadwick R.S., and Yamada K.M.Nonpolarized signaling reveals two distinct modes of 3D cell migration. J Cell Biol 197,439, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Even-Ram S., and Yamada K.M.Cell migration in 3D matrix. Curr Opin Cell Biol 17,524, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Baker B.M., and Chen C.S.Deconstructing the third dimension—how 3D culture microenvironments alter cellular cues. J Cell Sci 125,3015, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pampaloni F., Reynaud E.G., and Stelzer E.H.The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8,839, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Schmeichel K.L., and Bissell M.J.Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci 116,2377, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradbury P., Fabry B., and O'Neill G.M.Occupy tissue. The movement in cancer metastasis. Cell Adhes Migr 6,424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hakkinen K.M., Harunaga J.S., Doyle A.D., and Yamada K.M.Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng Part A 17,713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadjipanayi E., Mudera V., and Brown R.A.Guiding cell migration in 3D: a collagen matrix with graded directional stiffness. Cell Motil Cytoskeleton 66,121, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Isenberg B.C., DiMilla P.A., Walker M., Kim S., and Wong J.Y.Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys J 97,1313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf K., Alexander S., Schacht V., Coussens L.M., von Andrian U.H., van Rheenen J., et al. . Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol 20,931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu X.Z., and Machesky L.M.Cells assemble invadopodia-like structures and invade into Matrigel in a matrix metalloprotease dependent manner in the circular invasion assay. PLoS One 7,e30605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poincloux R., Collin O., Lizarraga F., Romao M., Debray M., Piel M., et al. . Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc Natl Acad Sci U S A 108,1943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao S.S., Bentil S., DeJesus J., Larison J., Hissong A., Dupaix R., et al. . Inherent interfacial mechanical gradients in 3D hydrogels influence tumor cell behaviors. PLoS One 7,e35852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeLong S.A., Gobin A.S., and West J.L.Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J Control Release 109,139, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Beningo K.A., Dembo M., Wang Y.-L.Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc Natl Acad Sci U S A 101,18024, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cukierman E., Pankov R., Stevens D.R., and Yamada K.M.Taking cell-matrix adhesions to the third dimension. Science 294,1708, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Friedl P., and Bröcker E.B.The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci 57,41, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedl P., Entschladen F., Conrad C., Niggemann B., and Zanker K.S.CD4(+) T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta 1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol 28,2331, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Friedl P., and Wolf K.Plasticity of cell migration: a multiscale tuning model. J Cell Biol 188,11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Starke J., Maaser K., Wehrle-Haller B., and Friedl P.Mechanotransduction of mesenchymal melanoma cell invasion into 3D collagen lattices: filopod-mediated extension-relaxation cycles and force anisotropy. Exp Cell Res 319,2424, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Raeber G.P., Lutolf M.P., and Hubbell J.A.Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J 89,1374, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orsi S., Guarnieri D., De Capua A., and Netti P.A.Gene-activated and cell-migration guiding PEG matrices based on three dimensional patterning of RGD peptides and DNA complexes. Acta Biomater 8,3228, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Leslie-Barbick J.E., Moon J.J., and West J.L.Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed 20,1763, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Grinnell F.Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 13,264, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Petrie R.J., and Yamada K.M.At the leading edge of three-dimensional cell migration. J Cell Sci 125,5917, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhee S., Jiang H., Ho C.H., and Grinnell F.Microtubule function in fibroblast spreading is modulated according to the tension state of cell-matrix interactions. Proc Natl Acad Sci U S A 104,5425, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martins G.G., and Kolega J.Endothelial cell protrusion and migration in three-dimensional collagen matrices. Cell Motil Cytoskeleton 63,101, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Van Goethem E., Poincloux R., Gauffre F., Maridonneau-Parini I., and Le Cabec V.Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol 184,1049, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Gray D.S., Tien J., and Chen C.S.Repositioning of cells by mechanotaxis on surfaces with micropatterned Young's modulus. J Biomed Mater Res Part A 66,605, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Harunaga J.S., and Yamada K.M.Cell-matrix adhesions in 3D. Matrix Biol 30,363, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S., Lao J., Chen B.P., Li Y.S., Zhao Y., Chu J., et al. . Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. FASEB J 17,97, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Wozniak M.A., Modzelewska K., Kwong L., and Keely P.J.Focal adhesion regulation of cell behavior. Biochim Biophys Acta Mol Cell Res 1692,103, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Zhong J., Baquiran J.B., Bonakdar N., Lees J., Ching Y.W., Pugacheva E., et al. . NEDD9 stabilizes focal adhesions, increases binding to the extra-cellular matrix and differentially effects 2D versus 3D cell migration. PLoS One 7,e35058, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jayo A., and Parsons M.Imaging of cell adhesion events in 3D matrix environments. Eur J Cell Biol 91,824, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Tamariz E., and Grinnell F.Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell 13,3915, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo Q.Y., Wang X.B., Tibbitt M.W., Anseth K.S., Montell D.J., and Elisseeff J.H.Light activated cell migration in synthetic extracellular matrices. Biomaterials 33,8040, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doyle A.D., Wang F.W., Matsumoto K., and Yamada K.M.One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol 184,481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf K., Te Lindert M., Krause M., Alexander S., Te Riet J., Willis A.L., et al. . Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 201,1069, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palmisano R., and Itoh Y.Analysis of MMP-dependent cell migration and invasion. Methods Mol Biol 622,379, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Koike T., Vernon R.B., Hamner M.A., Sadoun E., and Reed M.J.MT1-MMP, but not secreted MMPs, influences the migration of human microvascular endothelial cells in 3-dimensional collagen gels. J Cell Biochem 86,748, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Fraley S., Feng Y., Krishnamurthy R., Kim D.-H., Celedon A., Longmore G., et al. . A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 12,598, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kubow K.E., and Horwitz A.R.Reducing background fluorescence reveals adhesions in 3D matrices. Nat Cell Biol 13,3; author reply 7, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo Y., and Shoichet M.S.A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat Mater 3,249, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Frey M.T., Tsai I.Y., Russell T.P., Hanks S.K., and Wang Y.L.Cellular responses to substrate topography: role of myosin II and focal adhesion kinase. Biophys J 90,3774, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berry C.C., Campbell G., Spadiccino A., Robertson M., and Curtis A.S.G.The influence of microscale topography on fibroblast attachment and motility. Biomaterials 25,5781, 2004 [DOI] [PubMed] [Google Scholar]
- 83.Ghibaudo M., Di Meglio J.M., Hersen P., and Ladoux B.Mechanics of cell spreading within 3D-micropatterned environments. Lab Chip 11,805, 2011 [DOI] [PubMed] [Google Scholar]
- 84.Chang S.S., Guo W.H., Kim Y., and Wang Y.L.Guidance of cell migration by substrate dimension. Biophys J 104,313, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kloxin A.M., Tibbitt M.W., and Anseth K.S.Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat Protoc 5,1867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kloxin A.M., Kasko A.M., Salinas C.N., and Anseth K.S.Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324,59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee S.H., Moon J.J., and West J.L.Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials 29,2962, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tayalia P., Mazur E., and Mooney D.Controlled architectural and chemotactic studies of 3D cell migration. Biomaterials 32,2634, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fallica B., Maffei J.S., Villa S., Makin G., and Zaman M.Alteration of cellular behavior and response to PI3K pathway inhibition by culture in 3D collagen gels. PLoS One 7,e48024, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soman P., Kelber J.A., Lee J.W., Wright T.N., Vecchio K.S., Klemke R.L., et al. . Cancer cell migration within 3D layer-by-layer microfabricated photocrosslinked PEG scaffolds with tunable stiffness. Biomaterials 33,7064, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoffmann J.C., and West J.L.Three-dimensional photolithographic micropatterning: a novel tool to probe the complexities of cell migration. Integr Biol 5,817, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]