Abstract
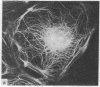
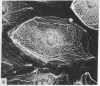
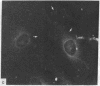
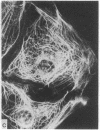
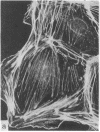
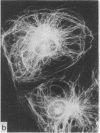
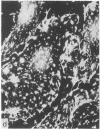
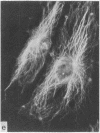
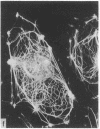
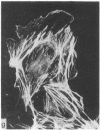
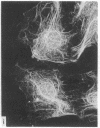
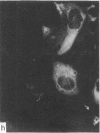
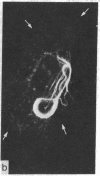
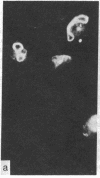
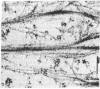
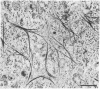
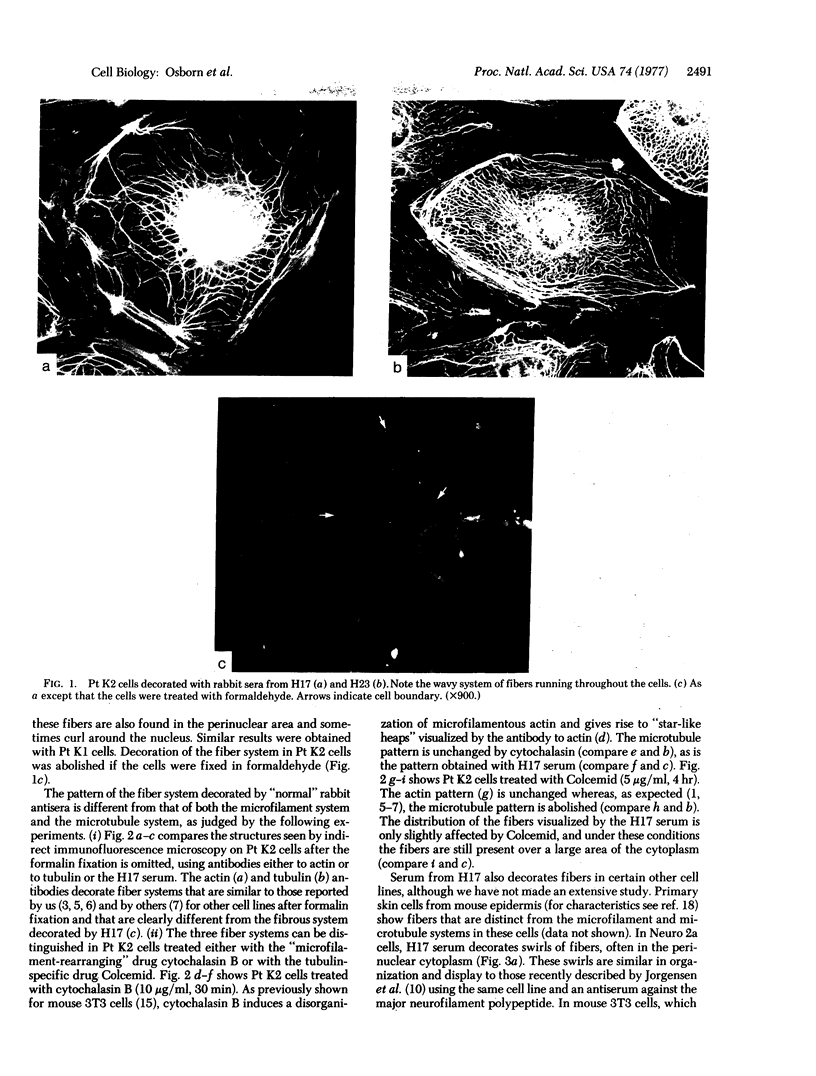
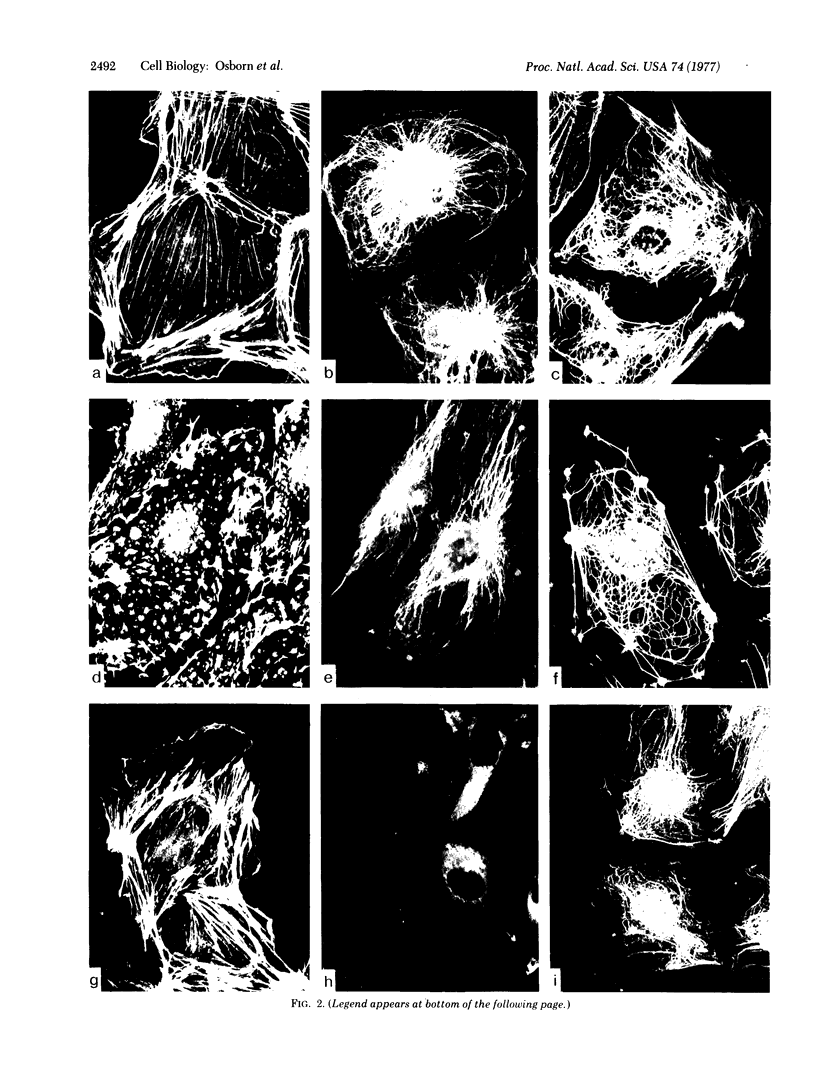
During our studies with antibodies against structural proteins of the cytoskeleton of eukaryotic cells we have observed that sera from many normal rabbits decorate a fiber system in cells of the established rat kangaroo cell line Pt K2. The display and organization of these fibers are different from those of microfilament bundles (decorated by antibody to actin) and microtubules (decorated by antibody to tubulin). This new fiber system can be further distinguished by its resistance to reorganization when cells are treated with Colcemid or cytochalasin B. The decoration of this fiber system is not detected if Pt K2 cells are fixed with formaldehyde. Such sera also appear to decorate swirls of perinuclear fibers in mouse Neuro 2a cells, and in mouse 3T3 cells treated with mitotic drugs. Comparison of the immunofluorescence pictures with electron microscopic data suggests that the sera are visualizing bundles of intermediate 7- to 10-nm filaments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brecher S. The occurrence and possible role of 80-100 A filaments in PtKl cells. Exp Cell Res. 1975 Dec;96(2):303–310. doi: 10.1016/0014-4827(75)90261-x. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Lüder M. R., Kartenbeck J., Zerban H., Keenan T. W. Involvement of vesicle coat material in casein secretion and surface regeneration. J Cell Biol. 1976 Apr;69(1):173–195. doi: 10.1083/jcb.69.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Lamelin J. P., Vassalli P., Majno G., Bouvier C. A., Cruchaud A., Lüscher E. F. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to "nonmuscular" cells. Am J Pathol. 1973 Sep;72(3):473–488. [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Holborow E. J., Glynn L. E. Antibody to smooth muscle in patients with liver disease. Lancet. 1965 Oct 30;2(7418):878–879. doi: 10.1016/s0140-6736(65)92505-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen A. O., Subrahmanyan L., Turnbull C., Kalnins V. I. Localization of the neurofilament protein in neuroblastoma cells by immunofluorescent staining. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3192–3196. doi: 10.1073/pnas.73.9.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Hubbard B. D. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4344–4348. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoltsy A. G. Desmosomes, filaments, and keratohyaline granules: their role in the stabilization and keratinization of the epidermis. J Invest Dermatol. 1975 Jul;65(1):127–142. doi: 10.1111/1523-1747.ep12598093. [DOI] [PubMed] [Google Scholar]
- Orwin D. F., Thomson R. W., Flower N. E. Plasma membrane differentiations of keratinizing cells of the wool follicle. II. Desmosomes. J Ultrastruct Res. 1973 Oct;45(1):15–29. doi: 10.1016/s0022-5320(73)90029-4. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Cytoplasmic microtubules in tissue culture cells appear to grow from an organizing structure towards the plasma membrane. Proc Natl Acad Sci U S A. 1976 Mar;73(3):867–871. doi: 10.1073/pnas.73.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenberg C. F., Needham D. M. A study of the mechanism of contraction in vertebrate smooth muscle. Biol Rev Camb Philos Soc. 1976 Feb;51(1):53–104. doi: 10.1111/j.1469-185x.1976.tb01120.x. [DOI] [PubMed] [Google Scholar]
- Snyder J. A., McIntosh J. R. Biochemistry and physiology of microtubules. Annu Rev Biochem. 1976;45:699–720. doi: 10.1146/annurev.bi.45.070176.003411. [DOI] [PubMed] [Google Scholar]
- Trenchev P., Holborow E. J. The specificity of anti-actin serum. Immunology. 1976 Oct;31(4):509–517. [PMC free article] [PubMed] [Google Scholar]
- Weber K., Bibring T., Osborn M. Specific visualization of tubulin-containing structures in tissue culture cells by immunofluorescence. Cytoplasmic microtubules, vinblastine-induced paracrystals, and mitotic figures. Exp Cell Res. 1975 Oct 1;95(1):111–120. doi: 10.1016/0014-4827(75)90615-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Wehland J., Herzog W. Griseofulvin interacts with microtubules both in vivo and in vitro. J Mol Biol. 1976 Apr 25;102(4):817–829. doi: 10.1016/0022-2836(76)90293-x. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Dahl D., Schachner M., Shelanski M. L. Biochemistry of the filaments of brain. Proc Natl Acad Sci U S A. 1976 Feb;73(2):529–533. doi: 10.1073/pnas.73.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELICKSON A. S. Normal human keratinization processes as demonstrated by electron microscopy. J Invest Dermatol. 1961 Nov;37:369–379. [PubMed] [Google Scholar]
- Zerban H., Franke W. W. Structures indicative of keratinization in lactating cells of bovine mammary gland. Differentiation. 1977 Jan 14;7(2):127–131. doi: 10.1111/j.1432-0436.1977.tb01505.x. [DOI] [PubMed] [Google Scholar]