Abstract
Extracellular vesicles (EVs)—comprising a heterogeneous population of cell-derived lipid vesicles including exosomes, microvesicles, and others—have recently emerged as both mediators of intercellular information transfer in numerous biological systems and vehicles for drug delivery. In both roles, EVs have immense potential to impact tissue engineering and regenerative medicine applications. For example, the therapeutic effects of several progenitor and stem cell-based therapies have been attributed primarily to EVs secreted by these cells, and EVs have been recently reported to play direct roles in injury-induced tissue regeneration processes in multiple physiological systems. In addition, EVs have been utilized for targeted drug delivery in regenerative applications and possess unique potential to be harnessed as patient-derived drug delivery vehicles for personalized medicine. This review discusses EVs in the context of tissue repair and regeneration, including their utilization as drug carriers and their crucial role in cell-based therapies. Furthermore, the article highlights the growing need for bioengineers to understand, consider, and ultimately design and specifically control the activity of EVs to maximize the efficacy of tissue engineering and regenerative therapies.
Introduction
Tissue engineering and regenerative medicine are strongly associated with the controlled application of cells for therapy, often in combination with biocompatible materials. Technological advances such as three-dimensional printing have opened up exciting possibilities in these fields; however, development of enhanced understanding of cellular function and especially intercellular communication also offers transformational potential for improving tissue engineering strategies. Additionally, tissue repair and regeneration through drug therapies—either as part of an integrated therapeutic approach involving cells or as an alternative to cellular therapies—remains a tantalizing option given potential advantages over cells with regard to reproducibility, scalability, quality control, and regulatory considerations.
One significant recent development in the understanding of intercellular communication is the appreciation of the role of extracellular vesicles (EVs).1 Once considered to be mere cellular debris, EVs—composed of exosomes, microvesicles, and other heterogeneous vesicles typically sized in the 30–2000 nm range that are shed from cells either by blebbing from the plasma membrane or by the endolysosomal pathway—are increasingly being recognized as vital mediators of information transfer between cells (we refer the reader to several excellent recent reviews covering the biogenesis of EVs and their role in cell–cell communication2–4). The authors note that many published studies describe activities or properties of specific EVs such as exosomes or microvesicles; however, due to variability in EV isolation and characterization methods as well as to the continuing development of knowledge of EV biogenesis, identification of these specific vesicle populations is problematic and terms are often used interchangeably. Thus, in this review, we will refer to all vesicle populations as EVs. Importantly, EVs have been demonstrated to be vital components of cell-based therapies; in some cases, the therapeutic efficacies of cell-based interventions are almost entirely attributable to paracrine effects through EVs.5,6 Yet, the literature reflects that the role of EVs in many cell-based therapies has yet to become a primary design consideration in tissue engineering, as issues such as the potential effects of culture conditions and cell–biomaterial interactions on EV biogenesis and content are scarcely reported.
Interestingly, EVs also have significant potential as drug and gene carriers for regenerative therapeutic applications. For example, seminal work by Wood and colleagues demonstrated that EVs can be loaded with a desired genetic cargo and targeted to a specific cell type by straightforward methods.7 Additional studies have shown that EVs are potentially versatile drug carriers that could be applied in wide-ranging applications toward regeneration.8–10 However, the full potential of EVs as mediators of tissue repair and regeneration by drug and gene delivery is still unknown.
Overall, EVs have substantial promise to play important roles in tissue engineering and regenerative therapies. Despite this promise, many questions remain to be answered before EVs are likely to be a significant consideration of a vast majority of scientists and engineers at the design stage of tissue engineering and regenerative therapeutic approaches. In this article, we review reports of EVs related to tissue engineering and regenerative therapies, with a focus on the most recent literature, and attempt to summarize current knowledge in this area. Specifically, the roles of EVs in cell therapies and as drug and gene carriers are highlighted and integrated into a discussion of what additional knowledge might lead to EVs becoming more broadly considered as crucial players in the fields of tissue engineering and regenerative medicine.
EVs as Paracrine Mediators of Cell-Based Therapeutic Regeneration
EVs are released from numerous cell types and can transfer information—including mRNA, miRNA, and proteins1,2,4 as well as DNA11,12—from cell to cell. In the context of cell therapy, EVs can act in a paracrine manner to alter the phenotypes of recipient cells, potentially promoting repair and regeneration. The therapeutic benefit of EVs in cell-based therapies has been observed in several different physiological systems that are of interest in tissue engineering and regenerative medicine, and cell source is a critical factor, with EVs derived from stem or progenitor cells—and especially from mesenchymal stromal cells (MSCs)6,13,14—having shown promising regenerative effects in several of these systems.
Nervous system
Nerve repair and regeneration has long been a significant goal in tissue engineering and regeneration.15–17 Most cell types within the nervous system (NS) are thought to release EVs, and the role of exosomes—a particular subset of EVs—in information transfer in the NS is well established.18–20 Pioneering work (discussed in more detail later in this article) in harnessing EVs for drug delivery was conducted in the NS,7 and the roles of exosomes and other EVs in NS development, normal function, and pathophysiology are expertly reviewed elsewhere.21 With regard to NS regenerative therapies, several recent reports have revealed the therapeutic potential of EVs. Xin et al. showed that EVs emanating from MSCs transfer miR-133b to neural cells, inducing neurite outgrowth and enhancing functional recovery from stroke in a rodent model.22–24 Kraig and colleagues reported the utility of dendritic cell-derived EVs in inducing remyelination of damaged nerve fibers, which could have significant implications for the treatment of multiple sclerosis and other NS pathophysiologies.25 In this study, interferon-γ stimulation was used to promote expression and EV packaging of miRNAs associated with myelin production, enhancing the regenerative nature of the EVs and demonstrating how EV cargo is susceptible to change based on source cell microenvironmental conditions.25 Schwann cell-derived EVs have also been shown to induce regeneration in the NS.26 Court and colleagues discovered that Schwann cells, well-known inducers of axonal regeneration, utilize EVs as part of their overall regulation and stimulation of axonal growth after injury. This study further demonstrates the capability of Schwann cell-derived EVs to enhance axonal regeneration after sciatic nerve injury in vivo,26 providing direct support for the potential application of NS cell-derived EVs for peripheral NS regenerative therapies.
Importantly, the study by Court and colleagues also suggests that EVs should be considered in peripheral NS tissue engineering strategies (e.g., for spinal cord repair). This suggestion is buttressed by the finding of Fruhbeis et al. that EVs play a critical role in oligodendrocyte–neuron communication toward promoting axonal integrity.20 Thus, the maintenance of appropriate EV biogenesis and signaling in cell-based tissue engineering approaches for treatment of NS injury may be a crucial factor in the potential long-term success of any intervention.
Vascular system
Therapeutic control of vascularization has long been sought as a critical enabling technology for complex tissue engineering strategies, and numerous drug delivery and cell transplantation strategies have been developed toward this goal.27–34 Recent studies have focused on exploring the roles of EVs in autocrine and paracrine signaling during vascular development and maturation and as potential drug delivery vehicles for therapeutic vascularization. A study by van Balkom et al. showed that endothelial cells (ECs) secrete miR-214-rich EVs, which promote EC migration and angiogenesis in vitro and in vivo through repression of ataxia telangiectasia mutated (ATM) expression in recipient cells,35 revealing a crucial role for EVs in EC autocrine signaling. Dimmeler and colleagues reported that miRNA transfer from ECs to smooth muscle cells (SMCs) through EVs could induce an atheroprotective SMC phenotype,36 indicative of a paracrine signaling role for EVs in the vascular system and establishing the potential for EC-derived EVs to be employed as therapeutic drug/gene carriers for treatment of vascular and cardiovascular diseases. EVs from endothelial progenitor cells (EPCs) also have physiological importance as demonstrated by Deregibus et al., who showed that EPC-derived EVs activate an angiogenic program in ECs through horizontal transfer of mRNA.37 Furthermore, Cantaluppi et al. showed that EPC-derived EVs carrying proangiogenic miR-126 and miR-296 sustain islet vascularization and maintain β-cell function by transfer of miRNAs to islet endothelium, resulting in activation of PI3K-Akt and eNOS signaling pathways.38 Also, as in other systems, MSC-derived EVs can therapeutically impact the vascular system. Salomon and colleagues showed that placental MSC-derived EVs induce human placental microvascular EC migration and tube formation,39 and Xin et al. demonstrated that, in addition to improving overall functional recovery, the administration of MSC-derived EVs to rats after stroke enhances angiogenesis.24 Additionally, Losordo and colleagues showed that the proangiogenic paracrine activity associated with human CD34+ stem cells can be almost entirely attributed to EVs secreted from these cells.5 In total, these studies solidify the importance of EVs in physiological vascular signaling and demonstrate the potential of EVs for therapeutic delivery to vascular cells. Both these roles merit consideration at the design stage of tissue engineering and regenerative medicine approaches involving vascularization.
Cardiovascular system
Heart disease and dysfunction remain leading causes of mortality and morbidity worldwide, and the innate regenerative capacity of the heart is inadequate to compensate for any significant decrease of function due to damage or death of cardiomyocytes40,41; thus, cardiac regeneration is currently a high priority goal within the fields of tissue engineering and regenerative medicine.42,43 Recent evidence suggests a potential role for EVs in cardiovascular homeostasis, and there have already been several demonstrations of the therapeutic efficacy of EVs on the cardiovascular system. Sahoo and Losordo demonstrated that both mouse and human cardiomyocytes are able to produce EVs under normal and ischemic conditions,44 and Barile et al. provided evidence, in mice, that cardiovascular progenitor cells secrete EVs.45 Further understanding of any differences in the distribution, function, and fate of secreted EVs in the cardiovascular system will undoubtedly enhance the design of multicellular tissue engineering strategies and provide insight into cell-based cardiac regeneration approaches.
In animal models, the uptake of EVs in the heart is supported by the therapeutic effects of MSC-derived EVs in reducing myocardial/ischemia reperfusion injury,6 enhancing blood flow recovery following acute myocardial infarction46 and reducing cardiac fibrosis in infarcted hearts.47 The proangiogenic effects of EVs also contribute to their cardiovascular therapeutic efficacy. Vrijsen et al. showed that cardiomyocyte progenitor cell-derived EVs stimulate EC migration.48 Furthermore, Mackie and colleagues engineered CD34+ stem cells to release EVs containing sonic hedgehog, a proangiogenic factor. Injection of these modified cells to the border zone of mice after myocardial infarction resulted in reduced infarct size, increased capillary density, and improved functional recovery.49 Thus, advances in the application and engineering of EVs as therapeutic delivery vehicles may lead to new possibilities for cardiovascular therapies.
Reproductive system
Tissue engineering has already begun to deliver on the promise of repairing or regenerating organs or tissues in the reproductive system, as exemplified by a recent report of successful functional engineering of vaginal tissues in human patients,50 and additional potential applications for reproductive tissue engineering and regeneration include male reproductive organs as well as the uterus and cervix.51 The direct regenerative potential of EVs emanating from reproductive tissues is unclear, although evidence has begun to emerge that EVs secreted by (uterine) endometrial epithelial cells are critical for successful embryo implantation in pregnancy52 and that EV-mediated miRNA transfer within the ovarian follicle is crucial for healthy oocyte development.53 In addition, placental EVs were previously identified as vital immunoregulatory agents in pregnancy by Taylor, Gercel-Taylor and colleagues.54,55 In the male reproductive system, EVs secreted by the epididymis and prostate function to protect sperm and promote its maturation,56 enhancing reproductive potential. All these findings suggest that the role of EVs should be considered in the design of tissue engineering or regenerative medicine approaches involving reproductive tissues or organs. For example, Campbell et al. reported a strategy for growth of uterine tissue in the peritoneal cavity of host animals before transplantation to the appropriate anatomical location.57 In this type of in vivo bioreactor approach to tissue engineering, it may be critical to examine what effect the generation of tissue in nonorthotopic locations has on EV biogenesis and content and to understand what information may be transferred to these tissues during their pretransplantation maturation by EVs. Additionally, Ulrich et al. recently reported the use of MSCs derived from human endometrium as a component of a tissue engineering strategy to address pelvic organ prolapse.58 It is likely that EVs play at least some role in the effects of the endometrial MSCs in this system, and thus, it may be of interest to compare the rates of biogenesis and, especially, the cargos of EVs derived from endometrial MSCs to those of other MSC-derived EVs.
Hepatic system
Although the liver has significant intrinsic regenerative capacity, liver failure is still a prevalent health problem worldwide and tissue engineering approaches are being sought to ease the shortage of donor organs. Many such strategies involve hepatocyte transplantation,59,60 and recent reports indicate a potential role for hepatocyte-derived EVs in liver regeneration. Nojima et al. demonstrated a dose-dependent increase in hepatocyte proliferation when ischemia/reperfusion (I/R)-injured mice were treated with hepatocyte-derived EVs.61 Likewise, Herrera et al. demonstrated the therapeutic properties of human liver stem cell-derived EVs to accelerate liver regeneration in a 70% hepatectomized rat model.62 In this study, RNA transfer from EVs into target hepatocytes was mediated by the presence of α4-integrin adhesion molecules on EV surfaces,62 indicative of specificity for this mode of intercellular information transfer. Additionally, Royo et al. demonstrated that EVs derived from primary hepatocytes activated hepatic stellate cells by means of RNA transfer.63 In this study, hepatic stellate cells treated with EVs derived from primary hepatocytes showed increased expression of nitric oxide synthase 2 (NOS2), an enzyme characteristic of stellate cell activation.63 RNase-pretreated hepatocyte EVs did not evince increased NOS2 expression,63 highlighting the essential role of EV-mediated RNA transfer in hepatic stellate cell activation, which is known to occur during the early stages of liver generation.64 Furthermore, proteomic analysis of EVs from animals following experimentally induced liver injury revealed that the liver-specific enzyme S-adenosylmethionine synthetase 1, essential for liver regeneration, was present in hepatocyte-derived EVs.65
All the studies referenced above point toward the need for consideration of EVs in liver tissue engineering applications, and some work has already been done to illuminate the mechanisms involved in EV biogenesis and secretion in hepatocytes. Nojima et al. recently demonstrated that CXCR1 and CXCR2, which have previously been shown to play a role in the recovery and regeneration of the liver after I/R injury, influence EV secretion by hepatocytes.61 This study revealed that CXCR2−/− hepatocytes produced EVs in similar quantities to those of wild-type hepatocytes, whereas CXCR1−/− hepatocytes produced considerably fewer EVs.61 Expansion of this genre of research would enable more thorough understanding and consideration of the role of EVs in liver regeneration, potentially leading to more effective liver regeneration and tissue engineering therapies.
Respiratory system
Given the diversity of cell types within the lungs, the establishment of appropriate intercellular communication is likely to be a key feature of any effective lung regeneration or tissue engineering approach. The importance of EVs in cell–cell communication1 suggests consideration of their effects, and indeed, EVs derived from MSCs have been shown to exert a pleiotropic protective effect on the lung resulting in the inhibition of pulmonary hypertension.66 It has also been established that EVs captured from fluid within the lungs have different cargoes in patients with lung diseases, such as asthma and sarcoidosis, compared with those in patients with typical lung function.67–69 Furthermore, EVs isolated from brocheoalveolar fluid of mice tolerized to an olive pollen antigen were shown to induce tolerance and protection in naive mice following intranasal administration,70 indicative that information from EVs collected in the lung can be processed to produce a physiological response. Most interestingly, Quesenberry and colleagues showed that lung-derived EVs stably reprogram marrow cells toward a pulmonary epithelial phenotype.71,72 Given the strong evidence of significant roles for EVs in lung physiology, EVs should be considered when designing strategies that build on the exciting initial efforts in lung tissue engineering.73,74
Renal system
EVs in the renal system are often discussed in the context of their potential use as biomarkers that can be isolated from urine.75,76 Also, as with many of the other physiological systems mentioned, MSC-derived EVs have been shown to have therapeutic effects on the kidneys,38,77–79 indicating that renal epithelial cells are capable of taking up EVs and processing information that results in phenotypic changes. In addition, pioneering work by Knepper and colleagues revealed that renal epithelial cells secrete EVs,80 providing a basis for the consideration of the role of EVs in renal system regenerative and tissue engineering approaches, although the role of EVs in intercellular communication between renal epithelial cells is not well defined. It has been shown, however, that renal fibrosis can lead to altered miR-29c content in urinary EVs,81 further evidence that EVs play a significant role in renal epithelial signaling and function and should be considered by tissue engineers employing differentiated or embryonic epithelial cell-based regenerative approaches to produce kidneys82,83 or other aspects of the renal (or urinary) system.84,85
Summary
The roles of EVs in tissue homeostasis and regeneration in numerous physiological systems are only beginning to be fully understood, yet the preponderance of evidence reported to date indicates that these roles are likely to be significant in at least some tissues and organs. Beyond what has been discussed above, the development of engineered tissues as both model systems for drug development86,87 or for study of complex biology in vitro would benefit from consideration of the roles of EVs. This is especially relevant for tissue engineering models of cancer,88 where roles for EVs in tumor growth and metastasis are well described.89–93
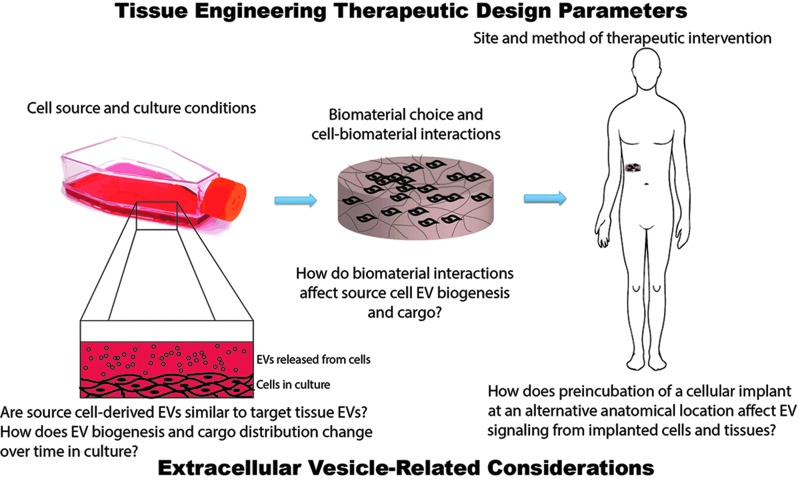
Overall, the consideration of EVs in the design stage of tissue engineering and regeneration approaches is warranted. For example, the choice of cell source for cell-based therapies might be impacted by knowledge of how EV signaling in the chosen cells relates to what is known about physiological EV signaling in the target organ or tissue. However, for such consideration to ever be undertaken generally, more fundamental studies of EV biology in the context of tissue engineering and regenerative medicine are needed. How are EV biogenesis and cargo content affected by cell culture conditions or cell–biomaterial interactions? Do induced pluripotent stem cells recapitulate EV biogenesis of the natural cells into which they have been differentiated? These and other questions await answers that have the potential to have a substantial impact on the practice of tissue engineering and regenerative medicine in the future (Fig. 1).
FIG. 1.
Consideration of extracellular vesicles (EVs) in the design stage of tissue engineering approaches. Tissue engineers consider many parameters in designing therapeutic approaches, including cell source and culture conditions, biomaterial–cell interactions, and the site and method of therapeutic intervention (e.g., injection vs. implantation, use of an alternative anatomical location as an in vivo bioreactor). Consideration of each of these parameters could be affected by further knowledge of how EV biogenesis and cargo may change based on the choices made at each step of the tissue engineering design process. Color images available online at www.liebertpub.com/teb
EVs as Therapeutic Delivery Vehicles for Tissue Regeneration
The emerging appreciation for the critical roles of EVs in mediating therapeutic effects of cell-based interventions not only highlights the need for consideration of EV biology in tissue engineering, it also reveals potential applications for EVs as natural delivery vehicles for biologics (e.g., mRNA, miRNA, DNA, and proteins) for tissue regeneration. As alternatives to cellular therapies, EVs have advantages with regard to safety and regulatory concerns—EVs do not have the replicative capacity of cells—and are also less complex and more amenable to quality control and, potentially, scale-up when compared to cells. Furthermore, EVs have the potential to be used generically as drug carriers and could be uniquely employed in personalized medicine if obtained from a patient's own cells. Thus, in addition to increased knowledge of EV biology, the fields of tissue engineering and regenerative medicine may benefit from advances in EV-related biotechnology.
Unmodified EVs as alternatives to cell therapies
Perhaps, the most straightforward utilization of EVs in therapeutic delivery involves the harvesting and administration of unmodified EVs containing their native cargo as surrogates in place of the source cells that produce the EVs. EVs are most commonly isolated from cell cultures by differential centrifugation, immunoprecipitation, or chromatographic methods,94,95 and it should be noted that current methods may not efficiently separate exosomes from microvesicles and/or other EVs; thus, reports of activities of specific EV populations should be taken with appropriate context. The potential efficacy of using unmodified EVs as surrogates is clearly dependent on cell source, and as highlighted earlier in this review, MSCs have proven to be a viable source of EVs for a variety of therapeutic interventions.8,14 Lim and colleagues have reported the development of a potentially infinite supply of MSC-derived EVs through oncogenic immortalization of human embryonic stem cell (ESC)-derived MSCs,96 an advance that may broaden the therapeutic applicability of MSC-derived EVs. However, data from our group suggest that cell immortalization strategies must be considered on a case-by-case basis, as EVs isolated from retrovirally telomerized ECs97 display vastly different RNA and protein profiles than those from native ECs derived from human umbilical vein and human dermal microvasculature (T.N.L. and S.M.J., unpublished). Nevertheless, the numerous reports of therapeutic effects of MSC-derived EVs demand their consideration as alternatives to MSC-based cellular regeneration strategies.
In addition to MSCs, stem and progenitor cells have been identified as producers of EVs with native therapeutic potential. This is not surprising, given that paracrine effects of implanted adult stem cells, like those of MSCs,98 are thought to account for the majority of their therapeutic impact in numerous studies.99,100 Working with murine ESCs, Ratajczak and colleagues showed that EVs derived from these cells induced enhanced survival and improved expansion of murine hematopoietic progenitor cells.101 As noted earlier, human CD34+ stem cell-derived EVs mediate the therapeutic effects of these cells,37 and both cardiomyocytes and EPCs produce EVs with biological activity that could be utilized therapeutically.38,48,102 It seems likely that, given the convergence of the increased understandings of the roles of paracrine factors in cell therapies and of EVs in paracrine effects of cells, stem cell-derived EVs will continue to emerge as viable alternatives to cellular regenerative therapies for many applications.
Engineering EVs for therapeutic delivery
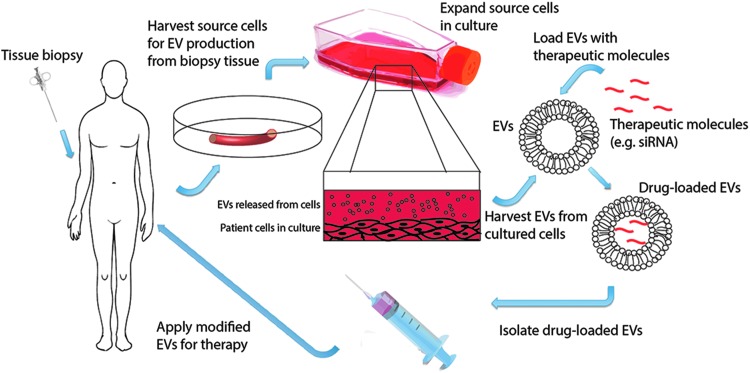
Although exploitation of the native capacity of EVs to mediate therapeutic effects is a promising avenue, the therapeutic efficacy and versatility of native EVs is limited by their native cargo and targeting capacity. Engineering of EVs for therapeutic delivery offers the potential to load non-native cargo—including nonbiologic molecules such as small molecule drugs (e.g., doxorubicin9)—and to add non-native abluminal moieties to affect pharmacokinetics and biodistribution. These possibilities, combined with the unique nature of EVs that allows for their isolation from a patient's own cells for application in a personalized medicine approach as self-derived drug delivery vehicles, endow EVs with the potential to revolutionize drug delivery (Fig. 2).
FIG. 2.
Engineering of EVs for use as therapeutic delivery vehicles in personalized medicine. EVs offer unique potential to be employed as delivery vehicles in personalized medicine. EVs could be obtained from cells harvested from biopsy tissue, loaded with a therapeutic molecule, and injected back into the patient. Such an approach would enable protection of labile therapeutic biologics while also avoiding the immunogenicity inherently associated with synthetic delivery systems. Color images available online at www.liebertpub.com/teb
A template for EV engineering was established in pioneering work by Wood and colleagues, who harvested EVs from dendritic cells of mice for ultimate injection into recipients with the same major histocompatibility complex haplotype.7 Before EV isolation, cells were transfected with plasmids encoding a fusion of either a muscle-specific or central NS-specific targeting peptide sequence, and Lamp2b, a protein known to associate with EV membranes, allowing for the targeting peptide sequences to be presented abluminally on EVs.7 This strategy was proven effective for multiple targeting peptides and has been expanded by others to target EGFR-expressing cancer cells for therapeutic delivery.103 Once isolated, the EVs were loaded with therapeutic siRNA molecules by electroporation before being administered to mice by intravenous injection.7 The result of this intervention was brain-specific knockdown of BACE1,7 a protease involved in Alzheimer's disease, serving as proof of concept that EV engineering has revolutionary potential for therapeutic delivery.
Excitingly, there are many paths forward to expand upon these initial groundbreaking findings. Gould and colleagues have elucidated mechanisms of protein targeting to EVs104–106 that could be utilized for controlled loading of protein cargo and/or expression of targeting ligands on EVs. Loading of EVs with nonbiologic therapeutic cargo has been reported,9,10,107 expanding the horizons for the types of therapeutic interventions that are possible with EVs. In addition to the myriad mammalian cell types that can be used as source cells for EVs, alternative sources of EVs such as fruit have been identified,108,109 which could open new doors for the scalable production of EVs for therapy.
Summary
The developments listed above, combined with the inherent advantages of EVs over synthetic drug delivery systems with regard to immunogenicity and ability to deliver cargo intracellularly and across biological barriers,110 spark great optimism for the potential of EVs in therapeutic biologic delivery. However, many challenges remain before widespread adoption of native or engineered EVs for therapeutic delivery applications. For example, standardization of EV isolation and characterization methods is needed to facilitate universality and reproducibility of findings,111 and large-scale production methods for therapeutic EVs must be developed. Additionally, better definition of EV pharmaceutical parameters, such as loading capacity and target ligand surface density, would help to clarify their therapeutic potential. Improved understanding of the biodistribution and trafficking of native EVs and how they are affected by cell source could enable more effective targeted therapeutic delivery. It is also important to consider how native cargo content is affected by cell source, cell culture conditions, and mechanical forces, and genomic and proteomic analyses should be applied to bring clarity to these issues. Finally, the drug release characteristics and tissue targeting and uptake efficiency of EVs must be directly compared to polymeric nanoparticle and liposomal delivery systems for specific cargoes to determine the true relevance of EVs as drug carriers. Resolution of these considerations along with continued development of EVs as therapeutic delivery vehicles and continued advances in understanding of EV biology will likely lead to new therapeutic opportunities involving EVs in tissue engineering and regenerative therapies in the future.
Conclusion
EVs have ubiquitous effects in biology and should be considered at the design stage of regenerative therapies and tissue engineering approaches. Additionally, EVs may be useful as delivery vehicles for biologics and as alternatives to cell-based tissue engineering or regenerative interventions. Overall, EVs should be recognized as key players in tissue engineering and regenerative medicine in a general sense.
Acknowledgments
We apologize to any author whose work was not included due to space considerations, and we acknowledge the support provided by funding from the National Institutes of Health (R00 HL112905) and the University of Maryland (Tier 1 Award).
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., and Lotvall J.O.Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9,654, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Andaloussi E.L., Mager I., Breakefield X.O., and Wood M.J.Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12,347, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Gyorgy B., Szabo T.G., Pasztoi M., Pal Z., Misjak P., Aradi B., Laszlo V., Pallinger E., Pap E., Kittel A., Nagy G., Falus A., and Buzas E.I.Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68,2667, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thery C.Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3,15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahoo S., Klychko E., Thorne T., Misener S., Schultz K.M., Millay M., Ito A., Liu T., Kamide C., Agrawal H., Perlman H., Qin G., Kishore R., and Losordo D.W.Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 109,724, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai R.C., Arslan F., Lee M.M., Sze N.S., Choo A., Chen T.S., Salto-Tellez M., Timmers L., Lee C.N., El Oakley R.M., Pasterkamp G., de Kleijn D.P., and Lim S.K.Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4,214, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., and Wood M.J.Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29,341, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Lai R.C., Yeo R.W., Tan K.H., and Lim S.K.Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv 31,543, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., and Nie G.A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35,2383, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Sun D., Zhuang X., Xiang X., Liu Y., Zhang S., Liu C., Barnes S., Grizzle W., Miller D., and Zhang H.G.A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18,1606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thakur B.K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B., Zheng Y., Hoshino A., Brazier H., Xiang J., Williams C., Rodriguez-Barrueco R., Silva J.M., Zhang W., Hearn S., Elemento O., Paknejad N., Manova-Todorova K., Welte K., Bromberg J., Peinado H., and Lyden D.Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 24,766, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldenstrom A., Genneback N., Hellman U., and Ronquist G.Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One 7,e34653, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai R.C., Chen T.S., and Lim S.K.Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med 6,481, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Yeo R.W., Lai R.C., Zhang B., Tan S.S., Yin Y., Teh B.J., and Lim S.K.Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 65,336, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt C.E., and Leach J.B.Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng 5,293, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Teng Y.D., Lavik E.B., Qu X., Park K.I., Ourednik J., Zurakowski D., Langer R., and Snyder E.Y.Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A 99,3024, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gage F.H.Mammalian neural stem cells. Science 287,1433, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Faure J., Lachenal G., Court M., Hirrlinger J., Chatellard-Causse C., Blot B., Grange J., Schoehn G., Goldberg Y., Boyer V., Kirchhoff F., Raposo G., Garin J., and Sadoul R.Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31,642, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Fruhbeis C., Frohlich D., and Kramer-Albers E.M.Emerging roles of exosomes in neuron-glia communication. Front Physiol 3,119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fruhbeis C., Frohlich D., Kuo W.P., Amphornrat J., Thilemann S., Saab A.S., Kirchhoff F., Mobius W., Goebbels S., Nave K.A., Schneider A., Simons M., Klugmann M., Trotter J., and Kramer-Albers E.M.Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 11,e1001604, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai C.P., and Breakefield X.O.Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol 3,228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xin H., Li Y., Buller B., Katakowski M., Zhang Y., Wang X., Shang X., Zhang Z.G., and Chopp M.Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30,1556, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin H., Li Y., Liu Z., Wang X., Shang X., Cui Y., Zhang Z.G., and Chopp M.MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31,2737, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xin H., Li Y., Cui Y., Yang J.J., Zhang Z.G., and Chopp M.Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33,1711, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pusic A.D., Pusic K.M., Clayton B.L., and Kraig R.P.IFNgamma-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J Neuroimmunol 266,12, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Verrilli M.A., Picou F., and Court F.A.Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 61,1795, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Richardson T.P., Peters M.C., Ennett A.B., and Mooney D.J.Polymeric system for dual growth factor delivery. Nat Biotechnol 19,1029, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Jay S.M., Shepherd B.R., Bertram J.P., Pober J.S., and Saltzman W.M.Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J 22,2949, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niklason L.E., Gao J., Abbott W.M., Hirschi K.K., Houser S., Marini R., and Langer R.Functional arteries grown in vitro. Science 284,489, 1999 [DOI] [PubMed] [Google Scholar]
- 30.L'Heureux N., Paquet S., Labbe R., Germain L., and Auger F.A.A completely biological tissue-engineered human blood vessel. FASEB J 12,47, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Takeshita S., Zheng L.P., Brogi E., Kearney M., Pu L.Q., Bunting S., Ferrara N., Symes J.F., and Isner J.M.Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest 93,662, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zisch A.H., Lutolf M.P., Ehrbar M., Raeber G.P., Rizzi S.C., Davies N., Schmokel H., Bezuidenhout D., Djonov V., Zilla P., and Hubbell J.A.Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J 17,2260, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Jain R.K., Au P., Tam J., Duda D.G., and Fukumura D.Engineering vascularized tissue. Nat Biotechnol 23,821, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Kaushal S., Amiel G.E., Guleserian K.J., Shapira O.M., Perry T., Sutherland F.W., Rabkin E., Moran A.M., Schoen F.J., Atala A., Soker S., Bischoff J., and Mayer J.E., Jr.Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med 7,1035, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Balkom B.W., de Jong O.G., Smits M., Brummelman J., den Ouden K., de Bree P.M., van Eijndhoven M.A., Pegtel D.M., Stoorvogel W., Wurdinger T., and Verhaar M.C.Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 121,3997, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Hergenreider E., Heydt S., Treguer K., Boettger T., Horrevoets A.J., Zeiher A.M., Scheffer M.P., Frangakis A.S., Yin X., Mayr M., Braun T., Urbich C., Boon R.A., and Dimmeler S.Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14,249, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Deregibus M.C., Cantaluppi V., Calogero R., Lo Iacono M., Tetta C., Biancone L., Bruno S., Bussolati B., and Camussi G.Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110,2440, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Cantaluppi V., Gatti S., Medica D., Figliolini F., Bruno S., Deregibus M.C., Sordi A., Biancone L., Tetta C., and Camussi G.Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 82,412, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Salomon C., Ryan J., Sobrevia L., Kobayashi M., Ashman K., Mitchell M., and Rice G.E.Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One 8,e68451, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergmann O., Bhardwaj R.D., Bernard S., Zdunek S., Barnabe-Heider F., Walsh S., Zupicich J., Alkass K., Buchholz B.A., Druid H., Jovinge S., and Frisen J.Evidence for cardiomyocyte renewal in humans. Science 324,98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senyo S.E., Steinhauser M.L., Pizzimenti C.L., Yang V.K., Cai L., Wang M., Wu T.D., Guerquin-Kern J.L., Lechene C.P., and Lee R.T.Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493,433, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segers V.F., and Lee R.T.Stem-cell therapy for cardiac disease. Nature 451,937, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Steinhauser M.L., and Lee R.T.Regeneration of the heart. EMBO Mol Med 3,701, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahoo S., and Losordo D.W.Exosomes and cardiac repair after myocardial infarction. Circ Res 114,333, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Barile L., Gherghiceanu M., Popescu L.M., Moccetti T., and Vassalli G.Ultrastructural evidence of exosome secretion by progenitor cells in adult mouse myocardium and adult human cardiospheres. J Biomed Biotechnol 2012,354605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bian S., Zhang L., Duan L., Wang X., Min Y., and Yu H.Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 92,387, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Feng Y., Huang W., Wani M., Yu X., and Ashraf M.Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One 9,e88685, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vrijsen K.R., Sluijter J.P., Schuchardt M.W., van Balkom B.W., Noort W.A., Chamuleau S.A., and Doevendans P.A.Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med 14,1064, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackie A.R., Klyachko E., Thorne T., Schultz K.M., Millay M., Ito A., Kamide C.E., Liu T., Gupta R., Sahoo S., Misener S., Kishore R., and Losordo D.W.Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res 111,312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raya-Rivera A.M., Esquiliano D., Fierro-Pastrana R., Lopez-Bayghen E., Valencia P., Ordorica-Flores R., Soker S., Yoo J.J., and Atala A.Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet 2014[Epub ahead of print]; DOI: 10.1016/S0140-6736(14)60542-0 [DOI] [PubMed] [Google Scholar]
- 51.Atala A.Tissue engineering of reproductive tissues and organs. Fertil Steril 98,21, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Ng Y.H., Rome S., Jalabert A., Forterre A., Singh H., Hincks C.L., and Salamonsen L.A.Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One 8,e58502, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.da Silveira J.C., Veeramachaneni D.N., Winger Q.A., Carnevale E.M., and Bouma G.J.Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol Reprod 86,71, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Taylor D.D., Akyol S., and Gercel-Taylor C.Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol 176,1534, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Sabapatha A., Gercel-Taylor C., and Taylor D.D.Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol 56,345, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Sullivan R., and Saez F.Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction 146,R21, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Campbell G.R., Turnbull G., Xiang L., Haines M., Armstrong S., Rolfe B.E., and Campbell J.H.The peritoneal cavity as a bioreactor for tissue engineering visceral organs: bladder, uterus and vas deferens. J Tissue Eng Regen Med 2,50, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Ulrich D., Edwards S.L., Su K., Tan K.S., White J.F., Ramshaw J.A., Lo C., Rosamilia A., Werkmeister J.A., and Gargett C.E.Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng Part A 20,785, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohashi K., Yokoyama T., Yamato M., Kuge H., Kanehiro H., Tsutsumi M., Amanuma T., Iwata H., Yang J., Okano T., and Nakajima Y.Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med 13,880, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Uygun B.E., Soto-Gutierrez A., Yagi H., Izamis M.L., Guzzardi M.A., Shulman C., Milwid J., Kobayashi N., Tilles A., Berthiaume F., Hertl M., Nahmias Y., Yarmush M.L., and Uygun K.Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 16,814, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nojima H., Wilson G., Quillin R., Schuster R., Blanchard J., Edwards M., Gulbins E., and Lentsch A.Hepatocyte-derived exosomes regulate liver recovery and regeneration after ischemia/reperfusion in mice. FASEB J 28,Supplement 398.3, 2014 [Google Scholar]
- 62.Herrera M.B., Fonsato V., Gatti S., Deregibus M.C., Sordi A., Cantarella D., Calogero R., Bussolati B., Tetta C., and Camussi G.Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med 14,1605, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Royo F., Schlangen K., Palomo L., Gonzalez E., Conde-Vancells J., Berisa A., Aransay A.M., and Falcon-Perez J.M.Transcriptome of extracellular vesicles released by hepatocytes. PLoS One 8,e68693, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mabuchi A., Mullaney I., Sheard P., Hessian P., Zimmermann A., Senoo H., and Wheatley A.M.Role of hepatic stellate cells in the early phase of liver regeneration in rat: formation of tight adhesion to parenchymal cells. Comp Hepatol 3Suppl 1,S29, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez-Suarez E., Gonzalez E., Hughes C., Conde-Vancells J., Rudella A., Royo F., Palomo L., Elortza F., Lu S.C., Mato J.M., Vissers J.P., and Falcon-Perez J.M.Quantitative proteomic analysis of hepatocyte-secreted extracellular vesicles reveals candidate markers for liver toxicity. J Proteomics 103,227, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee C., Mitsialis S.A., Aslam M., Vitali S.H., Vergadi E., Konstantinou G., Sdrimas K., Fernandez-Gonzalez A., and Kourembanas S.Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126,2601, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levanen B., Bhakta N.R., Torregrosa Paredes P., Barbeau R., Hiltbrunner S., Pollack J.L., Skold C.M., Svartengren M., Grunewald J., Gabrielsson S., Eklund A., Larsson B.M., Woodruff P.G., Erle D.J., and Wheelock A.M.Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol 131,894, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qazi K.R., Torregrosa Paredes P., Dahlberg B., Grunewald J., Eklund A., and Gabrielsson S.Proinflammatory exosomes in bronchoalveolar lavage fluid of patients with sarcoidosis. Thorax 65,1016, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Torregrosa Paredes P., Esser J., Admyre C., Nord M., Rahman Q.K., Lukic A., Radmark O., Gronneberg R., Grunewald J., Eklund A., Scheynius A., and Gabrielsson S.Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy 67,911, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Prado N., Marazuela E.G., Segura E., Fernandez-Garcia H., Villalba M., Thery C., Rodriguez R., and Batanero E.Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J Immunol 181,1519, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Aliotta J.M., Sanchez-Guijo F.M., Dooner G.J., Johnson K.W., Dooner M.S., Greer K.A., Greer D., Pimentel J., Kolankiewicz L.M., Puente N., Faradyan S., Ferland P., Bearer E.L., Passero M.A., Adedi M., Colvin G.A., and Quesenberry P.J.Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells 25,2245, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aliotta J.M., Pereira M., Li M., Amaral A., Sorokina A., Dooner M.S., Sears E.H., Brilliant K., Ramratnam B., Hixson D.C., and Quesenberry P.J.Stable cell fate changes in marrow cells induced by lung-derived microvesicles. J Extracell Vesicles 1,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., Gavrilov K., Yi T., Zhuang Z.W., Breuer C., Herzog E., and Niklason L.E.Tissue-engineered lungs for in vivo implantation. Science 329,538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ott H.C., Clippinger B., Conrad C., Schuetz C., Pomerantseva I., Ikonomou L., Kotton D., and Vacanti J.P.Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16,927, 2010 [DOI] [PubMed] [Google Scholar]
- 75.van Balkom B.W., Pisitkun T., Verhaar M.C., and Knepper M.A.Exosomes and the kidney: prospects for diagnosis and therapy of renal diseases. Kidney Int 80,1138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang D.Y., King H.W., Li J.Y., and Gleadle J.M.Exosomes and the kidney: blaming the messenger. Nephrology 18,1, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Camussi G., Cantaluppi V., Deregibus M.C., Gatti E., and Tetta C.Role of microvesicles in acute kidney injury. Contrib Nephrol 174,191, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Bruno S., Grange C., Collino F., Deregibus M.C., Cantaluppi V., Biancone L., Tetta C., and Camussi G.Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7,e33115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grange C., Tapparo M., Bruno S., Chatterjee D., Quesenberry P.J., Tetta C., and Camussi G.Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med 33,1055, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pisitkun T., Shen R.-F., and Knepper M.A.Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101,13368, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lv L.L., Cao Y.H., Ni H.F., Xu M., Liu D., Liu H., Chen P.S., and Liu B.C.MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol 305,F1220, 2013 [DOI] [PubMed] [Google Scholar]
- 82.Song J.J., Guyette J.P., Gilpin S.E., Gonzalez G., Vacanti J.P., and Ott H.C.Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19,646, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xinaris C., Benedetti V., Rizzo P., Abbate M., Corna D., Azzollini N., Conti S., Unbekandt M., Davies J.A., Morigi M., Benigni A., and Remuzzi G.In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol 23,1857, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Atala A., Bauer S.B., Soker S., Yoo J.J., and Retik A.B.Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367,1241, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Raya-Rivera A., Esquiliano D.R., Yoo J.J., Lopez-Bayghen E., Soker S., and Atala A.Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 377,1175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khetani S.R., and Bhatia S.N.Microscale culture of human liver cells for drug development. Nat Biotechnol 26,120, 2008 [DOI] [PubMed] [Google Scholar]
- 87.Sivaraman A., Leach J.K., Townsend S., Iida T., Hogan B.J., Stolz D.B., Fry R., Samson L.D., Tannenbaum S.R., and Griffith L.G.A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr Drug Metab 6,569, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Fischbach C., Chen R., Matsumoto T., Schmelzle T., Brugge J.S., Polverini P.J., and Mooney D.J.Engineering tumors with 3D scaffolds. Nat Methods 4,855, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Skog J., Wurdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., and Breakefield X.O.Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10,1470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Janowska-Wieczorek A., Wysoczynski M., Kijowski J., Marquez-Curtis L., Machalinski B., Ratajczak J., and Ratajczak M.Z.Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 113,752, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Taylor D.D., and Gercel-Taylor C.Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer 92,305, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor D.D., and Gercel-Taylor C.MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110,13, 2008 [DOI] [PubMed] [Google Scholar]
- 93.Andre F., Schartz N.E., Movassagh M., Flament C., Pautier P., Morice P., Pomel C., Lhomme C., Escudier B., Le Chevalier T., Tursz T., Amigorena S., Raposo G., Angevin E., and Zitvogel L.Malignant effusions and immunogenic tumour-derived exosomes. Lancet 360,295, 2002 [DOI] [PubMed] [Google Scholar]
- 94.Momen-Heravi F., Balaj L., Alian S., Mantel P.Y., Halleck A.E., Trachtenberg A.J., Soria C.E., Oquin S., Bonebreak C.M., Saracoglu E., Skog J., and Kuo W.P.Current methods for the isolation of extracellular vesicles. Biol Chem 394,1253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taylor D.D., Zacharias W., and Gercel-Taylor C.Exosome isolation for proteomic analyses and RNA profiling. Methods Mol Biol 728,235, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Chen T.S., Arslan F., Yin Y., Tan S.S., Lai R.C., Choo A.B., Padmanabhan J., Lee C.N., de Kleijn D.P., and Lim S.K.Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med 9,47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang J., Nagavarapu U., Relloma K., Sjaastad M.D., Moss W.C., Passaniti A., and Herron G.S.Telomerized human microvasculature is functional in vivo. Nat Biotechnol 19,219, 2001 [DOI] [PubMed] [Google Scholar]
- 98.Ranganath S.H., Levy O., Inamdar M.S., and Karp J.M.Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10,244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chimenti I., Smith R.R., Li T.S., Gerstenblith G., Messina E., Giacomello A., and Marban E.Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res 106,971, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gnecchi M., Zhang Z., Ni A., and Dzau V.J.Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103,1204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ratajczak J., Miekus K., Kucia M., Zhang J., Reca R., Dvorak P., and Ratajczak M.Z.Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20,847, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Cantaluppi V., Biancone L., Figliolini F., Beltramo S., Medica D., Deregibus M.C., Galimi F., Romagnoli R., Salizzoni M., Tetta C., Segoloni G.P., and Camussi G.Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant 21,1305, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Ohno S., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., Gotoh N., and Kuroda M.Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21,185, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fang Y., Wu N., Gan X., Yan W., Morrell J.C., and Gould S.J.Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol 5,e158, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen B., Wu N., Yang J.M., and Gould S.J.Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem 286,14383, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang J.M., and Gould S.J.The cis-acting signals that target proteins to exosomes and microvesicles. Biochem Soc Trans 41,277, 2013 [DOI] [PubMed] [Google Scholar]
- 107.Zhuang X., Xiang X., Grizzle W., Sun D., Zhang S., Axtell R.C., Ju S., Mu J., Zhang L., Steinman L., Miller D., and Zhang H.G.Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19,1769, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ju S., Mu J., Dokland T., Zhuang X., Wang Q., Jiang H., Xiang X., Deng Z.B., Wang B., Zhang L., Roth M., Welti R., Mobley J., Jun Y., Miller D., and Zhang H.G.Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther 21,1345, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Q., Zhuang X., Mu J., Deng Z.B., Jiang H., Zhang L., Xiang X., Wang B., Yan J., Miller D., and Zhang H.G.Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun 4,1867, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wood M.J., O'Loughlin A.J., and Samira L.Exosomes and the blood-brain barrier: implications for neurological diseases. Ther Deliv 2,1095, 2011 [DOI] [PubMed] [Google Scholar]
- 111.Witwer K.W., Buzas E.I., Bemis L.T., Bora A., Lasser C., Lotvall J., Nolte-'t Hoen E.N., Piper M.G., Sivaraman S., Skog J., Thery C., Wauben M.H., and Hochberg F.Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]