Abstract
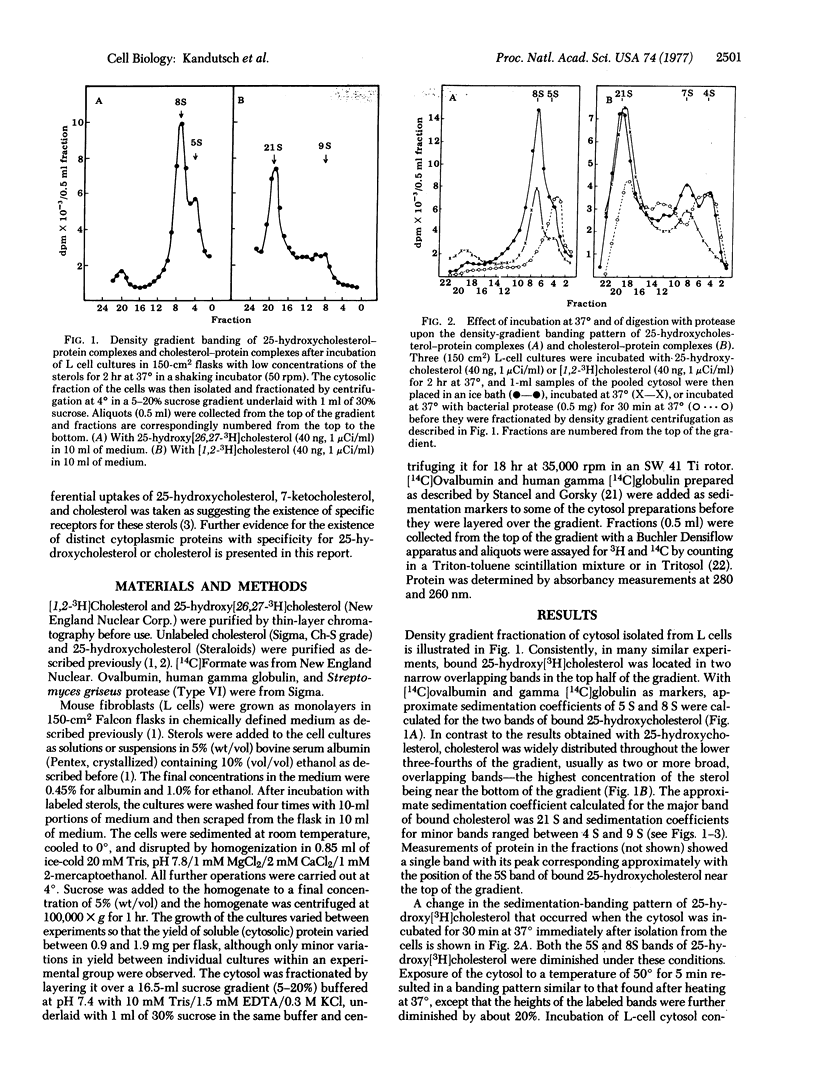
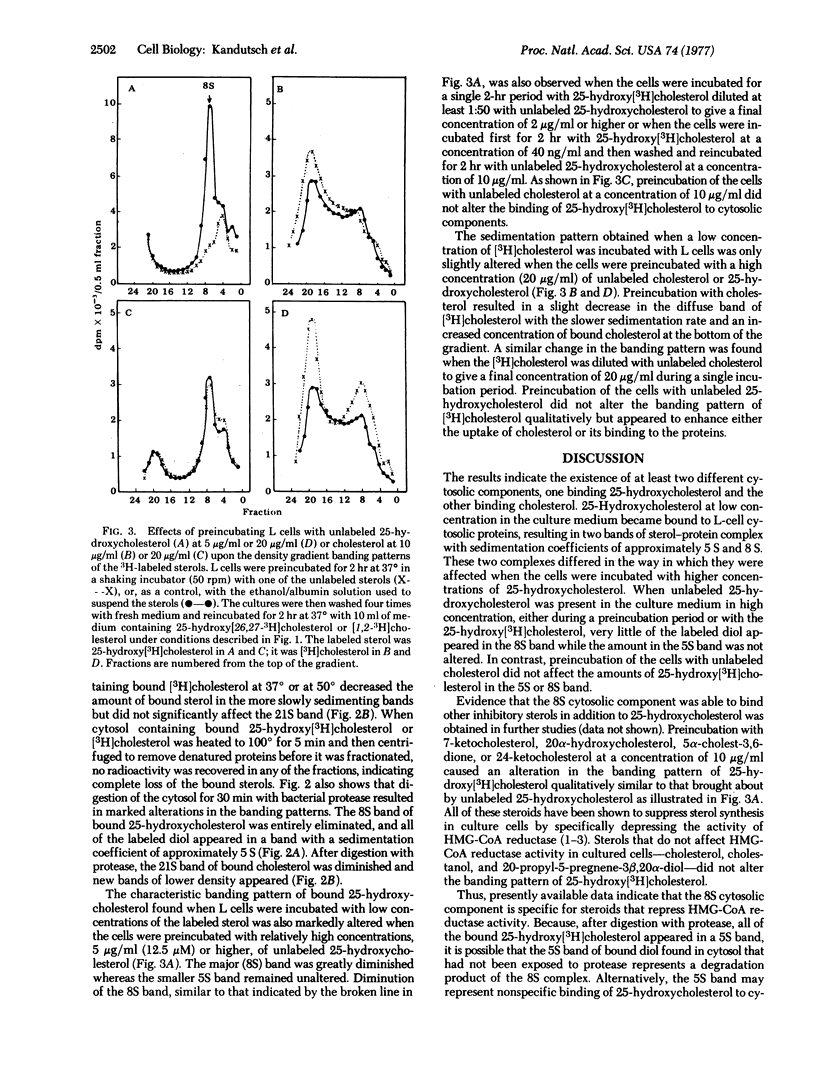
Studies were carried out to determine whether or not oxygenated derivatives of cholesterol (e.g., 25-hydroxycholesterol) that specifically suppress the activity of 3-hydroxy-3-methylglutaryl-CoA reductase [mevalonate:NADP+ oxidoreductase (CoA-acylating), EC 1.1.1.34], bind to a soluble component of the cytoplasm different from that which binds the nonsuppressor, cholesterol. Density gradient fractionation of the cytosolic fraction isolated from L cell cultures that had been incubated with low concentrations of 25-hydroxy[26,27-3H]cholesterol or [1,2-3H]cholesterol provided evidence for the existence of at least two different sterol-binding proteins. Bound cholesterol sedimented in a sucrose density gradient as two or more broad bands with coefficients of approximately 9 S and 21 S. Two relatively narrow bands of bound 25-hydroxycholesterol had sedimentation coefficients of 5 S and 8 S. Preincubation of the cells with a relatively high concentration of unlabeled 25-hydroxycholesterol altered the banding pattern of the 25-hydroxy[3H]cholesterol taken up during a subsequent incubation period by decreasing the size of the major (8S) band. Under these conditions, cholesterol did not affect the banding pattern of 25-hydroxy[3H]cholesterol. The density gradient banding pattern of bound [3H]cholesterol was only slightly affected by preincubating the cells with unlabeled cholesterol or 25-hydroxycholesterol. Both sterols appeared to be bound to proteins because the bound sterols were eliminated from cytosol that had been heated at 100°, and their sedimentation coefficients were altered by proteolysis.
Keywords: regulation, sterol synthesis, cell cultures
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter J. D., Tomkins G. M. Specific cytoplasmic glucocorticoid hormone receptors in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1971 May;68(5):932–937. doi: 10.1073/pnas.68.5.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. J., Sargeant T. E., Watson J. A. Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in hepatoma tissue culture cells by pure cholesterol and several cholesterol derivatives. Evidence supporting two distinct mechanisms.20l. J Biol Chem. 1976 Mar 25;251(6):1745–1758. [PubMed] [Google Scholar]
- Breslow J. L., Lothrop D. A., Spaulding D. R., Kandutsch A. A. Cholesterol, 7-ketocholesterol and 25-hydroxycholesterol uptake studies and effect on 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in human fibroblasts. Biochim Biophys Acta. 1975 Jul 22;398(1):10–17. doi: 10.1016/0005-2760(75)90165-4. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Brannan P. G., Bohmfalk H. A., Brunschede G. Y., Dana S. E., Helgeson J., Goldstein J. L. Use of mutant fibroblasts in the analysis of the regulation of cholesterol metabolism in human cells. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):425–436. doi: 10.1002/jcp.1040850409. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Chen H. W., Heiniger H. J., Kandutsch A. A. Relationship between sterol synthesis and DNA synthesis in phytohemagglutinin-stimulated mouse lymphocytes. Proc Natl Acad Sci U S A. 1975 May;72(5):1950–1954. doi: 10.1073/pnas.72.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey M. E. Regulation of steroid biosynthesis. Annu Rev Biochem. 1974;43(0):967–990. doi: 10.1146/annurev.bi.43.070174.004535. [DOI] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Higgins M. J., Brady D., Rudney H. Rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase: a comparison and immunological study of purified solubilized preparations, and alteration of enzyme levels by cholestyramine feeding. Arch Biochem Biophys. 1974 Jul;163(1):271–282. doi: 10.1016/0003-9861(74)90477-9. [DOI] [PubMed] [Google Scholar]
- Higgins M., Kawachi T., Rudney H. The mechanism of the diurnal variation of hepatic HMG-CoA reductase activity in the rat. Biochem Biophys Res Commun. 1971 Oct 1;45(1):138–144. doi: 10.1016/0006-291x(71)90061-1. [DOI] [PubMed] [Google Scholar]
- Higgins M., Rudney H. Regulation of rat liver beta-hydroxy-beta-methylglutaryl-CoA reductase activity by cholesterol. Nat New Biol. 1973 Nov 14;246(150):60–61. doi: 10.1038/newbio246060a0. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Consequences of blocked sterol synthesis in cultured cells. DNA synthesis and membrane composition. J Biol Chem. 1977 Jan 25;252(2):409–415. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Inhibition of sterol synthesis in cultured mouse cells by 7alpha-hydroxycholesterol, 7beta-hydroxycholesterol, and 7-ketocholesterol. J Biol Chem. 1973 Dec 25;248(24):8408–8417. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J Biol Chem. 1974 Oct 10;249(19):6057–6061. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Regulation of sterol synthesis in cultured cells by oxygenated derivatives of cholesterol. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):415–424. doi: 10.1002/jcp.1040850408. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Heiniger H. J., Chen H. W. Effects of 25-hydroxycholesterol and 7-ketocholesterol, inhibitors of sterol synthesis, administered orally to mice. Biochim Biophys Acta. 1977 Feb 23;486(2):260–272. doi: 10.1016/0005-2760(77)90022-4. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Saucier S. E. Prevention of cyclic and triton-induced increases in hydroxymethylglutaryl coenzyme A reductase and sterol synthesis by puromycin. J Biol Chem. 1969 May 10;244(9):2299–2305. [PubMed] [Google Scholar]
- Kirsten E. S., Watson J. A. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in hepatoma tissue culture cells by serum lipoproteins. J Biol Chem. 1974 Oct 10;249(19):6104–6109. [PubMed] [Google Scholar]
- Rothblat G. H., Buchko M. K. Effect of exogenous steroids on sterol synthesis in L-cell mouse fibroblasts. J Lipid Res. 1971 Nov;12(6):647–652. [PubMed] [Google Scholar]
- Saat Y. A., Bloch K. E. Effect of a supernatant protein on microsomal squalene epoxidase and 2,3-oxidosqualene-lanosterol cyclase. J Biol Chem. 1976 Sep 10;251(17):5155–5160. [PubMed] [Google Scholar]
- Scallen T. J., Srikantaiah M. V., Seetharam B., Hansbury E., Gavey K. L. Proceedings: Sterol carrier protein hypothesis. Fed Proc. 1974 Jun;33(6):1733–1746. [PubMed] [Google Scholar]
- Shapiro D. J., Rodwell V. W. Diurnal variation and cholesterol regulation of hepatic HMG-CoA reductase activity. Biochem Biophys Res Commun. 1969 Nov 20;37(5):867–872. doi: 10.1016/0006-291x(69)90972-3. [DOI] [PubMed] [Google Scholar]
- Srikantaiah M. V., Hansbury E., Loughran E. D., Scallen T. J. Purification and properties of sterol carrier protein1. J Biol Chem. 1976 Sep 25;251(18):5496–5504. [PubMed] [Google Scholar]
- Teng J. I., Kulig M. J., Smith L. L., Kan G., Van Lier J. E. Sterol metabolism. XX. Cholesterol 7 -hydroperoxide. J Org Chem. 1973 Jan 12;38(1):119–123. doi: 10.1021/jo00941a024. [DOI] [PubMed] [Google Scholar]
- Van Lier J. E., Da Costa A. L., Smith L. L. Cholesterol autoxidation: identification of the volatile fragments. Chem Phys Lipids. 1975 Aug;14(4):327–335. doi: 10.1016/0009-3084(75)90069-9. [DOI] [PubMed] [Google Scholar]



