Abstract
Transcatheter aortic valve replacement (TAVR) is a transformative innovation that provides treatment for high or prohibitive surgical risk patients with symptomatic severe aortic stenosis (AS) who were previously either not referred for or denied operative intervention. Trials have demonstrated improvements in survival and symptoms after TAVR compared to medical therapy, however there remains a sizable group of patients who die or lack improvement in quality of life soon after TAVR. This raises important questions about the need to identify and acknowledge the possibility of futility in some patients considered for TAVR. In this very elderly population, a number of factors in addition to traditional risk stratification need to be considered including multimorbidity, disability, frailty, and cognition in order to assess the anticipated benefit of TAVR. Consideration by a multidisciplinary heart valve team with broad areas of expertise is critical for assessing likely benefit from TAVR. Moreover, these complicated decisions should take place with clear communication around desired health outcomes on behalf of the patient and provider. The decision that treatment with TAVR is futile should include alternative plans to optimize the patient's health state or, in some cases, discussions related to end of life care. We review issues to be considered when making and communicating these difficult decisions.
Keywords: aortic valve stenosis, valve replacement, heart failure, frailty, outcomes
Introduction
The only treatment that improves survival and quality of life for patients with severe symptomatic aortic stenosis (AS) is valve replacement (1,2). Until recently, however, at least one-third of such patients did not undergo treatment either due to advanced age, left ventricular (LV) dysfunction, or numerous co-morbidities (3,4). Over the last decade, transcatheter aortic valve replacement (TAVR) has emerged as a less invasive alternative to surgical valve replacement (5-7). Randomized trials have demonstrated the superiority of TAVR compared to medical therapy for patients at prohibitive surgical risk and the non-inferiority of TAVR compared to surgical valve replacement for patients at high surgical risk (8,9). TAVR is a transformative innovation that has extended treatment to numerous patients with severe AS who previously were not referred for or were denied surgical treatment.
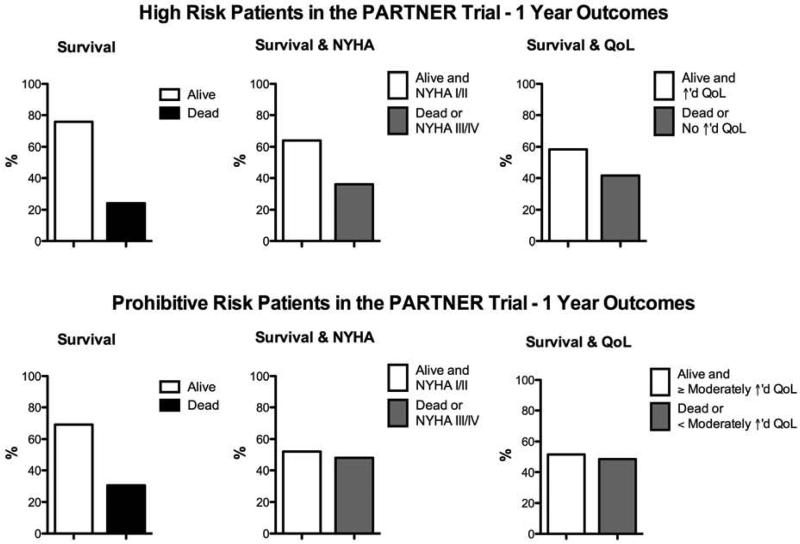
However, while there is a tremendous survival advantage and symptom benefit for many patients undergoing TAVR (compared to no valve replacement), growing clinical experience demonstrates that some patients die soon after the procedure or have little improvement in quality of life or functional status (8-11). For some, treatment of AS does little to alter the poor prognosis associated with numerous comorbidities (12). Among patients at prohibitive surgical risk treated with TAVR in the PARTNER IB Trial, 30% were dead at 1 year and approximately half were dead or had less than a moderate improvement in their quality of life or NYHA class (Figure 1) (8,10). Registries from Europe, including treatment with the Edwards SAPIEN valve and Medtronic CoreValve, have also demonstrated that approximately one-quarter of patients are dead by 1 year after TAVR (13-15).
Figure 1. Survival, heart failure symptoms, and quality of life at 1 year in patients treated with TAVR in the PARTNER trial.
Data shown is from the PARTNER Trial (8-11). Abbreviations: NYHA, New York Heart Association; QoL, quality of life.
Therapeutic futility has been defined as a lack of medical efficacy, particularly when the therapy is unlikely to produce its intended clinical result, as judged by the physician; or lack of a meaningful survival, as judged by the personal values of the patient (16). Ascertaining benefit-versus-futility in individual patients is therefore a multi-faceted exercise that must integrate information to facilitate a collective judgment (7,17). Considering the importance of doing no harm and the reality of limited resources, in each case tough questions about whether we should perform TAVR even if we can perform TAVR must be asked. The latter question is technical in nature and may be distilled into measurable facts; the former is less straightforward and includes value judgments and uncertainty, which extend beyond the individual cardiologist's or surgeon's technical or clinical expertise. We will review issues to be considered when making and communicating these difficult decisions.
Approach to the High / Prohibitive Risk TAVR Referral
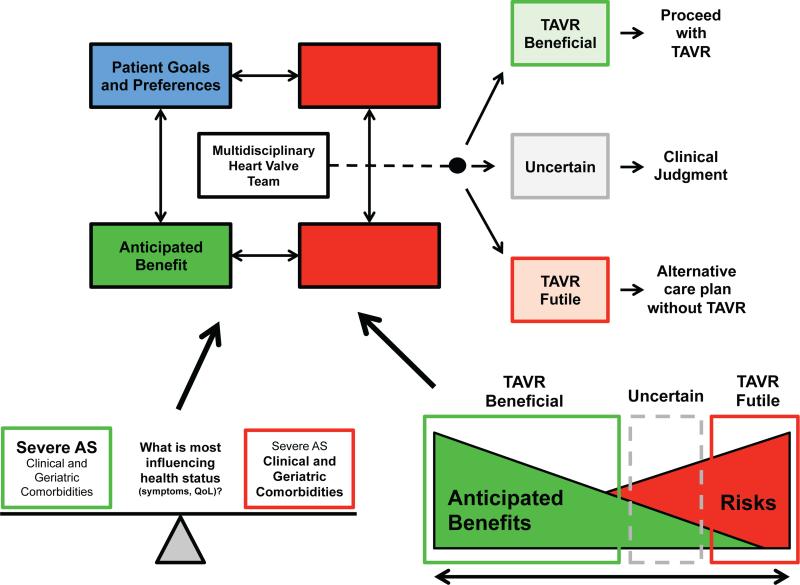
Patients with severe AS fall along a spectrum of risk for valve replacement. For those at the low-risk end of the spectrum, such as the 65 year-old asymptomatic patient with normal LV function and no significant co-morbidities, the preeminent question is when to perform valve replacement, and risk stratification is employed to determine the optimal timing of surgery (18,19). In contrast, at the high-risk end of the spectrum, the question is more often whether to perform valve replacement. These are the patients in whom advanced age, the number and severity of co-morbidities, and poor functional status make it difficult to determine whether valve replacement will be beneficial (20). There is no firm line drawn between those who will and will not benefit meaningfully from TAVR; rather, in the transitional zone, acknowledgement of likely outcomes in the context of the patient's preferences becomes central. We propose the following framework be considered: 1) clinical risk stratification; 2) geriatric risk stratification; 3) anticipated clinical benefit; and 4) assessment of patient goals and preferences (Figure 2).
Figure 2. Decision making by the multidisciplinary heart valve team on patients referred for TAVR.
The multidisciplinary heart valve team considers and weighs the various factors shown and makes a decision regarding whether TAVR will likely be beneficial or futile. Areas of uncertainty require clinical judgment. What factors are thought to most influence the patient's current health status affects assessment of the anticipated benefit of TAVR. Anticipated benefits or risks may clearly outweigh the other, but in some cases there is uncertainty when patient goals and preferences are especially important to incorporate into decision-making regarding whether to perform TAVR.
Clinical Risk Stratification
There are numerous ways to clinically risk stratify patients with severe AS being considered for aortic valve replacement (2). The following are some of the factors that are associated with a marked increase in risk (Table 1). We do not include anatomic factors that can make a patient prohibitively high risk for conventional surgery (eg. porcelain aorta or hostile chest due to prior radiation) as these factors generally do not suggest potential futility of TAVR.
Table 1.
Clinical Predictors of Increased Risk
| • Severely reduced left ventricular function |
| • Very low transvalvular gradient (mean gradient <20 mmHg) |
| • Low flow (low stroke volume index, <35 ml/m2) |
| • Severe myocardial fibrosis |
| • Severe concomitant mitral and/or tricuspid valve disease |
| • Severe pulmonary hypertension (PASP ≥60 mmHg) |
| • Severe lung disease, particularly oxygen dependent |
| • Advanced renal impairment (stages 4 and 5) |
| • Liver disease |
| • Very high STS score (predicted risk of mortality >15%) |
Abbreviations: PASP, pulmonary artery systolic pressure; STS, Society of Thoracic Surgery.
Very high STS score
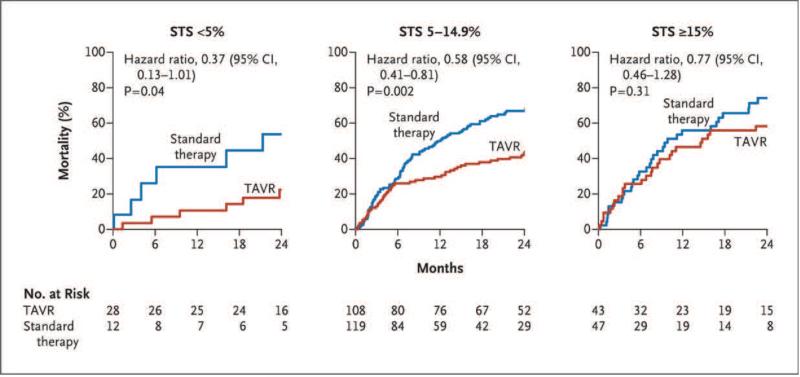
The Society of Thoracic Surgery (STS) risk score is widely used as a starting point to stratify patients in need of aortic valve replacement, both at the clinical and research levels. It integrates several clinical parameters to yield predicted probabilities of mortality and major morbidity (8,9,15). The STS score has well characterized limitations in predicting surgical risk in elderly patients with AS undergoing valve replacement (21), particularly in terms of calibration (i.e. predicted risk that significantly exceeds observed mortality rates). Relevant to the issue of futility, a sub-group analysis of the PARTNER I trial (inoperable cohort B) showed that in patients with an STS predicted risk of mortality >15%, there was no survival benefit from TAVR compared to medical therapy (22). The logistic EuroSCORE is also commonly used to risk stratify patients evaluated for TAVR and an increased score is independently associated with worse survival after TAVR (15).
Impaired LV systolic function, low valve gradients, and reduced stroke volume
Patients with low-flow, low-gradient, low ejection fraction severe AS with no contractile reserve on dobutamine stress echocardiography have an operative mortality of 22-36% (23,24). Although there appears to be a survival benefit with valve replacement compared to medical therapy even in those without contractile reserve, patients with a resting mean gradient <20 mmHg have a markedly increased risk of 1-year mortality (23,24). A low stroke volume index (<35 ml/m2) recently was shown to be an independent predictor of mortality, even after adjustment for ejection fraction, in high risk or prohibitive risk patients undergoing transcatheter or surgical valve replacement (25,26). Whether TAVR can be done at lower risk than surgery in these patients is a promising hypothesis that requires study.
Severe myocardial fibrosis
The presence and extent of interstitial myocardial fibrosis (due to chronic pressure overload rather than infarction from coronary disease) is an independent predictor of mortality in patients with AS (27,28). Weidmann et al. showed that in patients with AS undergoing valve replacement, those with severe cardiac fibrosis determined by cardiac magnetic resonance imaging pre-operatively did not experience symptomatic improvement from valve replacement and had less improvement in LV function (27).
Severe concomitant valve disease
Moderate to severe mitral regurgitation has been associated with increased mortality after TAVR at 30 days (29,30) and 1 year (30), despite the fact that approximately half of the patients with significant pre-procedural mitral regurgitation have improvement in regurgitation severity post-TAVR (29,30). However, a more recent report provided somewhat conflicting data showing that moderate or severe mitral regurgitation was not associated with increased 2-year mortality in patients undergoing TAVR in the PARTNER trial (31). With respect to tricuspid regurgitation, there are emerging data showing that moderate or severe tricuspid regurgitation is an independent predictor of increased mortality after TAVR or surgical AVR (32,33). The impact of untreated concomitant severe mitral or tricuspid valve disease on symptomatic improvement and survival after TAVR requires further study.
Severe pulmonary hypertension
The presence and severity of pulmonary hypertension in patients with AS may reflect a more advanced state of disease. Pulmonary hypertension is an independent predictor for mortality after surgical aortic valve replacement (34) and severe pulmonary hypertension (pulmonary artery systolic pressure >60 mmHg) is associated with a 2-fold higher adjusted risk of short- and long-term mortality in very high-risk patients undergoing TAVR (35).
Severe lung, renal, and/or liver disease
Advanced disease of other organs is independently associated with increased mortality in patients undergoing TAVR, including severe obstructive lung disease – particularly when oxygen dependent (22,35,36), advanced chronic kidney disease (37,38), and cirrhosis (38).
Geriatric Risk Stratification
Beyond the traditional co-morbidities, a number of age-associated conditions are prevalent in TAVR patients and are often overlooked. These include: frailty, disability, mobility impairment, cognitive impairment, mood disturbance, malnutrition, polypharmacy, fall risk, and social isolation (Tables 2 and 3). A multidimensional prognostic index based on this type of comprehensive geriatric assessment was shown to outperform traditional risk scores in a cohort of elderly heart failure patients (39) and similar indices are beginning to be tested in elderly TAVR patients (40-42).
Table 2.
Geriatric Predictors of Increased Risk
| • Advanced frailty |
| • Disability in activities of daily living |
| • Malnutrition |
| • Mobility impairment |
| • Low muscle mass and strength (“sarcopenia”) |
| • Cognitive impairment |
| • Mood disorders (depression, anxiety) |
Table 3.
Selected Geriatric Assessment Tools
| • Frality |
| 5-meter gait speed |
| Fried's frailty scale |
| Short physical performance battery (SPPB) |
| • Disability |
| Activities of daily living (ADL) |
| Instrumental activities of daily living (IADL) |
| • Cognitive impairment |
| Mini-Mental Status Examination (MMSE) |
| Montreal Cognitive Assessment (MoCA) |
| • Mood disturbance |
| Geriatric Depression Scale (GDS) |
| Hospital Anxiety and Depression Scale (HADS) |
| • Malnutrition |
| Albumin |
| Mini-nutritional assessment |
| • Polypharmacy |
| • Fall risk |
| • Social isolation |
Stortecky and Schoenenberger found that 30-40% of patients referred for TAVR had impairments in cognitive function as assessed by the mini-mental status examination, mobility limitation as assessed by the timed-up-and-go test, disability as assessed by activities of daily living (ADL's), or malnutrition as assessed by the mini-nutritional assessment (40,41). Each of these impairments predicted functional decline at 6 months and major adverse events at 1 year after adjusting for predicted risk scores. Green found that disability as assessed by ADL's and malnutrition as assessed by serum albumin were especially predictive of mortality at 1 year (42). Slow walking speed using the common cutoff of 0.8 meters/sec was almost universal in this severely compromised population and did not seem to be predictive of increased risk; a more stringent cutoff of 0.5 meters/sec fared slightly better.
Frailty is a geriatric syndrome defined as impaired resilience to stressors due to subclinical impairments in multiple organ systems particularly the cardiovascular and musculoskeletal systems (43,44). It is manifest at the clinical level as a constellation of slow walking speed, weak handgrip strength, low physical activity, exhaustion, and weight loss, and has been operationalized by Fried into a 5-item scale with 3 or more positive items required to make the diagnosis (45). In particular, walking speed may be considered a “geriatric vital sign” that predicts death and major adverse cardiac events in several populations (46-48). Frailty is often a precursor to adverse events observed after a physiological stressor (e.g. surgery).
Disability is defined as inability or dependency to carry out activities essential for independent living such as dressing or eating (termed activities of daily living; ADL), taking medications or managing finances (termed instrumental activities of daily living; IADL). Frail patients are at risk to develop disability in later stages, although the two entities are not interchangeable (49). Patients who have substantial disability are less likely to be referred and less likely to derive functional and quality of life benefit after valve replacement, regardless of whether it is technically successful. The extent of disability burden associated with minimal-or-no likelihood of benefit has not yet been quantified.
The impact of frailty is less clear in the setting of TAVR; frail patients are 3-5 fold less likely to survive up to 1 year of follow-up, yet they do not necessarily experience a higher rate of 30-day peri-procedural mortality or morbidity after TAVR (40-42). This finding is in contrast to what has been reported in the setting of cardiac surgery where frailty strongly predicts peri-procedural complications (46,50,51), and may be due to the less physiologically stressful nature of TAVR compared to surgery. Moreover, the feasibility of physical-performance frailty measures in TAVR patients is debated, with some authors reporting that as many as 30% of patients cannot complete the walking speed test, and others reporting a figure <10% (40-42). Further studies will clarify the impact of frailty on short- vs. long-term outcomes after TAVR.
At the present time, it is recommended to measure 5-meter walking speed (alone or as part of the 5-item Fried frailty scale) before TAVR as a simple and prognostic metric of frailty – albeit with the proviso that the cutoff to define elevated risk is not yet known in this population. It is also recommended to consider the burden of disability and cognitive impairment with an ADL/IADL scale and a Mini-Mental Status Exam or Montreal Cognitive Assessment (MoCA), respectively. Given the preliminary evidence surrounding malnutrition, it would seem wise to involve a dietician when this is suspected (40). In summary, the current body of evidence suggests that the presence of significant disability, cognitive impairment, and/or malnutrition tilts the decisional balance towards futility.
Anticipated Benefit of TAVR
We are used to thinking about the benefit of a cardiovascular procedure, in this case valve replacement, in terms of longer survival and improved symptoms (usually characterized as less shortness of breath and angina for patients with AS). To the degree that a patient's only or main medical problem can be ascribed to severe symptomatic AS, anticipating benefit from valve replacement is relatively straightforward. Also, within the classification of severe AS, there is a gradation of severity of valve obstruction such that a more critically narrowed valve and higher transvalvular gradient is suggestive of the potential for more pronounced clinical benefit from LV unloading after valve replacement.
In some difficult cases, such as when there is severe concomitant lung disease (particularly with a low B-type natriuretic peptide level) or left ventricular dysfunction with low transvalvular gradients, some clinicians have found it useful to perform a balloon aortic valvuloplasty to assess for any clinical improvement as demonstrated by an increase in six minute walk distance and improved symptoms (52). This may suggest that whatever else may be wrong with the patient, the AS is at least contributing in a substantive way to the patient's functional and symptomatic impairment and that TAVR may be of benefit. Even with the aid of assessing one's response to a “diagnostic valvuloplasty”, anticipating benefit—promotion and enhancement of well-being—from TAVR is often complex and difficult to predict given the very elderly population with numerous co-morbidities and poor functional status (Figures 2).
With respect to mortality, in the inoperable cohort of the PARTNER Trial, patients with an STS score >15% had no survival benefit from TAVR compared to medical management (22) (Figure 3). Arnold et al. have proposed a score to predict a poor clinical outcome at 6 months after TAVR defined as death, KCCQ <45 (comparable to NYHA class IV), or a decrease of ≥10 points in the KCCQ from baseline (53). By this definition, 35% of patients treated with TAVR in the PARTNER trial (including both cohorts) had a poor outcome (53). Integration of the KCCQ into the assessment and follow-up of patients with TAVR (it is a required component of the TVT Registry) is an important step toward developing a broader assessment of the effect of TAVR beyond survival and change in NYHA class (54).
Figure 3. Outcome of patients at prohibitive surgical risk in the PARTNER Trial.
From Makkar et al.(22) (PERMISSION NEEDED).
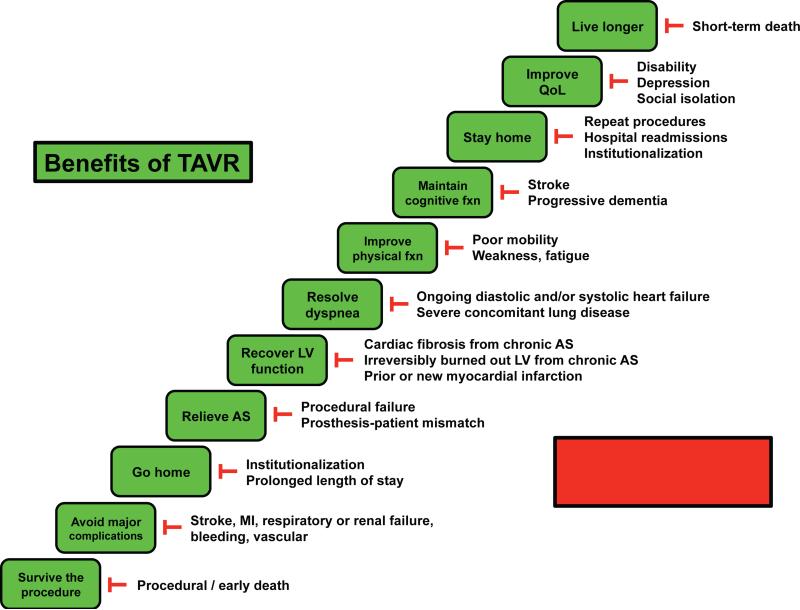
In addition, there may be other metrics that will help provide a more holistic, informative picture of the impact of TAVR on patient well-being. Going forward, it will be important to develop models and scores for predicting poor/good outcomes that are broadly defined, patient-centered, and particularly relevant to older adults (Figure 4).
Figure 4. Obstacles to benefits of TAVR.
The step-wise, progressive benefits and milestones anticipated to result from TAVR may not be realized due to several potential obstacles.
Patient Goals and Preferences
The assessment of futility is inherently value driven and must include consideration of patient's values, goals and preferences. Shared decision-making requires both patient and provider share information, work toward a consensus, and reach agreement on the course of action (55). This requires communication and a provider-patient relationship in which patients are empowered to question therapeutic options and areas of uncertainty. This exchange of goals and preferences assumes voluntary participation as well as intact cognitive functioning and no significant depression (56,57). Health care goals are as individual as the life circumstances, priorities, social setting and beliefs of the patients who set them. Some studies suggest that a majority of elderly patients with heart failure are not willing to trade survival time for improved quality of life, although this preference is difficult to predict and tends to shift with increasing age, decreasing activity level, and worsening symptoms (58). In general, life phase impacts the views of the elderly in terms of the relative priority of quantity or quality of life. As shown in the SUPPORT study, older patients prefer less aggressive care than younger patients, but may receive even less aggressive care than they might have chosen (59).
Therefore, age may impact healthcare priorities, but incorporating that into assumptions is apt to be misguided. Studies have shown that patient wishes often differ from those their families and healthcare providers estimated (60). Being in a more reflective life phase, ability to communicate with loved ones, maintaining independence and preserving quality of life often become as important as lengthening life. This is particularly true in situations where risks, such as stroke, disability, and cognitive impairment, increase. In the TAVR population, when benefit in symptom relief aligns with a patient's goals, care is not futile; however, when life prolongation and symptom relief is not anticipated, care is futile. In situations where disagreement about the treatment course remains, a fair process including ethics consultation when necessary has been proposed (61).
Multidisciplinary Heart Valve Team
Given the diversity and breadth of issues to consider when evaluating candidates for TAVR—aside from the technical expertise to perform the procedure successfully—it is critical to have a multidisciplinary heart valve team to provide additional insight and expertise to make the best decision regarding who is most likely to benefit from the procedure. This point cannot be emphasized enough: having the clinical volume and technical expertise should be a necessary but not sufficient requirement for a TAVR program (7,62). At the core, the heart valve team should include cardiac surgeons, interventional cardiologists, and non-invasive cardiologists, each with particular expertise in complicated structural/valvular heart disease. Consultation with imaging specialists, nurses, and geriatricians may be appropriate (7,63). Dieticians and physiotherapists offer complementary expertise, and ethicists may be needed if there is disagreement over the treatment plan. The size and composition of teams will differ, but these areas should be represented or available ad hoc. Every effort should be made to coordinate the evaluation of patients by multiple parties to limit the burden on the patient and providers alike. Regular valve team meetings are essential to discuss and review the patients and diagnostic data from multiple viewpoints representing the diverse areas of expertise delineated above. These teams should function much like those that exist in many institutions for tumor boards or transplantation networks. Difficult decisions integrating diverse types of information are made in a collaborative team. It is important for these teams to get comfortable with the decision to not pursue TAVR in patients for whom the anticipated benefits do not outweigh the risks. This will be an evolving area in which heart valve teams will need to grow and mature as we learn how to better identify patients who will benefit from therapy and those who will not.
Caring for Patients for Whom TAVR Has Been Deemed Futile
The decision not to offer TAVR should not be equated with abandoning care, yet when no other definitive treatment options remain it is important for the patient, family, and physician to be realistic about the poor prognosis and limited life expectancy of the patient. Just as for patients with advanced heart failure who are not candidates for a ventricular assist device or transplant, the focus should be on providing the patient and family with the appropriate palliative care resources (64,65). Restricting symptoms are common in the last year of life, particularly in elderly patients with chronic conditions. Chaudhry et al. found that while 27% of patients had physical or psychological symptoms leading to restrictions in daily activities 5 months prior to death, this rose to >55% in the month prior to death (66). Therefore, addressing symptoms should always be a part of the treatment plan, regardless of the futility of TAVR, and should be expected in this population. As important is ensuring continued access to clinical care, so arranging a future clinical visit (likely back with their primary care physician) can ease the transition between TAVR evaluation and the decision to not pursue that treatment.
Conclusion
The transformative innovation of TAVR has provided a tremendous opportunity to treat patients with severe symptomatic AS at increased surgical risk, but no therapy is universally beneficial. Clinical trials and our growing experience have demonstrated that certain patients who undergo TAVR do not benefit, either due to death or a lack of improvement in symptoms, functionality, or quality of life. Through clinical and geriatric risk stratification, estimation of clinical benefit, and a discussion of the patient's goals and preferences, the multidisciplinary heart valve team must discern who will benefit from the procedure, who will not, and those in the gray zone where the most patient engagement is needed. When the heart team determines that TAVR would be futile, it is imperative to emphasize the plans for providing continuing care for patients and their families. TAVR is an immensely promising therapeutic tool, but as we work on technological innovations to improve the devices we must also employ it responsibly within a framework of care which enables shared decision making and promotes patient goals and well-being.
Condensed abstract.
Transcatheter aortic valve replacement (TAVR) is a transformative innovation that benefits many patients with aortic stenosis who were previously untreated. However, many patients die soon after the procedure or lack improvement in quality of life. This emphasizes the need to identify and acknowledge the possibility of futility in some patients considered for TAVR. The multidisciplinary heart valve team needs to weigh a number of factors such as multimorbidity, frailty, and disability in addition to traditional risk factors in order to assess the anticipated benefit of TAVR. We review issues to be considered when making and communicating these difficult decisions.
Acknowledgments
The authors thank Michael W. Rich, MD, and Douglas L. Mann, MD, for their feedback on the manuscript.
Funding: Dr. Lindman is supported by K23 HL116660 and the Washington University Institute of Clinical and Translational Sciences grant (UL1 TR000448, KL2 TR000450) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Abbreviations and Acronyms
- ADL
activities of daily living
- AS
aortic stenosis
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- LV
left ventricular
- NYHA
New York Heart Association
- STS
Society of Thoracic Surgery
- TAVR
transcatheter aortic valve replacement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Lindman is a site co-investigator for the PARTNER Trial and has consulted for Gerson Lehrman Group Research.
References
- 1.Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–10. doi: 10.1161/01.cir.66.5.1105. [DOI] [PubMed] [Google Scholar]
- 2.Lindman BR, Bonow RO, Otto CM. Current management of calcific aortic stenosis. Circ Res. 2013;113:223–37. doi: 10.1161/CIRCRESAHA.111.300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–20. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 4.Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD, Jr., Gualano SK. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes. 2009;2:533–9. doi: 10.1161/CIRCOUTCOMES.109.848259. [DOI] [PubMed] [Google Scholar]
- 5.Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–8. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 6.Joint Task Force on the Management of Valvular Heart Disease of the European Society of C, European Association for Cardio-Thoracic S. Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–96. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 7.Holmes DR, Jr., Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200–54. doi: 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 9.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–72. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A). J Am Coll Cardiol. 2012;60:548–58. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 12.Salpeter SR, Luo EJ, Malter DS, Stuart B. Systematic review of noncancer presentations with a median survival of 6 months or less. Am J Med. 2012;125:512, e1–6. doi: 10.1016/j.amjmed.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–8. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Thomas M, Schymik G, Walther T, et al. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2011;124:425–33. doi: 10.1161/CIRCULATIONAHA.110.001545. [DOI] [PubMed] [Google Scholar]
- 15.Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–15. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic S. Withholding and withdrawing life-sustaining therapy. Ann Intern Med. 1991;115:478–85. doi: 10.7326/0003-4819-115-6-478. [DOI] [PubMed] [Google Scholar]
- 17.McDermid RC, Bagshaw SM. Prolonging life and delaying death: the role of physicians in the context of limited intensive care resources. Philosophy, ethics, and humanities in medicine : PEHM. 2009;4:3. doi: 10.1186/1747-5341-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carabello BA. Aortic valve replacement should be operated on before symptom onset. Circulation. 2012;126:112–7. doi: 10.1161/CIRCULATIONAHA.111.079350. [DOI] [PubMed] [Google Scholar]
- 19.Shah PK. Severe aortic stenosis should not be operated on before symptom onset. Circulation. 2012;126:118–25. doi: 10.1161/CIRCULATIONAHA.111.079368. [DOI] [PubMed] [Google Scholar]
- 20.American Geriatrics Society Expert Panel on the Care of Older Adults with M Patient-centered care for older adults with multiple chronic conditions: a stepwise approach from the American Geriatrics Society: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012;60:1957–68. doi: 10.1111/j.1532-5415.2012.04187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durand E, Borz B, Godin M, et al. Performance analysis of EuroSCORE II compared to the original logistic EuroSCORE and STS scores for predicting 30-day mortality after transcatheter aortic valve replacement. Am J Cardiol. 2013;111:891–7. doi: 10.1016/j.amjcard.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 22.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 23.Tribouilloy C, Levy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol. 2009;53:1865–73. doi: 10.1016/j.jacc.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Levy F, Laurent M, Monin JL, et al. Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: a European multicenter study. J Am Coll Cardiol. 2008;51:1466–72. doi: 10.1016/j.jacc.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann HC, Pibarot P, Hueter I, et al. Predictors of Mortality and Outcomes of Therapy in Low-Flow Severe Aortic Stenosis: A Placement of Aortic Transcatheter Valves (PARTNER) Trial Analysis. Circulation. 2013;127:2316–26. doi: 10.1161/CIRCULATIONAHA.112.001290. [DOI] [PubMed] [Google Scholar]
- 26.Le Ven F, Freeman M, Webb J, et al. Impact of low flow on the outcome of high-risk patients undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2013;62:782–8. doi: 10.1016/j.jacc.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 27.Weidemann F, Herrmann S, Stork S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–84. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 28.Dweck MR, Joshi S, Murigu T, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–9. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 29.Toggweiler S, Boone RH, Rodes-Cabau J, et al. Transcatheter aortic valve replacement: outcomes of patients with moderate or severe mitral regurgitation. J Am Coll Cardiol. 2012;59:2068–74. doi: 10.1016/j.jacc.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Bedogni F, Latib A, De Marco F, et al. Interplay Between Mitral Regurgitation and Transcatheter Aortic Valve Replacement With the CoreValve Revalving System: A Multicenter Registry. Circulation. 2013;128:2145–53. doi: 10.1161/CIRCULATIONAHA.113.001822. [DOI] [PubMed] [Google Scholar]
- 31.Barbanti M, Webb JG, Hahn RT, et al. Impact of Preoperative Moderate/Severe Mitral Regurgitation on 2-Year Outcome After Transcatheter and Surgical Aortic Valve Replacement: Insight From the Placement of Aortic Transcatheter Valve (PARTNER) Trial Cohort A. Circulation. 2013;128:2776–84. doi: 10.1161/CIRCULATIONAHA.113.003885. [DOI] [PubMed] [Google Scholar]
- 32.Tan TC, Flynn AW, Rincon LM, et al. Functional Tricuspid Regurgitation is an Independent Predictor of Mortality and Morbidity in Patients with Aortic Stenosis Undergoing Valve Replacement. Circulation. 2012;126:A19057. [Google Scholar]
- 33.Barbanti M, Freeman M, Binder R, et al. Impact of Preoperative Moderate/Severe Tricuspid Regurgitation on Patients Undergoing Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2013;61:E1889. doi: 10.1002/ccd.25512. [DOI] [PubMed] [Google Scholar]
- 34.Melby SJ, Moon MR, Lindman BR, Bailey MS, Hill LL, Damiano RJ., Jr Impact of pulmonary hypertension on outcomes after aortic valve replacement for aortic valve stenosis. J Thorac Cardiovasc Surg. 2011;141:1424–30. doi: 10.1016/j.jtcvs.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodes-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–90. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Dvir D, Waksman R, Barbash IM, et al. Outcomes of Patients with Chronic Lung Disease and Severe Aortic Stenosis Treated with Transcatheter- versus Surgical Aortic Valve Replacement or Standard Therapy: Insights from the PARTNER Trial. J Am Coll Cardiol. 2014;63:269–79. doi: 10.1016/j.jacc.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Sinning JM, Ghanem A, Steinhauser H, et al. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:1141–9. doi: 10.1016/j.jcin.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–95. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 39.Pilotto A, Addante F, Franceschi M, et al. Multidimensional Prognostic Index based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circ Heart Fail. 2010;3:14–20. doi: 10.1161/CIRCHEARTFAILURE.109.865022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489–96. doi: 10.1016/j.jcin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684–92. doi: 10.1093/eurheartj/ehs304. [DOI] [PubMed] [Google Scholar]
- 42.Green P, Woglom AE, Genereux P, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974–81. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm--issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–7. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–21. doi: 10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 45.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 46.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–76. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 47.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The journal of nutrition, health & aging. 2009;13:881–9. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 48.Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. Bmj. 2009;339:b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 50.Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222–8. doi: 10.1161/CIRCOUTCOMES.111.963157. [DOI] [PubMed] [Google Scholar]
- 51.Sundermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33–7. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 52.Kapadia SR, Goel SS, Yuksel U, et al. Lessons learned from balloon aortic valvuloplasty experience from the pre-transcatheter aortic valve implantation era. J Interv Cardiol. 2010;23:499–508. doi: 10.1111/j.1540-8183.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- 53.Arnold SV, Spertus JA, Lei Y, et al. How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes. 2013;6:591–7. doi: 10.1161/CIRCOUTCOMES.113.000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnold SV, Spertus JA, Lei Y, et al. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–7. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 55.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Social science & medicine. 1997;44:681–92. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 56.Kim SY, Karlawish JH, Caine ED. Current state of research on decision-making competence of cognitively impaired elderly persons. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2002;10:151–65. [PubMed] [Google Scholar]
- 57.Blank K, Robison J, Doherty E, Prigerson H, Duffy J, Schwartz HI. Life-sustaining treatment and assisted death choices in depressed older patients. J Am Geriatr Soc. 2001;49:153–61. doi: 10.1046/j.1532-5415.2001.49036.x. [DOI] [PubMed] [Google Scholar]
- 58.Brunner-La Rocca HP, Rickenbacher P, Muzzarelli S, et al. End-of-life preferences of elderly patients with chronic heart failure. Eur Heart J. 2012;33:752–9. doi: 10.1093/eurheartj/ehr404. [DOI] [PubMed] [Google Scholar]
- 59.Hamel MB, Lynn J, Teno JM, et al. Age-related differences in care preferences, treatment decisions, and clinical outcomes of seriously ill hospitalized adults: lessons from SUPPORT. J Am Geriatr Soc. 2000;48:S176–82. doi: 10.1111/j.1532-5415.2000.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 60.Bogardus ST, Bradley EH, Williams CS, Maciejewski PK, van Doorn C, Inouye SK. Goals for the care of frail older adults: do caregivers and clinicians agree? Am J Med. 2001;110:97–102. doi: 10.1016/s0002-9343(00)00668-9. [DOI] [PubMed] [Google Scholar]
- 61.Medical futility in end-of-life care: report of the Council on Ethical and Judicial Affairs. JAMA. 1999;281:937–41. [PubMed] [Google Scholar]
- 62.Holmes DR, Jr., Mack MJ. Transcatheter valve therapy a professional society overview from the american college of cardiology foundation and the society of thoracic surgeons. J Am Coll Cardiol. 2011;58:445–55. doi: 10.1016/j.jacc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 63.Holmes DR, Jr., Rich JB, Zoghbi WA, Mack MJ. The heart team of cardiovascular care. J Am Coll Cardiol. 2013;61:903–7. doi: 10.1016/j.jacc.2012.08.1034. [DOI] [PubMed] [Google Scholar]
- 64.Goodlin SJ. Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–96. doi: 10.1016/j.jacc.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 65.Lemond L, Allen LA. Palliative care and hospice in advanced heart failure. Prog Cardiovasc Dis. 2011;54:168–78. doi: 10.1016/j.pcad.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaudhry SI, Murphy TE, Gahbauer E, Sussman LS, Allore HG, Gill TM. Restricting symptoms in the last year of life: a prospective cohort study. JAMA internal medicine. 2013;173:1534–40. doi: 10.1001/jamainternmed.2013.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]