Abstract
Background
Familial cold autoinflammatory syndrome (FCAS) is characterized by rash, fever, and arthralgia in response to cold exposure. CIAS1, the gene that codes for cryopyrin, is mutated in FCAS. Treatment with anakinra (IL-1 receptor antagonist) prevents symptoms, indicating a crucial role for IL-1 in this disease.
Objective
To study cytokine responses to cold exposure in monocytes from subjects with FCAS.
Methods
Adherence-enriched monocytes were incubated at 32°C or 37°C. Transcription and release of IL-1β, IL-6, and TNF-α were monitored by quantitative PCR and ELISA.
Results
The FCAS monocytes but not control cells responded to 4 h incubation at 32°C with significant secretion of IL-1β. At 16 h, IL-1β, IL-6, and TNF-α were all significantly elevated in FCAS monocytes at 32°C. Increased cytokine transcription was observed in all monocytes at 4 hours, but at 16 hours it was only seen in FCAS monocytes incubated at 32°C. Incubation at 32°C for as little as 1 hour sufficed to induce measurable IL-1β release. Caspase-1 inhibitors prevented the cold-induced IL-1β release, whereas a purinergic antagonist did not. Anakinra had no effect on the early IL-1β release but significantly reduced the late-phase transcription and release of all cytokines.
Conclusion
FCAS monocytes respond to mild hypothermia with IL-1β release, which in turn induces autocrine transcription and secretion of IL-6 and TNF-α as well as stimulation of further IL-1β production.
Clinical implications
These results confirm the central role of IL-1β in FCAS and support the use of IL-1 targeted therapy in these patients.
Keywords: Cold, autoinflammatory syndrome, cryopyrin, CIAS1, interleukin-1β, monocytes, caspase-1, anakinra, inflammation
The protein cryopyrin (also known as NALP3 and PYPAF1) is the product of CIAS1, which was first described as the gene mutated in a rare genetic disorder, now termed familial cold autoinflammatory syndrome (FCAS),1 in which affected individuals respond to cold temperatures with rash, fever, and arthralgia. Mutations in CIAS1 are also associated with the more severe disorders Muckle-Wells syndrome (MWS)1–3 and chronic infantile neurological, cutaneous and articular syndrome (CINCA).4,5 Whereas MWS and CINCA are characterized by ongoing systemic inflammation resulting in significant neurologic or renal sequelae,6 FCAS patients often lead relatively normal lives with symptoms brought on by cold exposure.7 Disease-associated mutations in CIAS1 result in the expression of a form of cryopyrin that is believed to be constitutively activated or more easily activated than its native form, as illustrated by in vitro transfection experiments.8,9 Because FCAS patients express IL-1 in involved skin during a cold-induced attack, and cryopyrin was demonstrated to be involved in release of the proinflammatory cytokine IL-1β,10 we previously conducted a clinical trial in FCAS with the IL-1 receptor antagonist (IL-1Ra) anakinra after a controlled cold challenge.11 Anakinra treatment completely prevented development of the cold-induced symptoms, such as rash and fever, and inhibited leukocytosis,11 highlighting the central role of IL-1β in FCAS.
IL-1β is produced mainly by cells of the monocytic lineage. Its synthesis and secretion is regulated by a multistep process involving transcription and translation of pro-IL-1β, followed by cleavage of pro-IL-1β to mature IL-1β by caspase-1, with each process regulated by separate stimuli.12 For example, bacterial LPS stimulates transcription and translation of pro-IL-1β, and ATP activates the purinergic ion channel receptor P2X7, leading to caspase-1–mediated pro-IL-1β cleavage and subsequent IL-1β release.13,14 The IL-1–converting enzyme caspase-1 plays a critical role in IL-1 processing. It is normally found in a protein complex termed the inflammasome which also contains cryopyrin and other adaptor proteins.10,15,16 The crucial importance of the inflammasome in IL-1β release was recently confirmed by studies showing that inflammasome-deficient mice demonstrated resistance to lethal endotoxic shock.17,18 Similarly, macrophages from cryopyrin-deficient mice were unable to activate caspase-1 and release IL-1β in response to ATP,18 bacterial RNA, and certain bacterial toxins18,19 or to gout-associated crystals.20
Patients with FCAS develop symptoms after cold exposure, but the underlying mechanism for this cold-induced inflammation has remained elusive. Local dermal cold stimulus with a diagnostic ice cube test is typically negative,7 and a more systemic cold exposure appears necessary for development of symptoms. Biopsies of FCAS skin rash reveal a neutrophilic infiltrate with elevated levels of IL-1β and other interleukins, such as IL-6 and IL-8.11 We hypothesized that monocytic cells residing in the skin were responsible for sensing the cold and initiating the inflammatory symptoms. We report here the response of cold-challenged FCAS monocytes in vitro.
METHODS
Subjects
Study participants included 10 FCAS subjects (9 women, 1 man), with ages ranging from 21 to 86 years, and 11 age-matched subjects without CIAS1 mutations and in good general health (6 women, 5 men), with ages ranging from 19 to 83. In the FCAS group, CIAS1 mutations included L353P (n = 5), Y563N (n = 2), M659K (n = 2), and E525K/V198M (n = 1). All of the FCAS patients had a classic clinical presentation and met diagnostic criteria, as described previously.7 None of the FCAS or control subjects were experiencing significant inflammatory symptoms at the time of study, and none were on regular antiinflammatory medications. Three of the FCAS patients in the present study were treated previously with 2 doses of anakinra >12 months before the study. None of the FCAS subjects had evidence of neurologic symptoms, skeletal abnormalities, hearing loss, or amyloidosis.
Participants were evaluated in the University of California–San Diego General Clinical Research Center, and blood samples (20–60 mL) were obtained by venipuncture. The study received the approval of the University of California Human Research Protection Program committee, and informed consent was obtained from the subjects before the study.
Monocyte isolation and culture
Peripheral blood leukocytes were separated by Percoll-gradient centrifugation (Amersham Biosciences, Piscataway, NJ), and PBMCs were collected from the upper layer. After washing, cells were plated at 1 million/well in 12-well plates in serum-free RPMI at 37°C with 5% CO2. Nonadherent cells were removed by mild washing in RPMI 3–4 hours later, and the remaining monocytes were cultured in 1 mL RPMI/10% FBS with 5% CO2 at 37° or 32°C for 4 or 16 hours. In one experiment, FCAS monocytes from 2 subjects were incubated at 32°C for various time periods (5 min to 4 h) and were then returned to 37°C for a total incubation length of 16 hours. Caspase inhibitors (10 µmol/L z-VAD or z-YVAD; Alexis Biochemicals/Axxora, San Diego, Calif), the purinergic antagonist PPADS(pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid tetrasodium salt, 100 µmol/L; Alexis Biochemicals) or recombinant IL-1Ra (Anakinra, 10 µg/mL; C. Dinarello and Amgen Pharmaceuticals, Thousand Oaks, Calif) were added to the cultures for 1 hour before incubation. At the end of the incubation time, plates were briefly centrifuged, supernatants were removed, and adhering cells were dissolved in Trizol reagent (Invitrogen, Carlsbad, Calif) and immediately stored at −80°C along with supernatants. Mononuclear cell purity by cytospins was consistently >90%. There was no significant difference between patients and controls in monocyte percentages by manual white blood cell differential counts. There was also no significant difference in the number of adherent cells between FCAS subjects and normal controls at either temperature over the course of 16 hours, as determined by content of message for the housekeeping gene GAPDH.
Cytokine assays
Diluted monocyte supernatants were assayed for IL-1β, IL-6, and TNF-α by colorimetric ELISA (R&D Systems, Minneapolis, Minn). Sample concentrations were calculated from regression line fitting on log-log standard results. RNA was isolated from adherent cells and cDNA synthesized as previously described.21 Transcripts for IL-1β, IL-6, TNF-α, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were quantified by TaqMan qPCR using predeveloped reagents (Applied Biosystems, Foster City, Calif). Resulting threshold cycle data were normalized to standard curves constructed from ConA-stimulated PBMC cDNA,21 yielding cell equivalents. The ratios between the specific cytokine and GAPDH cell equivalents (relative expression units) are reported.
Statistical analysis
Data are reported as means ± SEMs. In experiments where 3 or more subjects were available, paired 2-tailed t tests were used to compare results. A P value ≤.05 was considered to be statistically significant.
RESULTS
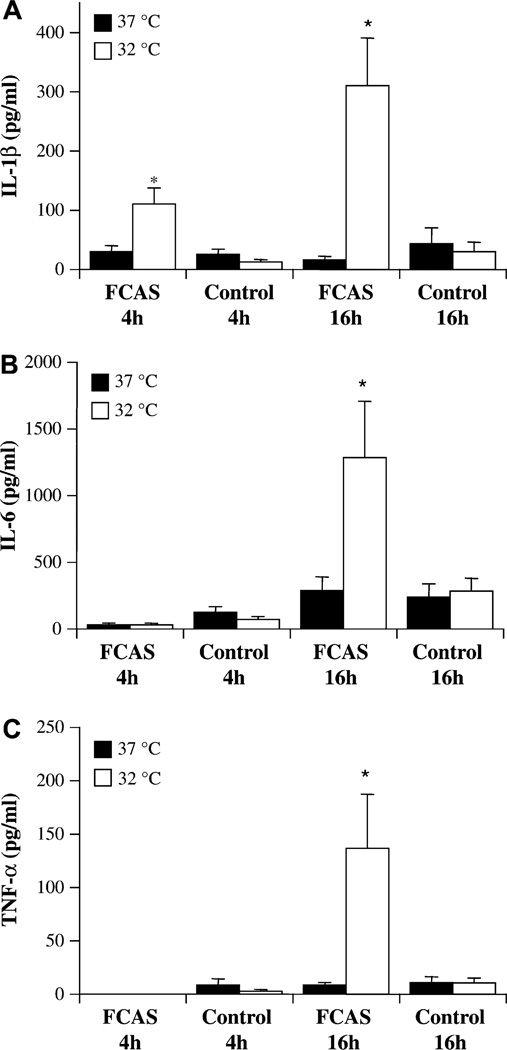
We studied adhesion-enriched monocytes in culture to determine whether they could be induced directly by cold temperature to synthesize and release cytokines. As seen in Fig 1, FCAS monocytes incubated at normal body temperature of 37°C for as much as 16 hours remained relatively quiescent and did not release more IL-1β, IL-6, or TNF-α than monocytes from control subjects. However, an incubation temperature of 32°C, simulating skin temperature of subjects exposed to cold, led to significantly enhanced cytokine release by FCAS monocytes. Secretion of IL-1β was increased at 4 hours and 16 hours (Fig 1, A), and release of IL-6 and TNF-α were increased at 16 hours only (Fig 1, B and C). Therefore, cold-induced IL-1β release appeared to precede release of IL-6 and TNF-α. Although there was some individual variation between FCAS subjects, there was no correlation with specific genotype; there was no significant difference in cytokine release in monocytes from subjects with L353P mutations (n = 5) versus other mutations collectively (n = 5) (data not shown). There was no relationship between age of the FCAS subject and the response to cold of his or her monocytes (correlation coefficient = −0.31; P = .46). When only female subjects were included in the analysis, the effect was still evident; IL-1β release after 16 hours at 32°C was 245 ± 52 pg/mL in FCAS and 13 ± 6 pg/mL in unaffected subjects. Control monocytes were not significantly activated by cold temperature (Fig 1), although they were capable of responding to 0.1 ng/mL LPS as a positive control by releasing 482 ± 136 pg/mL IL-1β over the course of 16 hours.
FIG 1.
Cytokine secretion from FCAS and control monocytes at 37°C and 32°C. Cells were incubated for 4 h or 16 h and cytokine levels in culture supernatants determined by ELISA. A, IL-1β. B, IL-6. C, TNF-α. Means and SEMs; n = 6. *P < .05 by paired t test, 37°C versus 32°C.
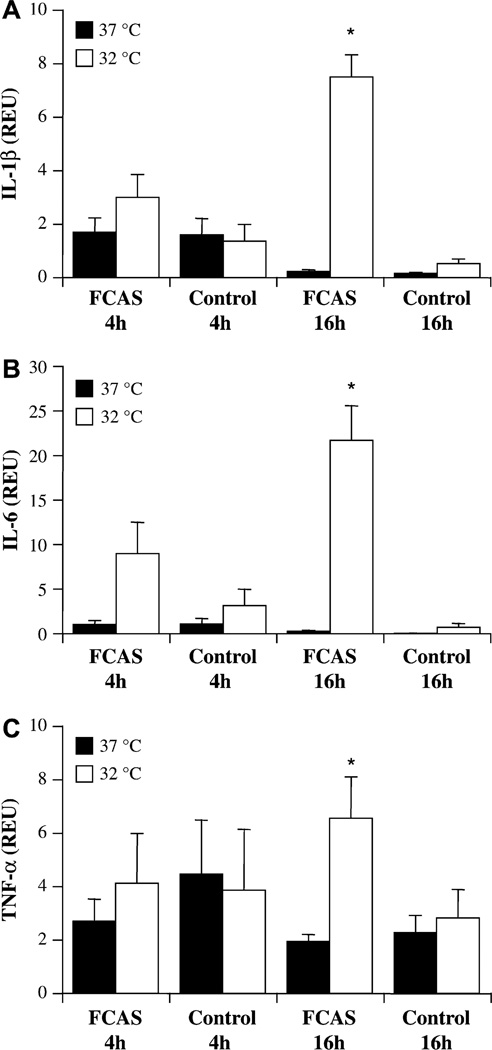
To determine whether the increase in cytokine release was accompanied by elevated transcription, cytokine mRNA was quantified by qPCR (Fig 2). At the earlier time point of 4 hours, both FCAS and control monocytes expressed readily detectable levels of IL-1β and TNF-α message at both temperatures, and there was no significant difference between 37°C and 32°C (Fig. 2, A and C). IL-6 mRNA appeared elevated in FCAS monocytes incubated at 32°C for 4 hours, but the effect did not reach statistical significance (P=.075; Fig 2, B). After 16 hours incubation at 37°C, IL1-β and IL-6 expression in both FCAS and control cells returned to low levels. In contrast, FCAS monocytes cultured at 32°C, but not control monocytes, showed dramatically increased transcription of all 3 cytokines at 16 hours (Fig 2). The enhanced early (4 hours) IL-1β protein release from FCAS monocytes at 32°C was accompanied by slightly elevated, but not statistically significant, IL-1β mRNA transcription, suggesting that most of the early IL-1β release was not due to increased message. However, the later (16 hours) release of all 3 cytokines was associated with significantly increased RNA expression.
FIG 2.
Cytokine mRNA expression by FCAS and control monocytes at 37°C and 32°C. Cells were incubated for 4 h or 16 h, cDNA prepared, and cytokine message levels determined by qPCR. A, IL-1β. B, IL-6. C, TNF-α. Means and SEMs; n = 6–7. *P < .05 by paired t test, 37°C versus 32°C.
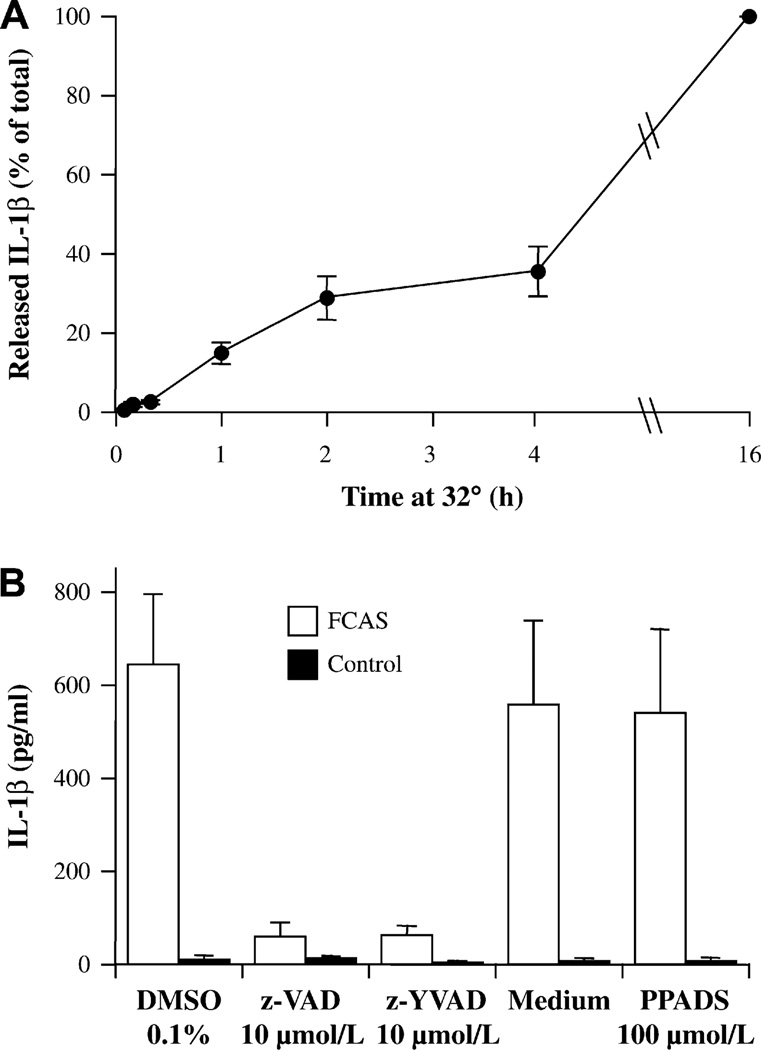
Next, we investigated the length of cold exposure that was required to elicit a response. Cold exposure as short as 1 h led to detectable release of IL-1β which comprised 15%–20% of the amount released when cells were kept at 32°C for the entire 16 hours (Fig 3, A). These data indicate that FCAS monocytes responded to cold temperatures by significant (265%) and rapid secretion of IL-1β, which was accompanied by modest (75%) and not statistically significant elevated transcription. Therefore, increased IL-1β release, even after short cold challenge, is predominately due to post-transcriptional regulation of IL-1β.
FIG 3.
Characterization of IL-1β secretion by FCAS monocytes at 32°C. A, Time requirement for cold effect. Cells were cultured at 32°C for the indicated time and then at 37°C for the remainder of 16 h. B, Effect of inhibitors of pan-caspase (z-VAD) and caspase-1 (z-YVAD) and a P2X7 antagonist (PPADS) on monocytes incubated 16 h at 32°C. Means and SEMs; n = 2.
IL-1β is formed by cleavage of pro-IL-1 by the inflammasome-associated caspase-1. To investigate the involvement of caspase-1 in the cold-induced IL-1β release, we pretreated monocytes for 1 hour with the nonspecific caspase inhibitor z-VAD or the caspase-1–specific inhibitor z-YVAD before 16 hours of culture at 32°C. Results (Fig 3, B) indicated that the cold-induced IL-1β release by FCAS monocytes was substantially inhibited by either caspase inhibitor compared with the vehicle 0.1% DMSO, illuminating the crucial role for the caspase-1 and the inflammasome in the cold response. On the other hand, the purinergic antagonist PPADS did not affect the response to cold (Fig 3, B), suggesting that the ATP-mediated step of caspase-1 activation did not play a role in the cold-induced inflammasome activation.
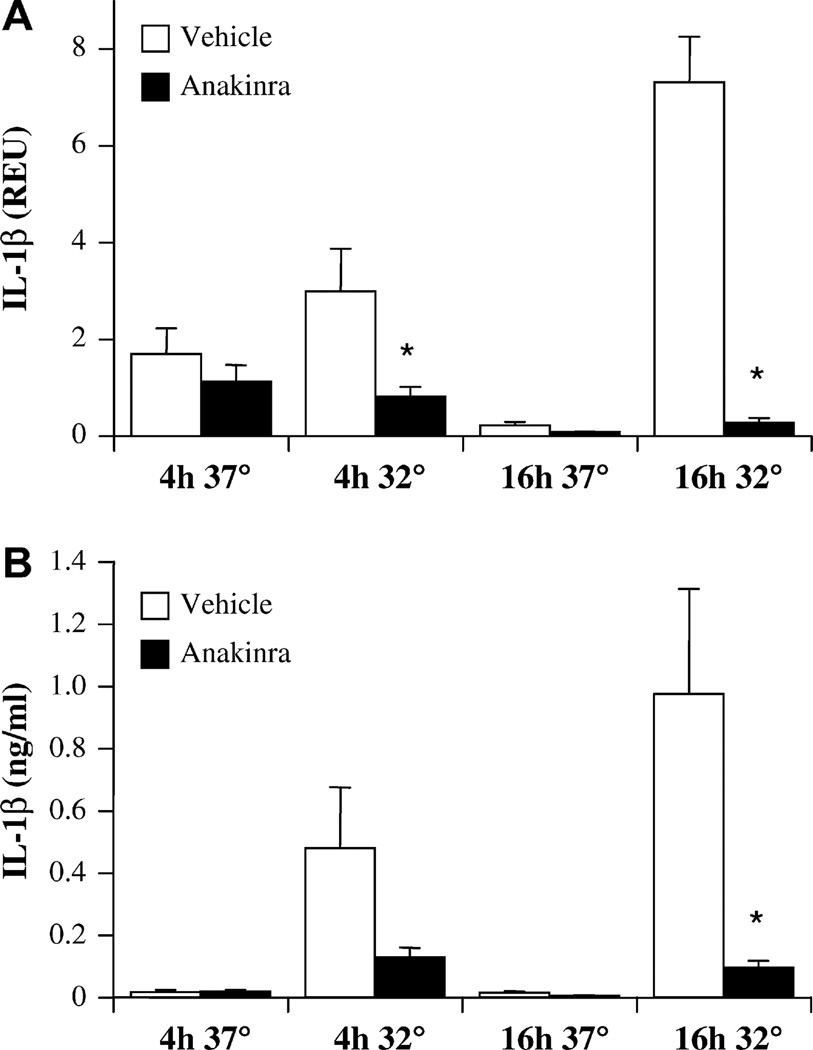
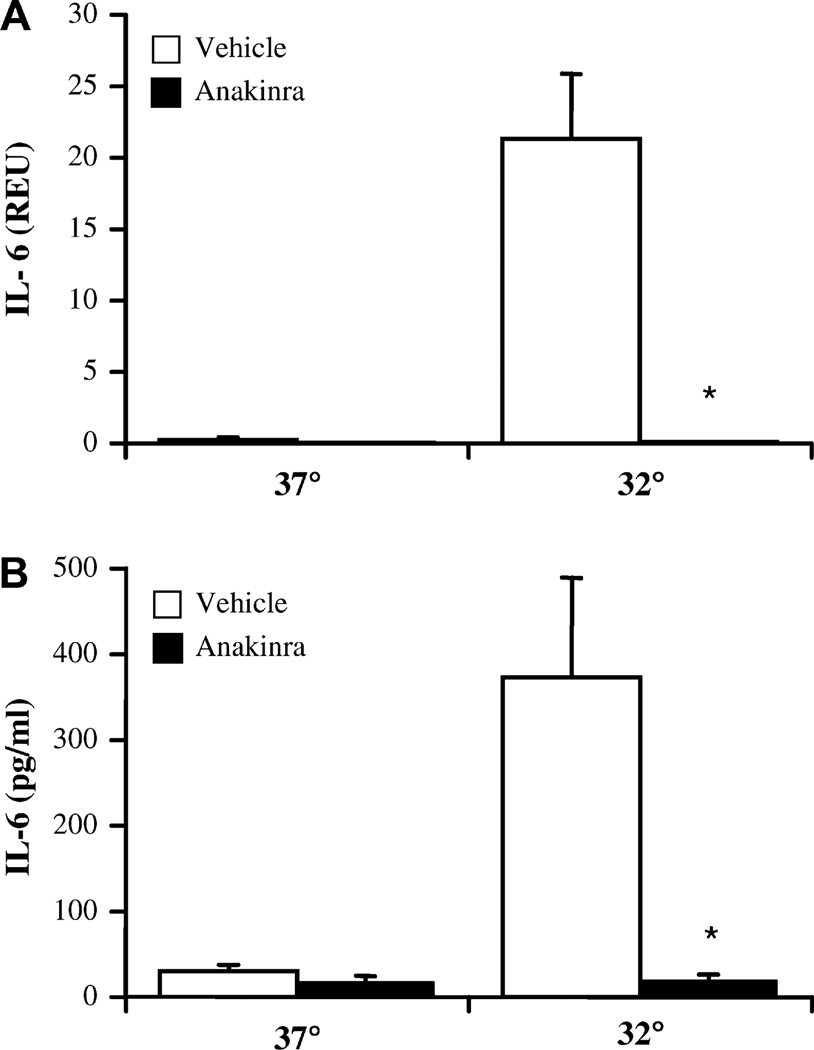
If early IL-1β release through aberrant inflammasome/caspase-1 activation was responsible for further activation of FCAS monocytes cultured for 16 hours at 32°C, an antagonist of IL-1 should have an inhibitory effect. This hypothesis was tested by the addition of anakinra, a recombinant IL-1Ra, to cultures of FCAS monocytes before cold exposure. At 4 h, anakinra significantly reduced IL-1β transcription of IL-1β at 32°C (Fig 4, A). Anakinra also appeared to decrease the release of IL-1β protein (Fig 4, B), but this was not statistically significant. In contrast, after 16 h the cold-induced response was highly dependent on autologous IL-1β receptor stimulation; both mRNA and protein levels were reduced by 90% or more by anakinra (Fig 4). IL-1 blockade also completely normalized the elevated transcription (Fig 5, A) and release (Fig 5 B) of IL-6 observed in FCAS monocytes cultured at 32°C for 16 hours, rendering IL-6 levels similar to those observed in cultures at 37°C. Therefore, the cold-induced response in FCAS monocytes was primarily due to inflammasome activation and release of IL-1β, which in turn led to autocrine activation and subsequent release of IL-1β and other monokines, such as IL-6.
FIG 4.
Anakinra inhibits cold-induced IL-1β release from FCAS monocytes only at the later time point. Cells were incubated for 4 h or 16 h at 37°C or 32°C with or without anakinra (10 µg/mL). A, IL-1β message by qPCR. B, [IL-1β] in supernatants by ELISA. Means and SEMs; n = 6. *P < .05 by paired t test, vehicle versus anakinra.
FIG 5.
Anakinra inhibits cold-induced IL-6 release from FCAS monocytes. Cells were incubated for 16 h at 37°C or 32°C with or without anakinra (10 µg/mL). A, IL-6 message by qPCR. B, [IL-6] in supernatants by ELISA. Means and SEMs; n = 6 (A) or 3 (B). *P < .05 by paired t test, vehicle versus anakinra.
DISCUSSION
Familial cold autoinflammatory syndrome is caused by mutations in the gene CIAS1, coding for cryopyrin, and is characterized by an inflammatory response to systemic cold exposure. The normal function of cryopyrin involves regulation of IL-1β production through caspase-1 activation.22 In FCAS, aberrant IL-1β release was shown to be responsible for the cold-induced systemic inflammation, because IL-1 blockade by anakinra abrogated all symptoms.11 In the present study, we demonstrated for the first time that the response to cold observed in the skin of FCAS patients could be replicated in vitro using monocytes derived from FCAS patients. There are several intriguing parallels between the in vivo and in vitro models. In subjects exposed to a controlled cold challenge,11 skin surface temperature reached 30°C–32°C at the end of the 45-min exposure time (Hoffman HM, unpublished data). This exposure was associated with the development of clinical symptoms within 1–2 hours, which could be effectively blocked by anakinra. In FCAS monocytes, the signal for IL-1β release at 32°C was induced in less than 1 hour, and despite being returned to 37°C, the cells were then sufficiently activated to release IL-1β over the following 16 hours. In addition, IL-1β release in vitro, which took place early, was responsible for induction of synthesis and release of several cytokines (including IL-1β itself), as demonstrated by the prevention of increased cytokine release after treatment with anakinra, as was seen in clinical studies. Therefore, the incubation of FCAS monocytes at 32°C in vitro constitutes a reasonable experimental model for some of the cold-induced symptoms in FCAS subjects and provides novel insights into the mechanisms underlying this disorder.
Unlike with most cytokines, transcription and translation of the IL-1β gene yields an inactive form, called pro-IL-1β, which requires cleavage by caspase-1 to produce mature IL-1β. In the present study, a measurable IL-1βmessage was present in FCAS and control monocytes at 37°C or 32°C at 4 hours, although little IL-1β release was observed except in FCAS cells at 32°C. It is possible that the adhesion step used to purify the monocytes induced transient increased transcription and accumulation of pro-IL-1β in the cytosol at the early time points. Because a caspase-1 inhibitor largely abolished the cold-induced IL-1β release in cold-challenged FCAS cells, it appears that the increased IL-1β release in FCAS is mediated by mutation-altered cryopyrin activation of caspase-1.
The purine ATP is often used in combination with other agents that induce pro-IL-1β synthesis to stimulate efficient caspase-1 activation through activation of the P2X7 purinergic ion channel receptor.23 Recently, cryopyrin was also shown to mediate this ATP response in mouse macrophage studies.18 We hypothesized that ATP, released from cells injured by incubation at the lower temperature, might stimulate purinergic receptors and thus mediate the enhanced IL-1β release from FCAS monocytes. However, this was not supported by our data; the purinergic antagonist PPADS had no effect on the cold response at a concentration that was shown in other studies to inhibit IL-1β release stimulated by LPS + ATP.14
To date, there are only a few reports of in vitro studies using cells from patients with cryopyrin-associated syndromes. In a single CINCA patient, IL-1β release from whole blood was significantly elevated in response to LPS or TNF-α, and elevated amounts of IL-1β were released without stimulation.24 Enhanced levels of IL-1β were also observed in unstimulated monocytes from a CINCA patient4 and an MWS patient.10 These findings are in accordance with in vitro cell line transfection studies using CIAS1 mutants associated with MWS and CINCA, which resulted in increased IL-1β processing by caspase-19 and IL-1β8 secretion. In contrast, unstimulated PBMC from FCAS patients incubated at 37°C did not release IL-1β or IL-18, although FCAS cells did release more of both cytokines in response to LPS than normal PBMC.25 This is in agreement with the present study, where the FCAS monocytes did not spontaneously produce more cytokine message or protein than normal cells at 37°C. It is possible that the increased baseline IL-1β release at 37°C by cells from CINCA and MWS patients, which is not seen in cells from FCAS patients, reflects the difference in clinical severity among these disorders, because FCAS represents the least severe of the 3 syndromes.
Inflammatory responses to hypothermic conditions have been studied previously using the promonocytic cell line THP-1 differentiated to a macrophage-like phenotype. The LPS-induced IL-1β and TNF-α release was significantly potentiated by an incubation temperature of 32°C through a mechanism that involved prolonged NF-κB activation.26,27 On the other hand, unstimulated cells did not release increased levels of cytokines.27 Similarly, in unstimulated PBMC cultures, no alteration in IL-6 release was observed at 32°C, and TNF-α was only minimally potentiated.28 Those data agree with the present findings that control monocytes did not produce elevated cytokine levels at 32°C.
Although we found that activation of FCAS monocytes incubated at 32°C is IL-1β dependent, the exact nature of how the cells sense the colder temperature is poorly understood. It is possible that mutated cryopyrin is simply less soluble, as earlier reported for different CIAS1 mutations,9 and as a result it precipitates or oligomerizes at 32°C, leading to inflammasome activation. Alternatively, some other intermediate mechanisms, such as cold shock proteins or cold receptors, may be involved. Culturing cells at 32°C is considered to represent a mild hypothermic condition and changes the expression of various proteins, as reviewed previously.29 Specifically, in promonocytic THP-1 cells, the cold shock protein CIBRP was induced at 32°C, and a multitude of other genes were enhanced or repressed as well.30 Given that elevated IL-1β from FCAS monocytes at 32°C can be detected in as little as 4 hours, it is unlikely that a major change in expression would have time to take place.
Although the exact mechanism remains unknown, monocytes from FCAS patients, but not from normal subjects, in the absence of any other stimuli respond to incubation at 32°C with a rapidly induced IL-1β release. This in turn results in autocrine stimulation and secondary induction of synthesis of other cytokines, such as IL-6 and TNF-α, as well as further stimulation of IL-1β production. The time requirement, the temperature at which the phenomenon occurs and the primary dependency on IL-1β parallel the clinical findings in FCAS patients after exposure to cold. Thus, this model is well suited for mechanistic studies to further enhance our understanding of FCAS.
Acknowledgments
Supported by National Institute of Allergy and Infectious Diseases (NIAID) grant R01 AI52430, Rheumatic Diseases Core Center grant P30 AR47360, University of California at San Diego General Clinical Research Center grant M01 RR00827, and the Ludwig Institute of Cancer Research, San Diego Branch.
We are grateful to the FCAS family members and other control subjects who participated in the research. We also wish to thank the staff of the General Clinical Research Center, University of California, San Diego, for phlebotomy support, Mamata Engineer and Jyothi Nayar for general laboratory support, Stephen Willingham for reagents, Gary Firestein for helpful advice, and Sherry Soefje for editorial support.
Abbreviations used
- CIAS1
Cold-induced autoinflammatory syndrome-1
- CINCA
Chronic infantile neurological, cutaneous, and articular syndrome
- FCAS
Familial cold autoinflammatory syndrome
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- IL-1Ra
IL-1 receptor antagonist
- MWS
Muckle-Wells syndrome
Footnotes
Disclosure of potential conflict of interest: H. M. Hoffman is a consultant for Regeneron Pharmaceuticals. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dode C, Le Du N, Cuisset L, Letourneur F, Berthelot JM, Vaudour G, et al. New mutations of CIAS1 that are responsible for Muckle-Wells syndrome and familial cold urticaria: a novel mutation underlies both syndromes. Am J Hum Genet. 2002;70:1498–1506. doi: 10.1086/340786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aganna E, Martinon F, Hawkins PN, Ross JB, Swan DC, Booth DR, et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 2002;46:2445–2452. doi: 10.1002/art.10509. [DOI] [PubMed] [Google Scholar]
- 4.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hull KM, Shoham N, Chae JJ, Aksentijevich I, Kastner DL. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61–69. doi: 10.1097/00002281-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman HM, Wanderer AA, Broide DH. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol. 2001;108:615–620. doi: 10.1067/mai.2001.118790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowds TA, Masumoto J, Zhu L, Inohara N, Nunez G. Cryopyrin induced IL-1 secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem. 2004;279:21924–21928. doi: 10.1074/jbc.M401178200. [DOI] [PubMed] [Google Scholar]
- 9.Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, et al. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006;13:236–249. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- 10.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 Forms an IL-1beta–processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364:1779–1785. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 14.Grahames CB, Michel AD, Chessell IP, Humphrey PP. Pharmacological characterization of ATP- and LPS-induced IL-1beta release in human monocytes. Br J Pharmacol. 1999;127:1915–1921. doi: 10.1038/sj.bjp.0702732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- 16.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 19.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/NALP3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 20.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 21.Boyle DL, Rosengren S, Bugbee W, Kavanaugh A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003;5:R352–R360. doi: 10.1186/ar1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lich JD, Arthur JC, Ting JP. Cryopyrin: in from the cold. Immunity. 2006;24:241–243. doi: 10.1016/j.immuni.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 24.Janssen R, Verhard E, Lankester A, Ten Cate R, van Dissel JT. Enhanced interleukin-1beta and interleukin-18 release in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2004;50:3329–3333. doi: 10.1002/art.20494. [DOI] [PubMed] [Google Scholar]
- 25.Stack JH, Beaumont K, Larsen PD, Straley KS, Henkel GW, Randle JC, et al. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J Immunol. 2005;175:2630–2634. doi: 10.4049/jimmunol.175.4.2630. [DOI] [PubMed] [Google Scholar]
- 26.Fairchild KD, Singh IS, Patel S, Drysdale BE, Viscardi RM, Hester L, et al. Hypothermia prolongs activation of NF-kappaB and augments generation of inflammatory cytokines. Am J Physiol Cell Physiol. 2004;287:C422–C431. doi: 10.1152/ajpcell.00507.2003. [DOI] [PubMed] [Google Scholar]
- 27.Fairchild KD, Singh IS, Carter HC, Hester L, Hasday JD. Hypothermia enhances phosphorylation of I(kappa)B kinase and prolongs nuclear localization of NF-(kappa)B in lipopolysaccharide-activated macrophages. Am J Physiol Cell Physiol. 2005;289:C1114–C1121. doi: 10.1152/ajpcell.00152.2005. [DOI] [PubMed] [Google Scholar]
- 28.Russwurm S, Stonans I, Schwerter K, Stonane E, Meissner W, Reinhart K. Direct influence of mild hypothermia on cytokine expression and release in cultures of human peripheral blood mononuclear cells. J Interferon Cytokine Res. 2002;22:215–221. doi: 10.1089/107999002753536185. [DOI] [PubMed] [Google Scholar]
- 29.Fujita J. Cold shock response in mammalian cells. J Mol Microbiol Biotechnol. 1999;1:243–255. [PubMed] [Google Scholar]
- 30.Sonna LA, Kuhlmeier MM, Carter HC, Hasday JD, Lilly CM, Fairchild KD. The effect of moderate hypothermia on gene expression by THP-1 cells: a cDNA microarray study. Physiol Genomics. 2006;26:91–98. doi: 10.1152/physiolgenomics.00296.2005. [DOI] [PubMed] [Google Scholar]