Abstract
Objective
The purpose of this case report is to describe a patient with post traumatic myositis ossificans (PTMO) of the anterior thigh following blunt trauma and discuss the incidence, clinical presentation, management, and imaging findings.
Clinical features
An 18-year-old male presented to a chiropractic clinic with a chief complaint of left knee pain and reduced range of motion after an impact injury to his left anterior thigh during hurdling 6 weeks earlier. Immediately after the injury, he presented to the emergency department where radiography of the left knee was negative and he was diagnosed with a muscle sprain. Follow-up radiography and ultrasonography of the left knee in a chiropractic radiology department revealed ossification consistent with PTMO within his vastus intermedius.
Intervention and outcome
The patient underwent a course of rehabilitation for 2 months including ice, class IV cold laser and vibration applied to his anterior thigh, and myofascial release of his quadriceps musculature with targeted and progressive rehabilitative exercises. His left knee pain resolved within 2 weeks of care. He resumed sports participation (American football) pain-free, while wearing protective padding over the affected thigh, 1 month after presentation, which was approximately 2 1/2 months following his injury.
Conclusion
This case demonstrates that ultrasonography may have the capability to detect early phases of PTMO approximately 2 weeks prior to radiographic evidence and to monitor progression throughout its course.
Key indexing terms: Myosistis ossificans, Athletic injuries, Heterotopic ossification, Chiropractic
Introduction
Post traumatic myositis ossificans (PTMO) is a well understood, although uncommon, clinical entity.1 There are several subtypes of myositis ossificans (MO) including PTMO, nontraumatic/pseudomalignant myositis ossificans, and myositis ossificans progressive (congenital form).2 Over 90% of sports related injuries are diagnosed as muscle strains or contusions with PTMO developing in approximately 9% to 20%.1,3,4 PTMO develops commonly after a single direct blow to the musculature or through repetitive minor trauma.5 Patients report a history of trauma in 60% to 75% of cases.5,6 The formation of MO without history of trauma may be related to increased levels of neuro-inflammatory Substance P.7 Although any muscle can be involved, the most common location for PTMO is the anterior thigh musculature.8 Signs and symptoms typically include pain, edema, swelling, and decreased range of motion with a palpable mass.9–11 This entity increases morbidity with its relationship to pain, loss of range of motion and function, and local tenderness lasting an average of 1.1 years.1,10,11 Ryan et al found that development of PTMO was associated with the following 5 risk factors: knee flexion less than 120°, injury during football, previous quadriceps injury, delay in treatment more than 3 days, and ipsilateral knee joint effusion.3
Histologically, PTMO represents degeneration and necrosis of damaged muscle tissue resulting in bone formation.5 Differential diagnosis includes hematoma, abscess, focal rhabdomyolysis, and malignant primary or secondary soft tissue tumors.12 Early and accurate diagnosis is imperative as many symptoms overlap and appropriate treatment is vital to an optimal outcome.12 Various imaging techniques are available to evaluate the presence and progression of PTMO including radiography, computed tomography, magnetic resonance imaging (MRI), skeletal scintigraphy, and diagnostic ultrasonography (US). US is a sensitive modality in the detection of PTMO and is at the forefront of investigation into musculoskeletal athletic injuries; however, only a few case reports describing the US appearance of PTMO have been reported.5,13 The purpose of this study is to present a case of an 18-year-old male hurdler and an American football player that was diagnosed with PTMO utilizing radiography as well as US and monitored with interval follow ups.
Case Report
An 18-year-old male track and American football athlete presented to our clinic with a chief complaint of left knee and thigh pain. Patient consent was provided for publication of de-identified clinical information and images. Six weeks prior, while running, he impacted his left anterior thigh on a hurdle. At that time, he went to the emergency department where radiography of the femur was performed and interpreted as negative. He was diagnosed with a strain of his left quadriceps. Approximately 1 week after the injury, his thigh pain subsided but his left knee developed severe swelling and pain which he rated 7/10 on the numerical pain rating scale. The patient did not report his knee pain to his parents or medical providers until his presentation at our clinic 4 weeks later. Physical examination revealed edema in the suprapatellar area of the left knee and tenderness with a hard mass palpable in his left thigh. A full orthopedic examination was not possible at that time due to pain and reduced knee flexion.
He underwent left knee and femur radiography which revealed a lamellated, poorly organized calcification adjacent to the anterior midshaft of the femur measuring approximately 12.5 cm by 2.2 cm (Fig 1). A large suprapatellar joint effusion was also evident. The following day knee and femur US was performed utilizing a GE Logiq E9 (GE Healthcare, Milwaukee, WI) linear array transducer operating at 8 to 15 MHz. The US exam revealed a mass within the vastus intermedius consisting of mixed hypoechoic and hyperechoic areas with disorganization of muscular fibers. The mass represented an intramuscular hematoma. Also noted was calcification within the vastus intermedius muscle measuring 11.5 by 4.5 cm consistent with lamellar bone (Fig 2). A normal US demonstration of the anterior thigh anatomy is provided for comparison (Fig 3). The hyperechoic mass created an artifact known as acoustic shadowing, which obscured the underlying femoral cortex. Also noted (but not shown) was a lateral joint effusion, a grade I sprain of the lateral collateral ligament and a suggested lateral meniscal tear. There was no evidence of increased power Doppler flow adjacent to the ossification, thus confirming a remote injury. Follow-up radiography and US of his left femur were performed approximately 2 months after presentation. Both imaging exams demonstrated an interval increase in size and organization of the muscular ossification, indicating anticipated progression. The ossification measured 18.33 cm by 4.04 cm on radiography and 15.47 cm by 5.79 cm on US (Figs 4-5).
Fig 1.

Conventional radiography of the femur approximately 6 weeks post injury demonstrating faint heterotopic ossification within the anterior thigh musculature (arrow).
Fig 2.

Longitudinal plane US image of the left vastus intermedius approximately 6 weeks post injury demonstrating ossification (lamellar rim) within the muscle and intramuscular hematoma.
Fig 3.

Longitudinal plane US imaging demonstrating normal muscular anatomy of the anterior thigh.
Fig 4.

Conventional radiography of the femur approximately 2 months post injury demonstrating heterotopic ossification within the musculature. The ossification is dense peripherally (white arrow) and somewhat radiolucent centrally. It does not attach to the adjacent cortex of the femur (cleavage plane: black arrow).
Fig 5.
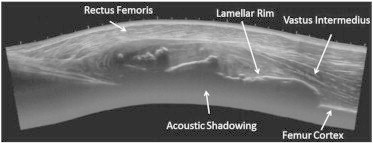
Longitudinal plane US imaging approximately 2 months post injury demonstrating further progression of the heterotopic ossification within the vastus intermedius and significant acoustic shadowing beneath the lamellar rim.
The goals of treatment were increased flexibility, strength, and proprioception.1,11,14 The frequency of treatment was 3 times per week utilizing ice, Class IV cold laser and vibration applied to his anterior thigh, myofascial release of his quadriceps musculature with targeted and progressive rehabilitative exercises.1,11,14 The rehabilitative exercises included whirlpool therapy while increasing range of motion, short arch exercises for the quadriceps musculature, and use of the stationary bike.1,11,14His left knee pain resolved within 2 weeks of care. He continued care for another 2 months. He resumed American football participation pain-free, 1 month after initial presentation, which was approximately 2 and a half months following his initial injury. He reported wearing protective gear over his left thigh while participating in sports. The patient discontinued care and was lost to follow-up.
Discussion
This case demonstrates the use of US and radiography to identify and monitor PTMO within the vastus intermedius. PTMO is a condition characterized by heterotopic bone formation in the soft tissues including muscle, fascia, tendons, joint capsules, and ligaments following trauma.9 Incidence is higher in males and usually between 30 to 40 years old.10,11 Symptoms include pain and local tenderness, edema, swelling, decreased range of motion, stiffness, and a palpable mass.10,11 In a study of 26 cases of PTMO, the most common presenting sign was a palpable mass present in 25 out of 26 subjects.10
Pathologic features can be divided into 3 categories known as the zone phenomenon: stage 1- pseudo-sarcoma, stage 2- differentiation, and stage 3- maturation.9 Following contusion to the soft tissues, cell death and metaplasia occur in stage 1. In the early stages, increased levels of neuro-inflammatory factor Substance P and bone morphogenetic protein facilitate heterotopic ossification.7 The sympathetic nervous system also plays a role since β-adrenergic and neuropeptide receptors are present on osteoblastic and osteoclastic cells.15 Within a month, proliferation of all mesenchymal cells occurs. The mixed echogenicity on the initial US images in this case demonstrates muscular contusion and hematoma findings consistent with metaplasia. In stage 2, the mesenchymal cells begin to differentiate into fibroblasts, chondroblasts, and osteoblasts and identification of osseous tissue may be evident on radiography. This occurs within the first 3 to 4 weeks following injury. The central portion of the lesion undergoes liquefactive necrosis but, the periphery ossifies as there is less damage in that area. The first images performed in this case, demonstrated ossification as well as hematoma. There was a rim of ossification noted on radiography and confirmed on US indicating further metaplasia. The final stage, maturation, results in peripheral periosteal formation with complete removal of the necrotic debris centrally.9 The second set of images demonstrated the third stage of PTMO with a dense ossification and a slightly lucent center. At this point, the ossification can be resected. If the lesion is not removed, within 1 to 2 years it may spontaneously regress although this occurs in only 30% of patients.6,16
Abate, et al characterized 2 phases of PTMO utilizing US and histological findings: early (17.5 days + 3.5 days) and mature (32.5 days + 17.5). The early phase may not be evident on radiography, but on US it demonstrates hypoechogenicity with an outer lamellar hyperechoic ill-defined rim.5 This appearance is consistent with the initial US images obtained in this case. The late phase shows lacy radiopacity followed by cloud-like ossification on radiography.9 Further progression reveals a bony mass with a radiopenic center and no connection to the adjacent bone, the so called cleavage plane.9 This finding aids in the differentiation of PTMO from malignant lesions of bone (Fig 4). Malignant osseous lesions would demonstrate a connection to the underlying bone. The cleavage plane refers only to malignant lesion of bone and cannot be applied to extraosseous sarcomas. In this instance the zone phenomenon aids in differentiation.9 On US, acoustic shadowing would be present deep to the ossific rim with a few internal calcific deposits.5
Early and accurate identification is a key factor in developing a treatment plan for patients with PTMO. The differential diagnosis includes malignant neoplasm, therefore it is important to establish the correct diagnosis and monitor progression.12,17 MRI findings can be non-specific and easily confused with soft tissue sarcoma or osteosarcoma.18 Early stage detection of PTMO by US occurs 2 weeks prior to the radiographic manifestations which develop 3 to 4 weeks after injury.5,9,18 US has several advantages over MRI, including lower cost, convenience, superior spatial resolution, portability, and dynamic evaluation of the injury.18
Biopsy of this lesion would reveal osteoblasts which could mimic a sarcomatous process, as the first pathologic stage “pseudo-sarcoma” implies. The osteoblasts would be more mature peripherally and immature or not formed at all at the center of the lesion. This is a key feature of PTMO and distinguishes it from a neoplastic lesion of bone which would not have immature cells centrally.19 There are radiographic diagnoses that are considered “leave me alone” lesions such as non-ossifying fibroma, osteochondroma or fibrous dysplasia, and PTMO would also fit in this category.20 These lesions, except for PTMO, need no intervention and complications may arise if surgical intervention or biopsy is performed. PTMO is a good example of a “leave me alone” lesion that would not benefit from biopsy and worse, it may complicate the differential diagnosis.
Limitations
Case reports have limitations. Generalization of the diagnostic findings represented in this case may not necessarily apply to other patients and the patient may have improved regardless of care rendered. The patient dropped out of care and was lost to follow up so further imaging was unattainable, thus we were not able to definitely report final outcomes.
Conclusion
This case demonstrates that ultrasonography may have the capability to detect early phases of PTMO approximately 2 weeks prior to radiographic evidence and to monitor progression throughout its course. Careful clinical evaluation is required along with an understanding of the pathophysiological timing of PTMO (zone phenomenon) in order to prevent a false-positive diagnosis of an extraosseous sarcoma or conversely overlook the presence of a malignant lesion.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
References
- 1.Torrance D.A., Degraauw C. Treatment of post-traumatic myositis ossificans of the anterior thigh with extracorporeal shock wave therapy. J Can Chiropr Assoc. 2011;55(4):240–246. [PMC free article] [PubMed] [Google Scholar]
- 2.Tyler P., Saifuddin A. The imaging of myositis ossificans. Semin Musculoskelet Radiol. 2010;14(2):201–216. doi: 10.1055/s-0030-1253161. [DOI] [PubMed] [Google Scholar]
- 3.Ryan J.B., Wheeler J.H., Hopkinson W.J., Arciero R.A., Kolakowski K.R. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299–304. doi: 10.1177/036354659101900316. [DOI] [PubMed] [Google Scholar]
- 4.Jackson D.W., Feagin J.A. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95–105. [PubMed] [Google Scholar]
- 5.Abate M., Salini V., Rimondi E. Post traumatic myositis ossificans: Sonographic findings. J Clin Ultrasound. 2011;39(3):135–140. doi: 10.1002/jcu.20792. [DOI] [PubMed] [Google Scholar]
- 6.Jung D., Cho K.T., Roh J.H. Non-traumatic myositis ossificans in the lumbosacral paravertebral muscle. J Korean Neurosurg Soc. 2013;53(5):305–308. doi: 10.3340/jkns.2013.53.5.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kan L., Lounev V.Y., Pignolo R.J. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem. 2011;112(10):2759–2772. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King J.B. Post-traumatic ectopic calcification in the muscles of athletes: a review. Br J Sports Med. 1998;32(4):287–290. doi: 10.1136/bjsm.32.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yochum T.R., Rowe L.J. 3rd ed. Lippincott/Williams & Wilkins; Philadelphia: 2005. Yochum and Rowe's essentials of skeletal radiology. [Google Scholar]
- 10.Li J., Zhu L., Hu Y., Liu M. Clinical analysis of 26 cases of myositis ossificans circumscripta. Chin J Traumatol. 2000;3(2):124–125. [PubMed] [Google Scholar]
- 11.Miller A.E., Davis B.A., Beckley O.A. Bilateral and recurrent myositis ossificans in an athlete: a case report and review of treatment options. Arch Phys Med Rehabil. 2006;87(2):286–290. doi: 10.1016/j.apmr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Damron T.A., Morris C., Rougraff B., Tamurian R. Diagnosis and treatment of joint-related tumors that mimic sports-related injuries. Instr Course Lect. 2009;58:833–847. [PubMed] [Google Scholar]
- 13.Koh E.S., McNally E.G. Ultrasound of skeletal muscle injury. Semin Musculoskelet Radiol. 2007;11(2):162–173. doi: 10.1055/s-2007-1001881. [DOI] [PubMed] [Google Scholar]
- 14.Trojian T.H. Muscle contusion (thigh) Clin Sports Med. 2013;32(2):317–324. doi: 10.1016/j.csm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 15.3 ed. vol. 117. Elsevier; 2013. (Handbook of Clinical Neurology). [Google Scholar]
- 16.Wang X.L., Malghem J., Parizel P.M., Gielen J.L., Vanhoenacker F., De Schepper A.M. Myositis ossificans circumscripta. Vol. 86. 5; 2003. Pictorial essay; pp. 278–285. (JBR-BTR). [PubMed] [Google Scholar]
- 17.Gindele A., Schwamborn D., Tsironis K., Benz-Bohm G. Myositis ossificans traumatica in young children: report of three cases and review of the literature. Pediatr Radiol. 2000;30(7):451–459. doi: 10.1007/s002479900168. [DOI] [PubMed] [Google Scholar]
- 18.Lee J.C., Mitchell A.W., Healy J.C. Imaging of muscle injury in the elite athlete. Br J Radiol. 2012;85(1016):1173–1185. doi: 10.1259/bjr/84622172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.E R . 2012. Rubin's Pathology: Clinicopathologic Foundations of Medicine; p. 1219. [Google Scholar]
- 20.Vanel D., Ruggieri P., Ferrari S. The incidental skeletal lesion: ignore or explore? Cancer Imaging. 2009;9:S38–S43. doi: 10.1102/1470-7330.2009.9009. [Spec No A] [DOI] [PMC free article] [PubMed] [Google Scholar]


