Abstract
Objective: Nanofibers for tissue scaffolding and wound dressings hold great potential in realizing enhanced healing of wounds in comparison with conventional counterparts. Previously, we demonstrated good fibroblast adherence and growth on a newly developed scaffold, Tegaderm™-Nanofiber (TG-NF), made from poly ɛ-caprolactone (PCL)/gelatin nanofibers electrospun onto Tegaderm (TG). The purpose of this study is to evaluate the performance and safety of TG-NF dressings in partial-thickness wound in a pig healing model.
Approach: To evaluate the rate of reepithelialization, control TG, human dermal fibroblast-seeded TG-NF(+) and -unseeded TG-NF(−) were randomly dressed onto 80 partial-thickness burns created on four female and four male pigs. Wound inspections and dressings were done after burns on day 7, 14, 21, and 28. On day 28, full-thickness biopsies were taken for histopathological evaluation by Masson-Trichrome staining for collagen and hematoxylin–eosin staining for cell counting.
Results: No infection and severe inflammation were recorded. Wounds treated with TG-NF(+) reepithelialized significantly faster than TG-NF(−) and control. Wound site inflammatory responses to study groups were similar as total cell counts on granulation tissues show no significant differences. Most of the wounds completely reepithelialized by day 28, except for two wounds in control and TG-NF(−). A higher collagen coverage was also recorded in the granulation tissues treated with TG-NF(+).
Innovation and Conclusion: With better reepithelialization achieved by TG-NF(+) and similar rates of wound closure by TG-NF(−) and control, and the absence of elevated inflammatory responses to TG-NF constructs, TG-NF constructs are safe and demonstrated good healing potentials that are comparable to Tegaderm.

Toan Thang Phan, MD, PhD
Introduction
The beneficial effects of occlusion and a moist wound environment on reepithelialization of both partial- and full-thickness wounds are widely known.1,2 Synthetic occlusive dressings are hence a popular and beneficial alternative in the treatment of burn injuries3 by reducing the wound healing time1,2 and the amount of pain experienced.1,4 This has motivated the development of various artificial skin substitutes in burn surgery.5
A wealth of materials, including natural (e.g., collagen, chitosan) and synthetic (e.g., polyurethane) polymers, have been intensively investigated and applied for wound healing and dermal reconstruction.3 However, many problems are associated with the current tissue scaffolds and/or skin substitutes. For instance, poor integration with host tissue, wound contraction,6 and scarring remain as major issues yet to be addressed. Whereas the overall composition of these scaffolding/dressing materials cannot be expected to dramatically change too much in the near term, continued exploration of novel processing strategies that can be used to produce extracellular matrix resembling scaffolds, may provide a gateway to the new generation of artificial skin substitutes.7 Likewise, extensive animal test evaluations based on those newly developed dermal analogues are critical in fully exploring their potential for clinical applications.
In recent years, electrospinning has emerged as one of the most attractive processing techniques to produce nanoscale fibers that can closely mimic the extracellular matrix components and to stimulate the natural functionalization of the cells. Many works using this nanofabrication technique demonstrated the great potential of applying such nanofibrous scaffolds for wound healing or skin tissue engineering.7–11 However, very limited in vivo animal tests for skin repair and regeneration are reported, which will obviously slow down the bench to bedside research.
Clinical Problem Addressed
Burn injury is a serious public health problem globally. Each year, over 300,000 people die from burn injuries caused by fire alone, with millions more suffering from burn-related disabilities.12 In Singapore, annual admissions for burn injuries stand at 288 from 1997 to 200313 and 161 from 2003 to 2005.14 Most of these burn injuries cover less than 10% of the total body surface area.13 Presently, split-thickness skin grafts (STSGs) are considered the best material for surgical repair of an excised burn wound.15 However, in patients with burns that affect greater than 50% of body surface area, there remains insufficient area of unaffected skin for autograft STSG harvest. The lack of donor site becomes a major factor in limiting rapid wound closure.
Previously, our group reported a scaffold, Tegaderm™-Nanofiber (TG-NF), made from poly ɛ-caprolactone (PCL)/gelatin electrospun onto a polyurethane dressing (Tegaderm; 3M Medical).16 Our in vitro results demonstrated that the TG-NF construct achieved significant cell adhesion, growth, and proliferation. In this study, we extend our work further by comparing the rate of wound closure, inflammation evaluation by total cell counts, and collagen deposition in granulation tissue of burn wounds treated by Tegaderm and TG-NF(−)/(+) in a porcine wound healing model.
Materials and Methods
Regulations
Dermal fibroblasts were obtained from skin tissue that was obtained from a female donor (14 months old, Malay) with consent from the parents of the donor. Staffs involved in the animal works have completed the Responsible Care and Use of Laboratory Animals course conducted by NUS Institutional Animal Care and Use (NUS-IACUC). Approvals from SingHealth-IACUC and NUH Institutional Review Board were obtained for the preclinical evaluation of TG-NF and the use of human dermal fibroblasts (HDFs) on a porcine wound healing model.
Culture of HDFs
Human's skin dermis was minced and incubated in a solution of collagenase type I (0.5 mg/mL) and trypsin (0.2 mg/mL) for 6 h at 37°C. HDFs were pelleted and grown in tissue culture flasks. The HDF strains were maintained and stored in liquid nitrogen until use. HDF strains were seeded at a density of 105 cells in Dulbecco's modified Eagle's Medium/10% fetal calf serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) maintained in a humidified incubator at 37°C with 5% CO2. Once the HDFs reached 70% confluence, they were trypsinized and passaged at 1:3 ratios. Only the second to fifth passages of HDFs were used in this study. Once HDFs reached confluence, the cultures were scrapped with a cell scraper and the number of cells was counted with a hemocytometer. The resulting cells in suspension were then seeded onto the TG-NF constructs.
Animal care
A total of eight domestic Yorkshire pigs (four females and four males, 3–6 months, 37–58 kg) were used. Animal housing and veterinary services were provided by the Department of Experimental Surgery, Singapore General Hospital. Animals were housed individually in pigpens with constant temperature and humidity and given free access to water ad libitum.
Burn wound model
Animals were acclimatized for a week before the start of experiment. The animals were fasted overnight and given atropine 0.05 mg/kg intramuscularly 15 min before sedating with ketamine 15 mg/kg IM, followed by masking down with 5% isofluorane and intubated with a size 7.0 endotracheal tube. Aseptic techniques were strictly practiced throughout the procedure. The hair on the back of the pigs was shaved and vacuumed. The skin was cleaned with 1% cetrimide wash, 0.05% chlorhexidine, and finally with 1% povidone–iodine. Ten circular deep dermal burns were created on the dorsal aspect on each pig's skin by applying a preheated 3-cm-diameter metal plate (5 min at 100°C water bath) for 1 min. The heat-coagulated tissues were scrapped off with a skin scrapper knife. This burning and scrapping procedure was repeated on the other burn sites. The depth of burned skin removed from each wound was ∼3 mm. These burn wounds were created 8 cm center-to-center apart to reduce cross-contamination of test materials and local effect of burns from one another.
Wound treatment
Wound sites were dressed using the control group: Tegaderm; Experimental groups: TG-NF(−)=TG-NF immersed in DMEM and TG-NF(+)=TG-NF seeded with 10,000 HDFs/cm2. Order of dressings on each wound: Burn wound—control/TG-NF(−)/TG-NF(+)—Tegaderm (except control)- Surgical Gauze—Opsite™ (Smith & Nephew)- Stockinette wrapped around the body and secured by adhesive tapes.
The control and experimental groups were randomly dressed onto different wound sites in each animal. This arrangement ensures that no one group will be positioned to heal better since the thickness of the skin at different locations, wound contraction,6 and healing can vary significantly from site to site. Also, to minimize the differences in wound healing between male and female animals, four females and four males were selected for this experiment.
Daily monitoring of animals was conducted to ensure that the animals are not in any pain and stress due to the wounds and their surroundings.
Wound inspection and documentation
On day 0, 7, 14, 21, and 28, old dressings and necrotic tissues were removed from the wounds. Wounds were digitally photographed alongside a sterile ruler for size standardization. Wound assessments were done by an experienced wound specialist blinded to the sample types. Wound margins were traced onto autoclaved transparencies for statistical comparison on the rate of wound closure. For postoperative wound evaluation, animals were given ketamine and then masked with isoflurane anesthesia at 3% concentration with oxygen. Animals were observed daily for any discomfort and localized/systemic infections.
Wound biopsy and histological stainings
Animals were euthanized with pentobarbitone 85 mg/kg intravenously on day 28. Full-thickness skin biopsies containing the entire wound site were excised and washed in 1% saline before storage in a −80°C freezer. Tissue samples 3 cm long by 0.5 cm thick were sectioned from the center of the wound to the outer boundary of unwounded skin and fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned.
Masson-trichrome staining
Paraffin sections were dewaxed and incubated in Bouin's solution for 1 h in a 56°C water bath. Sections were then stained with Weigert's iron hematoxylin solution (1 min), azophloxine solution, tungstophosphoric acid orange G solution, and light green SF. Tissue sections were then dehydrated by gradual immersion in 95% and 100% ethanol, air-dried, and finally mounted in Entellan®. Reepithelialization and a percentage of the collagen area were used to assess the wound healing for each group.
Harris hematoxylin–eosin Y staining
Paraffin sections were dewaxed in xylene and rehydrated in 100%, 95%, and 70% ethanol and distilled water. Deparaffinized sections were stained with the Harris hematoxylin solution (1 min), washed in running tap water, and then rinsed in 95% ethanol. Counterstained in the eosin-Y solution. Dehydrated through 95% alcohol and 100% ethanol. Air-dried and immersed in xylene before finally mounted in Entellan.
Computerized measurement of wound area
Wound tracings were scanned using an Epson image scanner at resolution 300 dpi. Gel-Pro® Analyzer ver. 4.5—MediaCybernetics was used to measure the wound areas in pixel units.
ImageJ %collagen area assessment and inflammatory cell counting
Using ImageJ ver 1.41o, the Masson-trichrome–stained tissue sections were quantified in %collagen area by measuring the collagen signal in the entire granulation tissue area at a constant threshold. Finally, the average %collagen coverage area was calculated and presented.
Using the cell counter in ImageJ ver 1.41o, hematoxylin-eosin–stained slides were used to estimate the total cell counts in the granulation tissue. To obtain robust estimates on the number of cells, counting was performed on multiple random areas (100 μm2) on the granulation tissues of each hematoxylin and eosin (H&E)-stained slide.
Statistical analysis
The one-way analysis of variance test was used to assess the statistical significance of the differences in the rate of wound reepithelialization (area closure-pixel/7 days), granulation tissue % collagen coverage area, and granulation tissue total cell counts of the wounds. The test was used for multiple comparisons between control versus TG-NF(−), control versus TG-NF(+), and TG-NF(−) versus TG-NF(+). A value of p<0.05 is considered statistically significant.
Results
Fabrication of TG-NF constructs
Briefly, TG-NF constructs consisting of a polymer blend of PCL/gelatin nanofibers were electrospun directly onto a Tegaderm sheet (3M Medical). Please refer to Figure 1 for schematic illustration. For detailed descriptions on the fabrication of this nanofibrous system, please refer to Chong et al.16
Figure 1.
Preparation of TG-NF constructs: (A) schematic illustration of the electrospinning of PCL/gelatin composite nanofibers onto a slowly rotating cylinder collector with the Tegaderm sheet wrapped around it; (B) a piece of such electrospun PCL/gelatin on a Tegaderm dressing mat; and (C) SEM image of the randomly oriented PCL/gelatin ultrafine fibers. PCL, poly ɛ-caprolactone; SEM, scanning electron microscopy; TG-NF, Tegaderm™-Nanofiber. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Animal assessments
In total, 80 partial-thickness wounds with characteristics of dry and white dermal beds were created on the dorsal aspects of eight pigs; 60 wounds were of sufficient quality to be used for analysis. Those 20 wounds of insufficient quality were mostly partial burn areas contributed by the fixed shape of the metal plate applied onto round contour parts of the pig. The 60 wounds of sufficient quality were further divided into three groups of 20 to be applied with control, TG-NF(−), and TG-NF(+). Dressings were applied and animals returned to the pen for rehabilitation and observation. The animals did not exhibit any sign of distress and were able to eat and sleep well coupled with good weight gain of ∼2 kg per week in all animals.
No localized or systemic infections/adverse reactions
Laboratory preparations of TG-NF constructs and HDFs were appropriate and safe for application on the animals. We did not observe any infection or anaphylactic reactions for all wounds in all eight animals. Antiseptic agents were not used in the length of this experiment.
Progress of wound closure
After day 0, the wounds were cleaned, assessed, and redressed on day 7, 14, 21, and 28. On day 7 (Fig. 2A–C), the pull back tension exerted by healthy skin tissues surrounding the wounds17 caused the areas to increase in control (25%), TG-NF(−) (12%), and TG-NF(+) (15%). From day 14 onward, wounds healed with no further sign of deterioration, with evidence of reepithelialization. On day 28, complete wound reepithelialization was observed in the three groups except for two wounds in control (Fig. 3A) and one wound in TG-NF(−). The burns dressed with TG-NF(+) were observed with less erythema and were drier and thicker to touch (Fig. 2C, day 28).
Figure 2.
Representative photographs of burn wounds taken on day 0, 7, 14, 21, and 28. (A) Control, (B) TG-NF(−), (C) TG-NF(+). Graphs of% average wound area (pixels) versus days (7-day intervals). (D) TG-NF(−), (E) TG-NF(+), (F) control. TG-NF(+), human dermal fibroblast-seeded Tegaderm-Nanofiber; TG-NF(−), human dermal fibroblast-unseeded Tegaderm-Nanofiber. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 3.
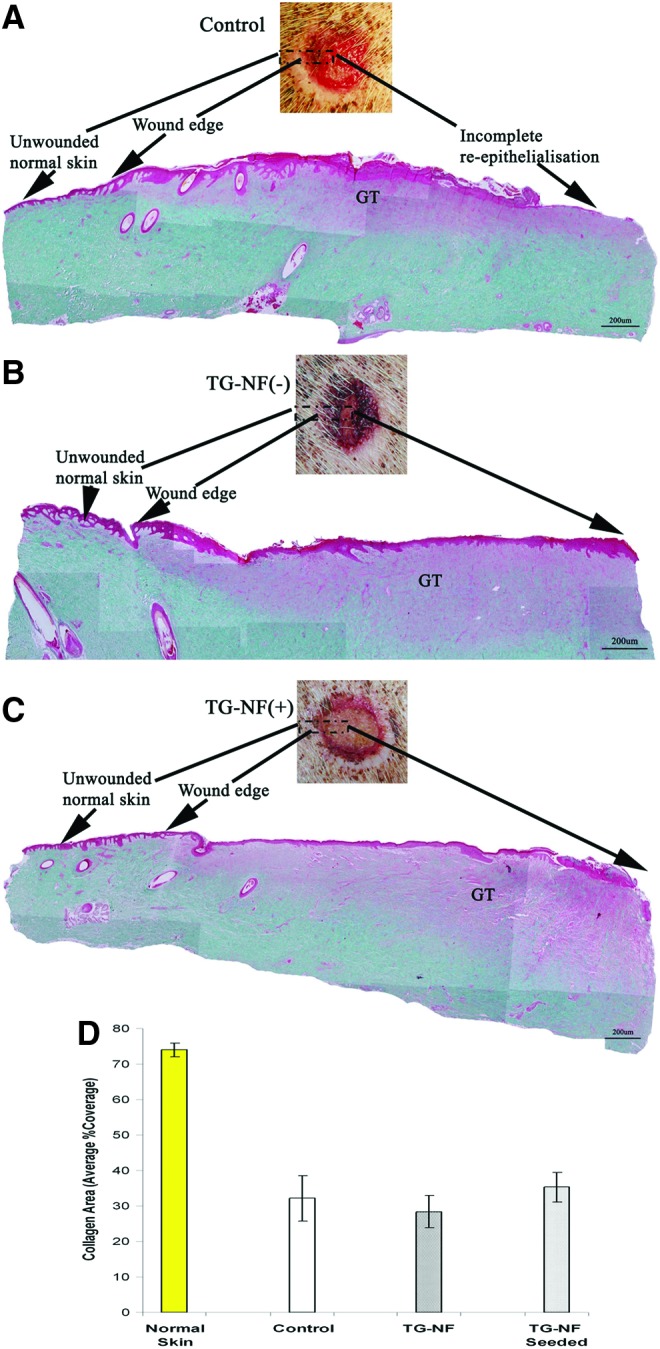
Histological examination of wound granulation tissues stained with Masson-trichrome staining. (A) Control (incomplete reepithelialization), (B) TG-NF(−), (C) TG-NF(+). (D) Histogram, collagen% average coverage in granulation tissue (GT). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The average rates of wound closure (Fig. 2D–F) taking only the trendline, starting from day 7 to 21, into consideration were control (44 closure area-pixels/7 days), TG-NF(−) (47 closure area-pixels/7 days), and TG-NF(+) (55 closure area-pixels/7 days). A significant increase in the rate of wound closure was observed in TG-NF(+) (p<0.05) when compared to control and TG-NF(−). The average rates of wound closure of TG-NF(−) and control group were not significantly different.
Collagen content
ImageJ was used to quantify the %collagen coverage area in the wound biopsy's granulation tissue sections stained in Masson-trichrome (Fig. 3). %Collagen coverage area in normal skin: 74% (standard deviation [STDV]:2); control: 32% (STDV:6.4); TG-NF(−): 28% (STDV:4.6); TG-NF(+): 35% (STDV:4.1). No statistically significant differences were observed between the control group versus TG-NF(−) and control group versus TG-NF(+), but a significant increase in %collagen coverage was observed in TG-NF(+) (p<0.05) as compared to TG-NF(−). %Collagen coverage in normal skin was significantly higher at approximately twofold and more than in granulation tissue.
Total cell counts
The wound site inflammatory response to each dressing was evaluated by total cell counts in H&E-stained tissue sections (Fig. 4). Total cell counts in control: mean 31.3 cells/100 μm2 (STDV: 6.1); TG-NF(−): mean 32 cells/100 μm2 (STDV: 3.6); TG-NF(+): 31.6 cells/100 μm2 (STDV: 4.7). No statistically significant differences were observed between the study groups.
Figure 4.
Histological examinations of wound granulation tissues stained with the Harris hematoxylin–eosin Y stain (40×). (A) Control, (B) TG-NF(−), (C) TG-NF(+). (D) Histogram, average total cell counts in granulation tissue. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Discussion
As an enabling technology, electrospinning has recently been widely recognized with its unique capability of making architecturally and biochemically extracellular matrix-mimicking scaffolds, which usually are difficult to achieve with those well-established conventional scaffold fabrication methods. Such bioinspired scaffolds are most promising in directing native wound healing processes and tissue regeneration. To enhance the efficacy of utilizing electrospun nanofibers for formation of biologically functional tissues, we had earlier on developed composite nanofibers of gelatin/PCL,11 which were found to be advantageous in attaining an optimal combination of mechanical, physicochemical, and biological performances. In the past few years, this hybrid nanofibrous material system had been employed as a versatile biomimetic substrate for engineering tissues such as skin, bone, nerve, muscle, cardiovascular, and for cancer and stem cell research.16,18–25 With the aim for wound healing and skin engineering through layered reconstitution, we previously prepared the Tegaderm-supported PCL/gelatin nanofiber construct TG-NF and evaluated in vitro its feasibility in achieving fibroblast populations. Following this, we attempted to test the feasibility of using the TG-NF construct for reepithelialization of partial-thickness wounds on a porcine wound healing model. The anatomy of the skin in a Yorkshire pig is very similar to a human and is a well-established model of normal skin wound healing, which closely approximates the healing process in humans.26,27 It is hoped that these consecutive investigations will form substantial evidences for the future assessment of our cost-effective TG-NF products in preclinical trials.
Results from this study indicate that partial-thickness burns in pigs treated with TG-NF(+) reepithelialized significantly faster than TG-NF(−) and control Tegaderm, a commercial polyurethane film. Most of the wounds were completely reepithelialized at day 28, except for two isolated cases that surfaced in the control group and in TG-NF(−). At day 28, the collagen coverage area in the granulation tissues treated with TG-NF(+) was also higher as compared to control and TG-NF(−), but was still less than 50% of coverage in normal skin.
Wound deterioration was observed on day 7, when healthy skin on the wound edges pull back causing the wound area to expand due to tension exerted by the surrounding healthy skin tissues.17 Day 14 onward, wounds were reepithelializing with no further sign of deterioration. TG-NF is a composition of PCL/gelatin electrospun on Tegaderm and wounds treated with it did not exhibit a different inflammatory response as compared to control. This observation was supported by the nonsignificant differences in the average total number of cells counted per 100 μm2 of granulation tissues for the three groups. Furthermore, we did not observe the severe inflammation seen in a wound healing experiment done on guinea pigs as reported by Khil et al.28 No infection or anaphylactic reactions for all wounds, in all the animals were observed. The complete reepithelialization and absence of wound deterioration, similar inflammation responses, and zero rate of infection proved that the laboratory production and handling of TG-NF, cell culture, and animal procedures were sterile and safe, thus passing the prerequisite for TG-NF to be used in a human clinical trial. It was also observed that during the inflammatory phase of healing (first 7 to 10 days), the TG-NF was unable to absorb the excess exudates due to the relatively less amount of the TG-NF mats used. This was circumvented by placing surgical gauze over Tegaderm.
Complete reepithelialized wounds dressed with TG-NF(+) have the appearance of matured skin that feels dry and thicker to touch. The better wound healing assessments in terms of collagen coverage in the granulation tissues and a higher rate of wound reepithelialization reported for TG-NF(+) could be attributed to the secretion of proteolytic and growth factors by the HDFs, which led to the faster degradation of the necrotic tissues, the formation of granulation tissues, and reepithelialization. Other factors that may have limited the reepithelialization of wounds treated by TG could have been that the dressing was removed prematurely and the adhesive coating may strip off newly forming epidermis.29–31
Innovation
The proven beneficial properties in wound healing of our newly developed composite nanofibers of gelatin/PCL have been amplified with the application of electrospinning, an emerging processing technology. Not only displaying the complete safety in the production, TG-NF also outperforms a commercial product in promoting wound healing. With these exciting results, a human clinical trial will be the next step we plan.
Key Findings.
• No severe inflammatory reaction was observed in wounds dressed with TG-NF.
• TG-NF is a safe wound dressing.
• Healing efficacy of TG-NF is comparable to Tegaderm.
Abbreviations and Acronyms
- HDFs
human dermal fibroblasts
- IACUC
Institutional Animal Care and Use
- PCL
poly ɛ-caprolactone
- STSGs
split-thickness skin grafts
- TG
Tegaderm™
- TG-NF(+)
human dermal fibroblast-seeded Tegaderm-Nanofiber
- TG-NF(−)
human dermal fibroblast-unseeded Tegaderm-Nanofiber
Acknowledgments and Funding Sources
The authors would like to thank Hairul Nizam from the Division of Bioengineering, National University of Singapore and Inria from the SingHealth Experimental Medicine Centre, Singapore General Hospital for their assistance in the project's administrative matters. This work was supported by the Proof of Concepts grant funded by the Singapore Economic Development Board.
Author Disclosure and Ghostwriting Statement
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Chee Tian Ong and Associate Professor Phan Toan Thang work closely in their research projects at the Stem Cell and Wound Healing Research Group in the National University of Singapore. Dr. Phan has been known worldwide for his discovery and applications of a novel source of stem cells from the umbilical cord lining membrane that holds translational potential for regenerative medicine, tissue engineering, and cell-based therapy.
References
- 1.James J, Watson A. The use of Op-site, a vapour permeable dressing, on skin graft donor sites. Br J Plast Surg 1975;28:107–110 [PubMed] [Google Scholar]
- 2.Lobe T, Anderson G, King D, Boles E. An improved method of wound management for pediatric patients. J Pediatr Surg 1980;15:886–889 [DOI] [PubMed] [Google Scholar]
- 3.Papini RP, Wilson AP, Steer JA, McGrouther DA, Parkhouse N. Wound management in burn centres in the United Kingdom. Br J Surg 1995;82:505–509 [DOI] [PubMed] [Google Scholar]
- 4.Neal D, Whalley P, Flowers M, Wilson D. The effects of an adherent polyurethane film and conventional absorbent dressing in patients with small partial thickness burns. Br J Clin Pract 1981;35:254–257 [PubMed] [Google Scholar]
- 5.Jones I, Currie L, Martin R. A guide to biological skin substitutes. Br J Plast Surg 2002;55:185–193 [DOI] [PubMed] [Google Scholar]
- 6.Middelkoop E, van den Bogaerdt AJ, Lamme EN, Hoekstra MJ, Brandsma K, Ulrich MM. Porcine wound models for skin substitution and burn treatment. Biomaterials 2004;25:1559–1567 [DOI] [PubMed] [Google Scholar]
- 7.Simpson DG. Dermal templates and the wound-healing paradigm: the promise of tissue regeneration. Expert Rev med Devices 2006;3:471–484 [DOI] [PubMed] [Google Scholar]
- 8.Zhong SP, Zhang YZ, Lim CT. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010;2:510–525 [DOI] [PubMed] [Google Scholar]
- 9.Zahedi P, Rezaeian I, Ranaei-Siadat S-O, Jafari S-H, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Technol 2010;21:77–95 [Google Scholar]
- 10.Hromadka M, Collins JB, Reed C, et al. Nanofiber applications for burn care. J Burn Care Res 2008;29:695–703 [DOI] [PubMed] [Google Scholar]
- 11.Zhang YZ, Ouyang HW, Lim CT, Ramakrishna S, Huang ZM. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res Part B 2005;72B:156–165 [DOI] [PubMed] [Google Scholar]
- 12.Peck M, Molnar J, Swart D. A global plan for burn prevention and care. Bull World Health Organ 2009;87:802–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song C, Chua A. Epidemiology of burn injuries in Singapore from 1997 to 2003. Burns 2005;31:S18–S26 [DOI] [PubMed] [Google Scholar]
- 14.Chong SJ, Song C, Tan TW, Kusumawijaja G, Chew KY. Multi-variate analysis of burns patients in the Singapore General Hospital Burns Centre (2003–2005). Burns 2009;35:215–220 [DOI] [PubMed] [Google Scholar]
- 15.Shakespeare P. Burn wound healing and skin substitutes. Burns 2001;27:517–522 [PubMed] [Google Scholar]
- 16.Chong EJ, Phan TT, Lim IJ, et al. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater 2007;3:321–330 [DOI] [PubMed] [Google Scholar]
- 17.Bush JA, Ferguson MW, Mason T, McGrouther DA. Skin tension or skin compression? Small circular wounds are likely to shrink, not gape. J Plast Reconstr Aesthet Surg 2008;61:529–534 [DOI] [PubMed] [Google Scholar]
- 18.Yang XC, Yang F, Walboomer XF, Bian ZA, Fan MW, Jansen JA. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J Biomed Mater Res Part A 2010;93A:247–257 [DOI] [PubMed] [Google Scholar]
- 19.Venugopal JR, Low S, Choon AT, Kumar AB, Ramakrishna S. Nanobioengineered electrospun composite nanofibers and osteoblasts for bone regeneration. Artificial Organs 2008;32:388–397 [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Perez MA, Guarino V, Cirillo V, Ambrosio L. Influence of gelatin cues in PCL electrospun membranes on nerve outgrowth. Biomacromolecules 2010;11:2238–2246 [DOI] [PubMed] [Google Scholar]
- 21.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrospun poly(epsilon-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008;29:4532–4539 [DOI] [PubMed] [Google Scholar]
- 22.Kim MS, Jun I, Shin YM, Jang W, Kim SI, Shin H. The development of genipin-crosslinked poly(caprolactone) (PCL)/gelatin nanofibers for tissue engineering applications. Macromol Biosci 2010;10:91–100 [DOI] [PubMed] [Google Scholar]
- 23.Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, et al. Three-dimensional electrospun ECM-based hybrid scaffolds for vascular tissue engineering. Biomaterials 2008;29:2907–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartman O, Zhang C, Adams EL, et al. Biofunctionalization of electrospun PCL-based scaffolds with perlecan domain IV peptide to create a 3-D pharmacokinetic cancer model. Biomaterials 2010;31:5700–5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauthaman K, Venugopal JR, Yee FC, Peh GSL, Ramakrishna S, Bongso A. Nanofibrous substrates support colony formation and maintain stemness of human embryonic stem cells. J Cell Mol Med 2009;13:3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JF, Olson ME, Reno CR, Kulyk W, Wright JB, Hart DA. Molecular and cell biology of skin wound healing in a pig model. Connect Tissue Res 2000;41:195–211 [DOI] [PubMed] [Google Scholar]
- 27.Wang JF, Olson ME, Reno CR, Wright JB, Hart DA. The pig as a model for excisional skin wound healing: characterization of the molecular and cellular biology, and bacteriology of the healing process. Comp Med 2001;51:341–348 [PubMed] [Google Scholar]
- 28.Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N. Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mater Res B Appl Biomater 2003;67:675–679 [DOI] [PubMed] [Google Scholar]
- 29.Zitelli JA. Delayed wound healing with adhesive wound dressings. J Dermatol Surg Oncol 1984;10:709–710 [DOI] [PubMed] [Google Scholar]
- 30.Leipziger LS, Glushko V, DiBernardo B, et al. Dermal wound repair: role of collagen matrix implants and synthetic polymer dressings. J Am Acad Dermatol 1985;12:409–419 [DOI] [PubMed] [Google Scholar]
- 31.Alvarez OM, Mertz PM, Eaglstein WH. The effect of occlusive dressings on collagen synthesis and re-epithelialization in superficial wounds. J Surg Res 1983;35:142–148 [DOI] [PubMed] [Google Scholar]





