Abstract
[Purpose]
The aim of this study was to investigate the effects of aerobic exercise training on a high fat diet (HFD)-induced fatty liver and its metabolic complications in C57BL/6 mice.
[Methods]
Mice at 5-month old (n = 30) were randomly assigned to standard chow (SC + CON, n = 10) and high-fat diet (HFD, n = 20), and they were subjected to SC and HFD, respectively, for 23-week. After 15-week of HFD, mice in the HFD group were further assigned to HFD (HFD + CON, n = 10) or exercise training (HFD + EX, n = 10) groups. The HFD + EX mice were subjected to aerobic treadmill running during the last 8-week of the 23-week HFD course. Outcomes included hepatic steatosis, insulin resistance, and expression of genes involved in mitochondrial function and/or fatty oxidation as well as de novo lipogenesis and/or triacylglycerol (TAG) synthesis.
[Results]
Treadmill running ameliorated impaired glucose tolerance and insulin resistance secondary to the HFD. The beneficial effects of treadmill running were associated with enhanced molecular markers of mitochondrial function and/or fatty acids oxidation (i.e., PPARα and CPT1a mRNAs, pAMPK/AMPK, pACC, and SIRT1 protein) as well as suppressed expression of de novo lipogenesis and/or TAG synthesis (i.e., SREBP1c, lipin1 and FAS mRNAs) in the liver.
[Conclusion]
The current findings suggest that aerobic exercise training is an effective and non-pharmacological means to combat fatty liver and its metabolic complications in HFD-induced obese mice.
Keywords: aerobic exercise, non-alcoholic fatty liver, high-fat diet, insulin resistance, AMPK
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of metabolic disorders characterized predominantly by macrovesicular hepatic steatosis that occurs in the absence of the consumption of alcohol in amounts considered harmful to the liver [1]. NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH), which may progress to endstage liver disease. It is characterized by elevated hepatic triacyglycerol (TAG) storage [1,2].
Hepatic accumulation of fat, mainly in its storage form of TAG, reflects an imbalance between pathways of fatty acid delivery into and fatty acid removal from the liver. Excessive carbohydrate intake is converted to fat by de novo lipogenesis. Recent studies indicate that hepatic de novo lipogenesis is increased in NAFLD as the result of overexpression of sterol-regulatory element binding protein (SREBP)-1c, which is an important transcription factor that regulates the genes to promote the fatty acid and TG synthesis and its target genes, such as fatty acid synthesis (FAS). Additionally, the hepatic fatty acid oxidative pathway is considered to be a part of the pathophysiological effects in the development of NAFLD. The entry flux of FA into the mitochondria is regulated by a carnitine palmitoyl transferase I (CPT I), which is induced transcriptionally by peroxisome proliferatoractivated receptor alpha (PPARα) [3-5].
NAFLD is a reversible condition characterized by obesity, hyperglycemia, and type-2 diabetes mellitus (T2DM). Thus, a healthy lifestyle such as increased physical activity has been recommended as a non-pharmacological means against NAFLD. Increased physical activity (PA) is known to be beneficial because it reduces the risks of T2DM, insulin resistance, hypertension, dyslipidemia, and metabolic syndrome [6,7]. Similarly, exercise training is a healthy lifestyle means to decrease body weight and serum TAG levels and increase insulin sensitivity and high density lipoprotein-cholesterol (HDL-C) [6,7]. In an accelerometer-based PA study involving 3056 subjects, Gerber et al. [8] showed that NAFLD patients with diabetes were in the lowest quartile of average PA as well as moderate to vigorous PA. In another population-based cross-sectional study, Zelber-Sagi et al. [9] showed that NAFLD patients engaged in less aerobic, resistance, or other kinds of PA. In male C57BL/6 mice, Schultz et al. [10] showed that progressive swimming exercise (6 min/day to 60 min/day, 40-60% VO2max, 5 days/week) as an intervention during the last 10-week period of a 22-week standard chow or high-fat-diet course resulted in reduced total body mass and improved insulin sensitivity. The beneficial changes of exercise training were associated with significantly suppressed expression of lipogenic genes and increased expression of oxidative genes in the liver.
Given the increasing prevalence of overweight and obesity, along with physical inactivity, NAFLD is recognized as a major threat to public health in Korea [11]. As a consequence, obtaining mechanistic insights into the beneficial effects of exercise training for NAFLD could contribute to the development of new and improved options to prevent and/or treat clinical conditions associated with the metabolic disorder. However, little is known regarding the molecular mechanism( s) underlying the benefits of exercise training in NAFLD. In this study, thus, we investigated the effects of aerobic exercise training on hepatic fat accumulation and its underlying molecular mechanism(s) in the C57BL/6 obese mice.
METHODS
Animals
In total, 30 male mice (C57BL/6 strain and 5-week old) were purchased from Charles River Laboratories and kept at a pathogen-free facility located at the Sungkyunkwan University School of Medicine (12/12-h light/dark cycle) with free access to tap water and chow. Mice were fed with a high fat for 15-week. The high-fat diet consisted of 20% protein, 60% fat, and 20% carbohydrate diet (D12492, Research Diet). Body weight was recorded weekly. All procedures were approved by the Sungkyunkwan University School of Medicine Institutional Animal Care and Use Committee (IACUC).
Experimental design and exercise protocol
Mice at 5-month old (n = 30) were randomly assigned to standard chow (SC + CON, n = 10) and high-fat diet (HFD, n = 20), and they were subjected to SC and HFD, respectively, for 23-week. After 15-week of HFD, mice in the HFD group were further assigned to HFD (HFD + CON, n = 10) or exercise training (HFD + EX, n = 10) groups. Mice in the HFD + EX group were forced to run on a motorized rodent treadmill (Columbus Instruments, Inc., Columbus, OH) during the last 8-week of the 23-week HFD course at a frequency of 5 days per week. Each time, the HFD + EX mice warmed up for 5 min at a speed of 8 m/min, ran for 45 min at a speed of 10 m/min and at an inclination of 5°, and cooled down for 5 min at a speed of 8 m/min, so a total 55 min duration per session.
Liver histology
Optimal cutting temperature (OCT)-frozen liver samples were cut at 5 μm (CM3050S, Leica Microsystems, Nussloch, Germany). Liver slides were stained with hematoxylin and eosin (H&E) for routine histological examination. Morphological analyses of stained slides were performed using the Leica Qwin image analyzer system with a Leica DMLS microscope.
Glucose and insulin tolerance test
After a 16-h overnight fast, a glucose tolerance test (GTT) was conducted with intraperitoneal injection of glucose at 1.5 g/kg of body weight. Blood was sampled from tail vein before injection (time 0) and 15, 30, 60, and 120 min after glucose injection. After a 4-h fast on another day, an insulin tolerance test (ITT) was conducted with an intraperitoneal injection of 1 U/kg human insulin (Sigma, St. Louis, MO, USA). Blood glucose was checked at 0, 15, 30, 45, and 60 min. Blood glucose was measured with a One Touch II glucose meter (One Touch; Lifescan, Mountain View, CA).
Biochemical assays for blood samples
Blood fasting glucose was checked from tail-vein blood using an automatic glucose monitor (One Touch). Plasma triglycerides, and total cholesterol and liver triglyceride were measured with colorimetric assay kits (Wako, Osaka, Japan). Total liver lipids were extracted with chloroform-methanol (2:1, v/v) mixture according to the Folch method [12]. Plasma insulin levels were measured with a commercial insulin assay ELISA kit (ALPCO Diagnostics, Salem, NH, USA). Serum levels of alanine aminotransferase (ALT) were determined using a Beckman DXC 800 analyzer (Brea, CA, USA).
Total RNA isolation and real-time PCR analysis
Total RNA was extracted from liver tissue using an RNA extraction kit (Ambion; Applied Biosystems, Foster City, CA). Complementary DNA was generated by reverse transcriptase (Invitrogen, Carlsbad, CA) with 1 μg of RNA. Real-time PCR was performed in ABI 7500 PCR system using the TaqMan primer and probe sets, based on 5’ nuclease chemistry using TaqMan minor groove binder (MGB) probes (Table 1). Target mRNA levels were normalized to β-actin as an endogenous control gene.
Table 1.
Mouse Taqman probes
| Gene symbol | Assay ID |
|---|---|
| Peroxisome proliferator-activated receptor alpha (PPARα) | Mm00440939_m1 |
| NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Mm00490758_m1 |
| Carnitine palmitoyltransferase I (CPT1A) | Mm01231183_m1 |
| Fatty acid synthase (FAS) | Mm01204974_m 1 |
| Sterol regulatory element-binding transcription factor 1c (SREBP1c) | Mm00550338_m1 |
| Lipin1 | Mm00550511_m1 |
| Beta-actin (β-actin) | Mm00607939_s 1 |
Western blot
Protein extracts from liver tissues were prepared and homogenized in RIPA buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4 and 1 μg/mL leupeptin) containing a protease inhibitor cocktail. Protein concentrations were measured with the Bradford assay. Briefly, 15 μg of total protein was loaded on a 7.515% sodium dodecyl sulfate/ polyacrylamide gel electrophoresis, separated, and transferred to nitrocellulose membranes. Membranes were blocked in 5% non-fat dry milk/0.05% TBST (0.02 M Tris, 0.15 M NaCl, 0.05% Tween 20, pH 7.2) and incubated overnight at 4℃ with the primary antibody. The primary antibodies for p-AMPKα (Thr172), AMPKα, and p-ACC were obtained from Cell Signaling (Danvers, MA). The primary antibody for SIRT1 was from Abcam (Cambridge, UK). A β-actin (Bethyl Laboratories, Montgomery, TX, USA) antibody was used as loading control. Western blot bands were analyzed using the ImageJ software (ver. 1.42; National Institutes of Health, Bethesda, MD, USA).
Statistical analyses
Data were analyzed using the SPSS software (ver. 16.0). All values are shown as means ± SD. Statistical comparisons of groups were analyzed using unpaired Student’s t-test and one-way ANOVA. In all statistical comparisons, a p value of < 0.05 was considered to indicate statistical significance.
RESULTS
Effects of aerobic exercise training on HFD-induced metabolic complications
Table 2 shows the outcomes of body weight and metabolic parameters. At 23-week, HFD + CON (p < 0.001) and HFD + EX (p < 0.001) mice had significantly higher body weights compared with SC + CON mice. The HFD + EX mice had significantly (p < 0.05) lower body weights compared with HFD + CON mice. There were no differences in the food intake along with treadmill exercise.
Table 2.
Metabolic characteristics
| SC + CON (n = 10) | HFD + CON (n = 10) | HFD + EX (n = 10) | |
|---|---|---|---|
| Body weight (g) | 29.7 ± 2.5a | 47.9 ± 1.0b | 44.7 ± 1.9c |
| Food intake (g/week/mouse) | 17.7 ± 0.9a | 13.1 ± 1.1b | 12.7 ± 1.4b |
| Fasting glucose (mg/dL) | 74.2 ± 9.1a | 145.4 ± 11.6b | 135.4 ± 4.5c |
| Fasting insulin (μU/dL) | 0.09 ± 0.05a | 0.39 ± 0.09b | 0.28 ± 0.05c |
| Serum TG (mg/dL) | 73.2 ± 9.0 | 71.4 ± 9.2 | 73.7 ± 10.0 |
| Serum cholesterol (mg/dL) | 76.6 ± 7.4a | 245.8 ± 48.7b | 198.7 ± 20.0c |
| Liver TG (mg/mg liver tissue) | 24.7 ± 2.4a | 60.1 ± 5.9b | 46.8 ± 6.0c |
TG: triglycerides. Data are presented as means ± SD. Superscripts with different letters (i.e., a-b, b-c) indicate significant group differences.
Multiple comparison used LSD.
With respect to lipid parameters, the HFD + CON (p < 0.001) and HFD + EX (p < 0.001) mice had significantly higher values of total cholesterol than the SC + CON mice. The HFD + EX mice had a significantly (p < 0.05) lower total cholesterol concentration than the HFD + CON mice (Table 2). No significant group differences were found in serum triglyceride (TG) levels among the SC + CON, HFD + CON, and HFD + EX mice (Table 2).
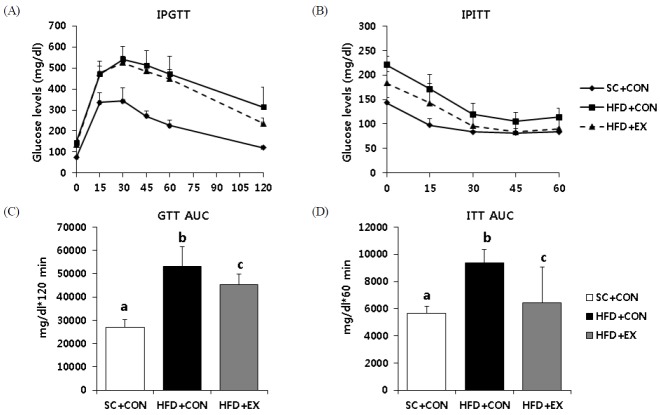
Fig. 1 represents the outcomes of insulin resistance parameters. The HFD + CON (p < 0.001) and HFD + EX (p < 0.001) mice had significantly higher values in areas under the curve (AUC) for the glucose tolerance test (GTT) and the insulin tolerance test (ITT) than the SC mice. The HFD + EX mice had significantly (p < 0.05) lower values of AUC for the GTT and ITT versus HFD + CON mice. Similarly, the HFD + CON and HFD + EX mice had significantly higher values of fasting glucose (p < 0.001) and insulin (p < 0.001) levels compared with SC + CON (Table 2). The HFD + EX mice had significantly lower values of fasting glucose (p < 0.05) and insulin (p < 0.05) concentration versus HFD + CON mice (Table 2).
Fig. 1.
Effects of aerobic exercise training on metabolic parameters (A) Glucose homeostasis was assessed by an intraperitoneal glucose tolerance test (IPGTT) and (B) intraperitoneal insulin tolerance test (IPITT). (C) The area under the curves for GTT and (D) ITT were calculated. Data are presented as means ± SD. Superscripts with different letters (i.e., a-b, b-c) indicate significant group differences. LSD post hoc tests were used for multiple comparisons.
Effects of aerobic exercise training on HFD-induced hepatic steatosis
Non-alcoholic fatty liver disease (NAFLD) is characterized by excess fat accumulation and steatosis in the liver. We determined hepatic steatosis using H&E staining (Fig. 2A). The HFD group had more micro- and macrovesicular steatosis than the SC group. However, 8-week of aerobic exercise training attenuated hepatic steatosis in comparison with HFD + CON (Fig. 2A). Similarly, intrahepatic TG contents, using the Folch method, showed that HFD + CON and HFD + EX mice had greater amounts of hepatic TG content than the SC + CON mice (Fig. 2B). The HFD + EX mice had significantly (p < 0.05) suppressed increases of hepatic TG levels than mice subjected to the HFD only (Fig. 2B).
Fig. 2.
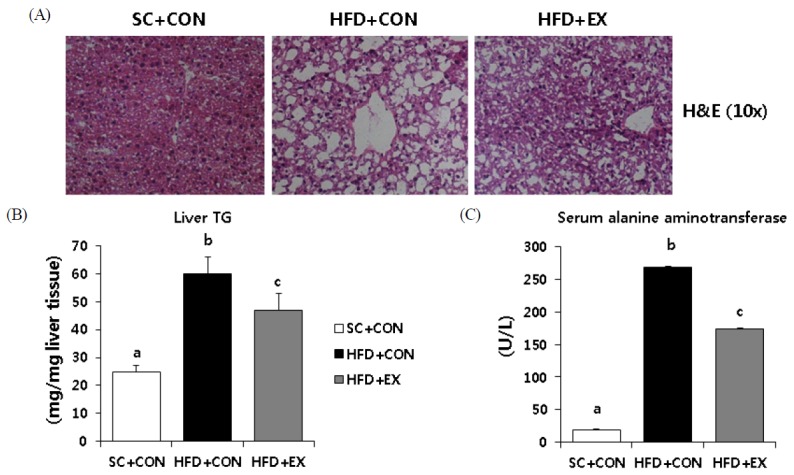
Effects of aerobic exercise training on hepatic steatosis (A) Representative images of hematoxylin and eosin (H&E). Quantification of liver TG and ALT levels is shown in B and C. Data are presented as means ± SD. Superscripts with different letters (i.e., ab, b-c) indicate significant group differences. LSD post hoc tests were used for multiple comparisons.
With respect to liver damage markers, the HFD + CON and HFD + EX mice had significantly (p < 0.001) higher values of serum ALT levels than SC + CON mice (Fig. 2C). The HFD + EX mice had significantly (p < 0.001) lower values of serum ALT levels than the HFD + CON mice (Fig. 2C).
Effects of aerobic exercise training on markers of mitochondrial function and fatty acid oxidation in the liver
Expression of CPT-1a, a rate-limiting step in mitochondrial fatty acid entry, was significantly (p < 0.05) suppressed in HFD + CON mice versus SC + CON and HFD + EX mice (Fig. 3A). However, the HFD + EX mice had significantly (p < 0.05) higher values of CPT-1a mRNA expression than the HFD + CON mice. Additionally, PPARα expression was significantly reduced in HFD + CON (p < 0.001) and HFD + EX (p < 0.05) mice than SC + CON mice. However, the HFD + EX mice had significantly (p < 0.05) higher values of PPARα expression compared with HFD + CON mice (Fig. 3A).
Fig. 3.
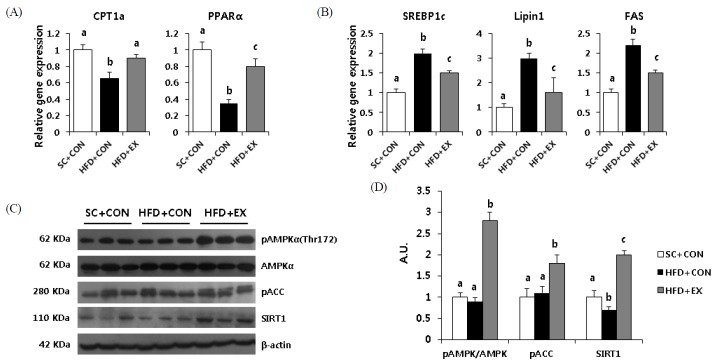
Effects of aerobic exercise training on markers of hepatic lipogenesis (A) Taqman real-time PCR data represent levels of CPT1a and PPARα related to fatty acid oxidation and (B) SREBP1c, lipin1, and FAS including TG synthesis. (C, D) Liver protein was immunoblotted for pAMPK, total AMPK, pACC, and SIRT1. Data were normalized to βactin levels. Data are presented as means ± SD. Superscripts with different letters (i.e., a-b, b-c) indicate significant group differences. LSD post hoc tests were used for multiple comparisons.
Effects of aerobic exercise training on markers of de novo lipogenesis and/or TAG synthesis in the liver
To determine the effects of exercise training on lipid metabolism in the liver, we analyzed the expression of SREBP-1c, lipin1, and FAS (Fig. 3B). Compared with the SC + CON mice, the HFD + CON and HFD + EX mice showed significant increases in SREBP-1c, lipin1, and FAS. On the other hand, the trained mice had markedly normalized levels of SREBP-1c (p < 0.05), lipin1 (p < 0.05), and FAS (p < 0.05) mRNAs compared with the HFD + CON mice.
As in skeletal muscles, AMPK acts as a metabolic master switch regulating the cellular uptake of glucose, the β-oxidation of fatty acids, and mitochondrial biogenesis in the liver. Thus, we determined the effects of exercise training on AMPK and its primary downstream target, ACC, in the liver. The exercise training resulted in a significant (p < 0.001) increase in hepatic pAMPKα (Thr172) and pACC protein levels compared with SC + CON and HFD + CON mice. Similarly, the 23-week HFD, compared with the SC + CON mice, resulted in a significant (p < 0.05) decrease in hepatic SIRT1 protein levels. HFD-induced decreases in the SIRT1 protein level were normalized in the trained mice (p < 0.001).
DISCUSSION
Increased habitual physical activity leads to improve liver histology and blood biochemical variables, such as aminotransferase (ALT), albumin, and glucose, in patients with NAFLD [13]. Cardio/respiratory fitness, independently of total body fat and adiposity, is inversely associated with liver fat content [14]. However, little is known about mechanism(s) by which exercise training induces improvements in fatty liver or the clinical consequences.
In this study, we investigated the effects of exercise training on fatty liver and its metabolic complications induced by a high-fat diet (HFD) in C57BL/6 mice. Compared to SC + CON mice, the mice fed HFD for 23-week had significantly higher body weights. HFD + CON mice showed significantly increased insulin resistance markers, including elevated fasting serum glucose and insulin, along with impaired glucose homeostasis, based on areas under the curves for glucose during both the glucose tolerance test (GTT) and the insulin tolerance test (ITT). ALT and serum total cholesterol were also significantly elevated after the 23-week HFD regimen. Liver histology showed clinical features of hepatic steatosis along with elevated hepatic TG contents. On the other hand, the treadmill running intervention in the last 8-week of the 23-week treatment course ameliorated hepatic steatosis and impaired glucose tolerance and insulin tolerance secondary to HFD. Treadmill running significantly increased PPARα and CPT-1a mRNAs and significantly decreased SREBP-1c, lipin1, and FAS mRNAs. Additionally, treadmill running resulted in higher pAMPK/AMPK, pACC and SIRT1 protein levels in the liver. The findings of this study suggest that exercise training may play an important role in attenuating hepatic steatosis and its metabolic complications, secondary to a high fat diet, by increasing molecular markers of mitochondrial biogenesis and/or fatty acids oxidation and suppressing de novo lipogenesis and/or TG synthesis.
Chronic HFD-induced obese C57BL/6 mice have been used as an experimental model of human metabolic syndrome. HFD promotes hyperglycemia and insulin resistance and increase the body mass [15]. Increased fasting serum insulin and impaired glucose tolerance are two well-known clinical features of HFD-induced insulin resistance [16]. Insulin is a key regulator of sterol regulatory element binding protein-1c (SREBP-1c), which is a key transcription factor of the genes encoding key enzymes for de novo lipogenesis, such as fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) [17]. In this study, we found that levels of SREBP-1c were suppressed significantly in the trained mice. Thus, it seems reasonable to suggest that treadmill running could contribute to attenuating hepatic TG accumulation, via suppressed de novo lipogenesis [18].
PPARα is a lipid-sensing member of the nuclear receptor superfamily that regulates the oxidation of fatty acids [19]. Hashimoto et al. (2000) reported that mice with insufficiently expressed PPARα had excessive accumulation of hepatic TG [20]. Purushotham et al. (2009) showed that liver-specific deletion of SIRT1, which is an important regulator of energy homeostasis in response to nutrient availability, impaired PPARα signaling [21]. In this study, HFD-induced downregulation of hepatic PPARα expression was alleviated with the exercise training, suggesting that exercise training could contribute to the oxidation of fatty acids and hepatic TG accumulation via enhanced expression of PPARα and its target genes, such as CPT1a.
Recently, several studies demonstrated that 5’ AMP-activated protein kinase (AMPK) plays an important role in lipid metabolism in the liver. AMPK is well known as an enzyme that plays a role in cellular energy homeostasis [22]. A previous study by Takekoshi et al. (2006) reported that a long-term exercise training induced increased phosphorylation of hepatic AMPK in rats, and thereby the training-induced activation of AMPK stimulated oxidation of fatty acids while inhibiting lipid synthesis. Increased AMPK induces the βoxidation of fatty acids and attenuates lipogenesis [23]. In this study, we found that HFD + EX mice had significantly increased pAMPKα and pACC protein levels in the liver, versus SC + CON and HFD + CON mice. Compared with SC + CON or HFD + CON mice, HFD + EX mice also had significantly increased hepatic SIRT1 levels. SIRT1, a NAD + dependent histone/protein deacetylase, is a stimulator of pAMPK expression, implying that increased SIRT1 expression could have contributed to attenuated HFD-induced lipid accumulation in the liver by upregulating and/or activating AMPK [24].
CONCLUSIONS
In summary, we found that the beneficial effects of exercise training on the reversal of HFD-induced fatty liver were associated with enhanced mitochondrial biogenesis and/or fatty acid oxidation (i.e., increased AMPK/SIRT1, PPARα, and CPT1a genes) as well as suppressed de novo lipogenesis and/or TG synthesis (decreased SREBP-1c, FAS, and lipin1 genes), supporting aerobic exercise training as an effective and non-pharmacological means to combat HFD-induced fatty liver and its metabolic complications.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant, funded by the Korean Government (NRF-2011-32A-G00049).
REFERENCES
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Kim S. H., Lee J. W., Hwang H. J. Associations between combinations of body mass index plus nonalcoholic fatty liver disease and diabetes mellitus among Korean adults. Asia Pac J Clin Nutr. 2011;20:14–20. [PubMed] [Google Scholar]
- 3.Tessari P., Coracina A., Cosma A., Tiengo A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2009;19:291–302. doi: 10.1016/j.numecd.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Greco D., Kotronen A., Westerbacka J., Puig O., Arkkila P., Kiviluoto T., Laitinen S., Kolak M., Fisher R. M., Hamsten A., Auvinen P., Yki-Järvinen H. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 5.Fabbrini E., Magkos F., Mohammed B. S., Pietka T., Abumrad N. A., Patterson B. W., Okunade A., Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueno T., Sugawara H., Sujaku K., Hashimoto O., Tsuji R., Tamaki S., Torimura T., Inuzuka S., Sata M., Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 7.Hickman I. J., Jonsson J. R., Prins J. B., Ash S., Purdie D. M., Clouston A. D., Powell E. E. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–419. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber L., Otgonsuren M., Mishra A., Escheik C., Birerdinc A., Stepanova M., Younossi Z. M. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36:772–781. doi: 10.1111/apt.12038. [DOI] [PubMed] [Google Scholar]
- 9.Zelber-Sagi S., Nitzan-Kaluski D., Goldsmith R., Webb M., Zvibel I., Goldiner I., Blendis L., Halpern Z., Oren R. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology. 2008;48:1791–1798. doi: 10.1002/hep.22525. [DOI] [PubMed] [Google Scholar]
- 10.Schultz A., Mendonca L. S., Aguila M. B., Mandarim-de-Lacerda C. A. Swimming training beneficial effects in a mice model of nonalcoholic fatty liver disease. Exp Toxicol Pathol. 2012;64:273–282. doi: 10.1016/j.etp.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Johnson N. A., Sachinwalla T., Walton D. W., Smith K., Armstrong A., Thompson M. W., George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 12.Folch J., Lees M., Sloane, Stanley G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 13.American Gastroenterological Association American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1702–1704. doi: 10.1053/gast.2002.36569. [DOI] [PubMed] [Google Scholar]
- 14.Church T. S., Kuk J. L., Ross R., Priest E. L., Biltoff E., Blair S. N. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023–2030. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Do G. M., Oh H. Y., Kwon E. Y., Cho Y. Y., Shin S. K., Park H. J., Jeon S. M., Kim E., Hur C. G., Park T. S., Sung M. K., McGregor R. A., Choi M. S. Long-term adaptation of global transcription and metabolism in the liver of high-fat diet-fed C57BL/6J mice. Mol Nutr Food Res. 2011;55(Suppl. 2):S173–185. doi: 10.1002/mnfr.201100064. [DOI] [PubMed] [Google Scholar]
- 16.Brown M. S., Goldstein J. L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Chen G., Liang G., Ou J., Goldstein J. L., Brown M. S. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Utzschneider K. M., K. M. S. E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 19.Gulick T., Cresci S., Caira T., Moore D. D., Kelly D. P. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc Natl Acad Sci U S A. 1994;91:11012–11016. doi: 10.1073/pnas.91.23.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto T., Cook W. S., Qi C., Yeldandi A. V., Reddy J. K., Rao M. S. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem. 2000;275:28918–28928. doi: 10.1074/jbc.M910350199. [DOI] [PubMed] [Google Scholar]
- 21.Purushotham A., Schug T. T., Xu Q., Surapureddi S., Guo X., Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viollet B., Foretz M., Guigas B., Horman S., Dentin R., Bertrand L., Hue L., Andreelli F. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol. 2006;574(Pt 1):41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takekoshi K., Fukuhara M., Quin Z., Nissato S., Isobe K., Kawakami Y., Ohmori H. Long-term exercise stimulates adenosine monophosphate-activated protein kinase activity and subunit expression in rat visceral adipose tissue and liver. Metabolism. 2006;55:1122–1128. doi: 10.1016/j.metabol.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Hou X., Xu S., Maitland-Toolan K. A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T. J., Cohen R. A., Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]