Abstract
[Purpose]
Attention-deficit/hyperactivity disorder (ADHD) is a heritable, chronic, neurobehavioral disorder that is characterized by hyperactivity, inattention, and impulsivity. It is commonly believed that the symptoms of ADHD are closely associated with hypo-function of the dopamine system. Dopamine D2 receptor activation decreases the excitability of dopamine neurons, as well as the release of dopamine. Physical exercise is known to improve structural and functional impairments in neuropsychiatric disorders. We investigated the therapeutic effect of exercise on ADHD.
[Methods]
Open field task and elevated-plus maze task were used in the evaluation of hyperactivity and impulsivity, respectively. Dopamine D2 receptor expression in the substantia nigra and striatum were evaluated by western blotting.
[Results]
The present results indicated that ADHD rats showed hyperactivity and impulsivity. Dopamine D2 receptor expression in the substantia nigra and striatum were increased in ADHD rats. Exercise alleviated hyperactivity and impulsivity in ADHD rats. Furthermore, dopamine D2 receptor expression in ADHD rats was also decreased by exercise.
[Conclusion]
We thus showed that exercise effectively alleviates ADHD-induced symptoms through enhancing dopamine D2 expression in the brain.
Keywords: attention-deficit/hyperactivity disorder, hyperactivity, impulsivity, substantia nigra, dopamine D2 receptors, exercise
INTRODUCTION
Attention deficit-hyperactivity disorder (ADHD) is the most common neurobehavioral disorder affecting children between 6 and 17 years of age. It occurs in about 3-6% of school-aged children [1]. ADHD is a heritable, chronic, neurobehavioral disorder that is characterized by hyperactivity, inattention, and impulsivity [2]. It is commonly believed that the symptoms of ADHD are closely associated with hypo-function of the dopamine system [3].
Dopamine plays a critical role in mediating neuronal motor control, cognition, emotion, vascular function, and event prediction. Dysfunction of the dopaminergic system in the brain is implicated in several neuropsychological diseases, such as Parkinson's disease, ADHD, and schizophrenia [1]. Several studies have suggested that dysfunctional dopamine signaling in the substantia nigra is one of the most likely mechanisms of behavioral symptoms of ADHD [4]. Substantia nigra is the crucial region of dopamine production and signaling, and its dopamine neurons project abundantly into the striatum. Dopamine receptors and their downstream signals play a pivotal role in the dopamine pathway. Dopamine receptors are divided into 2 distinct subtypes: D1-like receptor family including dopamine D1 and D5 receptors, and D2-like receptor family including dopamine D2, D3, and D4 receptors [5]. Dopamine D2 receptors are inhibitory and their activation decreases the excitability of dopamine neurons and the release of dopamine [6].
Stimulants including methylphenidate and amphetamines are considered as first-line therapy for ADHD patients. The stimulants interact with and inhibit the dopamine and norepinephrine transporter, thereby inhibiting the reuptake of dopamine and norepinephrine. However, these medications have multiple side effects, toxicity, drug interaction or potential for abuse [5].
Several studies have suggested that exercise or physical activity improves structural and functional impairments in neuropsychiatric disorders [7-9]. Animal studies have shown that exercise activates the dopaminergic system and increases dopamine availability in the substantia nigra and striatum [10,11].Physical activity improves motor performance and social behavioral problems, and alleviates hyperactivity in ADHD children [12].
Despite the many studies on the effect of physical exercise on abnormal brain function, none has addressed the effect of exercise on behavioral symptoms and dopamine pathway in ADHD rats. Therefore, we investigated the effect of exercise on hyperactivity, impulsivity and nigrostriatal dopamine D2 receptor expression in ADHD rats.
METHODS
Animals and treatments
Adult male spontaneously hypertensive rats (SHR) and Wistar-Kyoto (WKY) rats, weighing 210 ± 10 g (7 weeks old) were obtained from a commercial breeder (Orient Bio Co., Seoul, Korea). SHRs were used as the ADHD animal model, since SHR displays the major symptoms of ADHD, such as inattention, hyperactivity, and impulsiveness [13]. WKYs were used as the control, as in the previous study [14]. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. The animals were housed under controlled temperature (23 ± 5℃) and lighting conditions (07:00-19:00), with food and water made available ad libitum throughout the experiments. Animals were randomly divided into 4 groups (n = 12 in each group): Control group (WKY rats), ADHD group (SHR rats), ADHD & Exercise group, and ADHD & Methylphenidate- treated group. The rats in the methylphenidate-treated group received 1 mg/kg MPH (Ritalin®, Novartis Co., Basel, Switzerland) orally once a day for 28 consecutive days, as in a previous study [9].
Treadmill exercise protocol
Rats in the exercise groups were forced to run on a treadmill for 30 min once a day, 7 times a week, for a total of 28 days. Exercise load for the exercise groups consisted of running at a speed of 2 meters/min for the first 5 min, at a speed of 5 meters/min for the next 5 min, and at a speed of 8 meters/min for the last 20 min, with 0° inclination. This intensity corresponded to the low-intensity treadmill exercise (% maximal oxygen consumption) according to the rats in this age [15].
Open-field task
The open-field task was conducted 1 day prior to the initiation of the experiment using a previously described method, in order to confirm hyperactivity in the ADHD rats [9]. The animals were randomly assigned to an order of testing and placed in a white square open-field arena (100×100 cm) made of wood, enclosed by 40-cm-high walls and exposed to strong illumination (200 lux). The arena was divided into 25 squares (20×20 cm), consisting of 9 central and 16 peripheral squares. The animals were placed in the center of the arena; they were subsequently allowed to explore the environment for 1 min. The number of squares crossed was recorded for the next 5 min. The activity of each rat was repeatedly assessed using the open-field task at 9, 18 and 26 days following the initiation of the experiment.
Elevated plus maze task
The elevated plus maze task is a well-established test to determine the levels of impulsivity in rats. The elevated plus maze task was performed 28 days following the initiation of the experiment, according to a previously described method [16]. The plus maze consisted of black plexiglass with 2 open arms (50×10×36 cm) and 2 closed arms (50×10×36 cm), arranged such that the 2 arms were opposite and connected by a central platform (10×10 cm). The whole plus maze was elevated 60 cm above the floor and illuminated by a 100-watt light bulb fixed 2 m above the maze floor. During the test, each rat was placed on the central platform of the maze with their head facing an open arm. The total time spent in the open arms was collected over a period of 7 min.
Western blotting for the detection of dopamine D2 receptor expression
The substantia nigra and striatum tissues were immediately frozen at -80℃ following collection. The tissues of each brain area were homogenized on ice and lysed in a lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% deoxycholic acid, 1% Nonidet P40, 0.1% SDS, 1 mM PMSF and 100 mg/ml leupeptin. The protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). A total of 30 μg protein was separated on a SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane. Mouse anti-actin (1:500; Santa Cruz Biotechnology, Inc.) and mouse anti-dopamine D2 receptor antibody (1:1,000; Santa Cruz Biotechnology, Inc.) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibodies for actin and dopamine D2 receptor (1:2,000; Vector Laboratories) were used as the secondary antibodies. Experiments were performed in normal lab conditions and at room temperature, with the exception of the membrane transfer. The membrane transfer was performed at 4℃ using a cold pack and prechilled buffer. Bands were detected with an enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology, Inc.).
Data analysis
The expression levels of the dopamine D2 receptor in the substantia nigra and striatum were assessed by band densitometry using Image-Pro® Plus software (Media Cybernetics Inc., Silver Spring, MD, USA). Statistical analysis was performed by 1-way ANOVA followed by Duncan’s post-hoc test, and the results were expressed as the mean ± standard error of the mean (SEM). Significance was set as p <.05.
RESULTS
Effect of exercise on hyperactivity in spontaneous hypertensive rats
The levels of activity were determined by the open-field task performed 1 day prior to the initiation of the experiment. Activity scores were 11.22 ± 1.67 in the Control group, and 63.35 ± 1.02 in the ADHD group (Fig. 1a). ADHD rats were hyperactive, demonstrating symptoms of ADHD. The activity score on the open-field task was determined during the experimental period. The activity scores in the first task were 11.32 ± 1.53 in the Control group, 61.57 ± 3.01 in the ADHD group, 61.03 ± 2.96 in the ADHD & Exercise group, and 53.68 ± 3.96 in the ADHD & Methylphenidate-treated group. The activity scores in the second task were 9.68 ± 1.78 in the Control group, 56.58 ± 3.11 in the ADHD group, 58.63 ± 3.63 in the ADHD & Exercise group, and 49.52 ± 2.65 in the ADHD & Methylphenidate-treated group. The activity scores in the third task were 9.76 ± 1.48 in the Control group, 50.39 ± 2.67 in the ADHD group, 37.29 ± 1.62 in the ADHD & Exercise group, and 39.25 ± 2.91 in the ADHD & Methylphenidate-treated group.
Fig. 1.
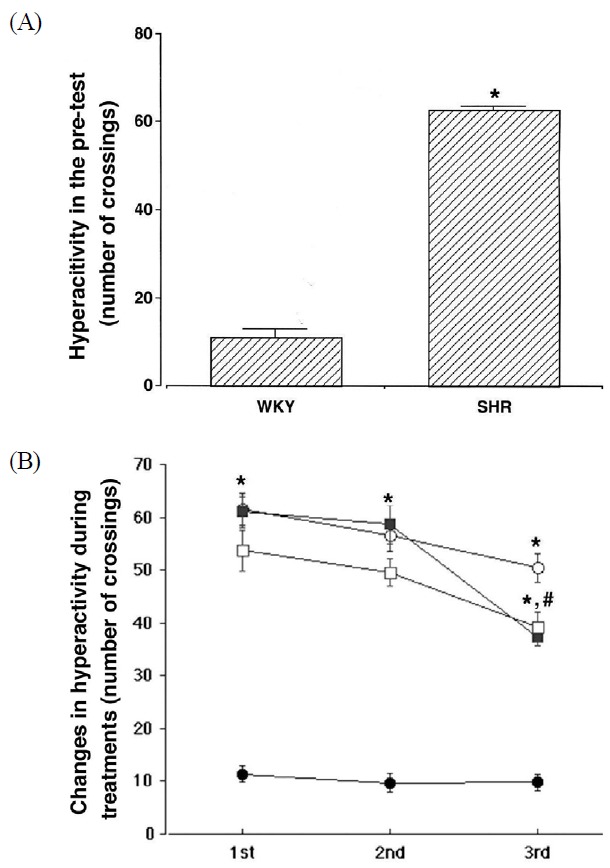
Results of the behavioral tests for hyperactivity in the open-field task. The results are presented as the means ± standard error of the mean. (a) Hyperactivity in the pre-test, (b) Changes in hyperactivity during treatments; (●) Control group, (○) ADHD group, (■) ADHD & Exercise group, (□) ADHD & Methylphenidate-treated group. * represents P < 0.05 compared to Control group. # represents P < 0.05 compared to the ADHD group.
The activity score in the ADHD group was higher, as compared to the Control group (p < .05), however the activity score was decreased in both, the exercise, and methylphenidate-treated groups (p < .05; Fig. 1b). The results also suggested that hyperactivity was further reduced with exercise and long term methylphenidate treatment.
Effect of exercise on impulsivity in the spontaneous hypertensive rats
Impulsivity was assessed using the elevated plus maze task. The latency of staying in the open arm in the elevated plus maze task was presented in Fig. 2. The latency time in the open arm was 52.23 ± 9.58 in the Control group, and 189.35 ± 20.68 in the ADHD group, 154.21 ± 8.02 in the ADHD & Exercise group, and 143.96 ± 15.29 in the ADHD & Methylphenidate-treated group. The number of entrances into the open arm was 1.71 ± 0.81 in the Control group, 6.91 ± 0.93 in the ADHD group, 5.79 ± 0.58 in the ADHD & Exercise group, and 5.63 ± 0.86 in the ADHD & Methylphenidate-treated group.
Fig. 2.
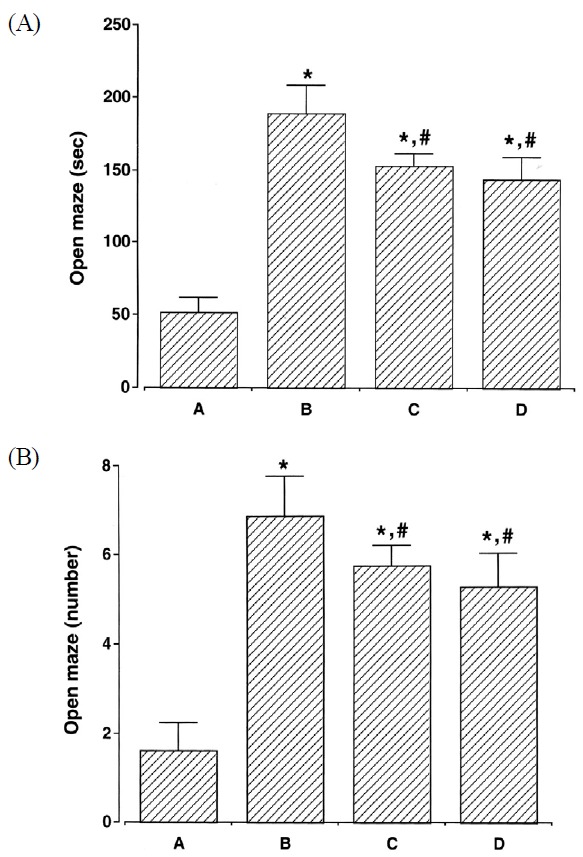
Results of the behavioral test for impulsivity in the elevated plus maze task. The results are presented as the means ± standard error of the mean. (a) Time spent in open arms, (b) Number of arm entries in open arms; (A) Control group, (B) ADHD group, (C) ADHD & Exercise group, (D) ADHD & Methylphenidate-treated group. * represents P < 0.05 compared to Control group. # represents P < 0.05 compared to the ADHD group.
The results showed that the ADHD group demonstrated a significant increase in the latency time and the number of entrances into the open arm, as compared with the Control group (p < .05), however these were significantly decreased in the exercise and methylphenidate-treated groups (p < .05). Interestingly, the latency time and the number of entrances into the open arm were not significantly different between the ADHD & Exercise and the ADHD & Methylphenidate-treated groups. The ADHD group thus demonstrated enhanced impulsive activity, as compared to the Control group (p < .05), whereas impulsivity was inhibited in both, the exercise, and methylphenidate-treated groups (p <.05).
Effect of exercise on dopamine D2 receptor expression in the substantia nigra of spontaneous hypertensive rats
Western blot analysis results of dopamine D2 receptor expression in the substantia nigra were presented in Fig. 3. When the Control group band density was expressed as 1.00, densitometry values were 2.03 ± 0.05 in the ADHD group, 1.15 ± 0.07 in the ADHD & Exercise group, and 1.09 ± 0.05 in the ADHD & Methylphenidate-treated group.
Fig. 3.
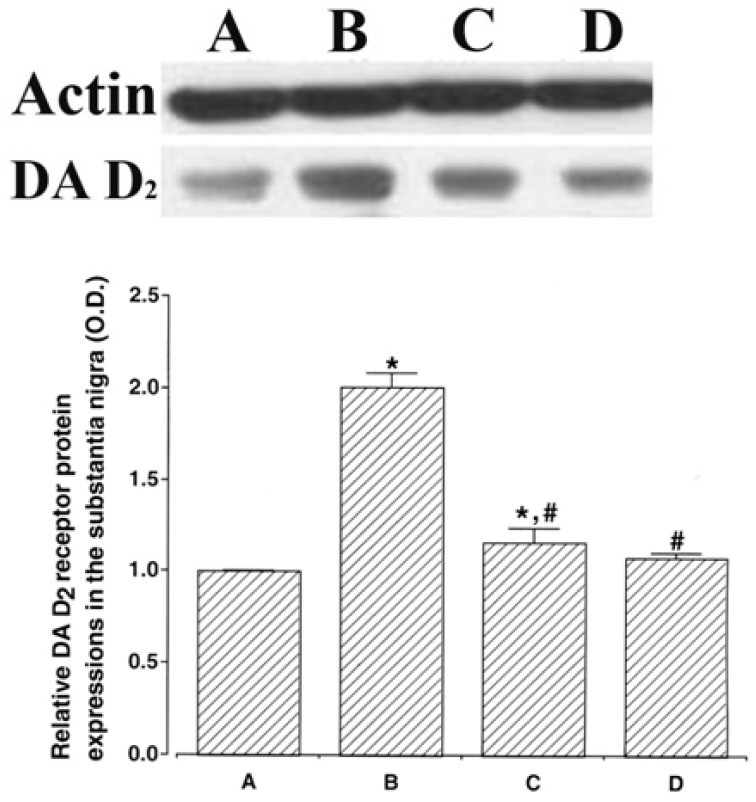
Dopamine D2 receptor (DA D2) expression levels in the substantia nigra of spontaneous hypertensive rats. Actin (43 kDa) was used as the internal control. The results are presented as the means ± standard error of the mean. (A) Control group, (B) ADHD group, (C) ADHD & Exercise group, (D) ADHD & Methylphenidate-treated group. * represents P < 0.05 compared to Control group. # represents P < 0.05 compared to the ADHD group; O.D., optical density.
The dopamine D2 receptor expression levels in the substantia nigra were higher in the ADHD group, as compared to the Control group (p < .05). By contrast, the dopamine D2 receptor expression levels in the ADHD group were suppressed in the ADHD & Exercise group and the ADHD & Methylphenidate-treated group (p <.05).
Effect of exercise on dopamine D2 receptor expression in the striatum of spontaneous hypertensive rats
Western blot analysis results of dopamine D2 receptor expression in the striatum were presented in Fig. 4. When the Control group band density was expressed as 1.00, densitometry values were 5.95 ± 0.04 in the ADHD group, 4.11 ± 0.13 in the ADHD & Exercise group, and 3.83 ± 0.07 in the ADHD & Methylphenidate-treated group.
Fig. 4.
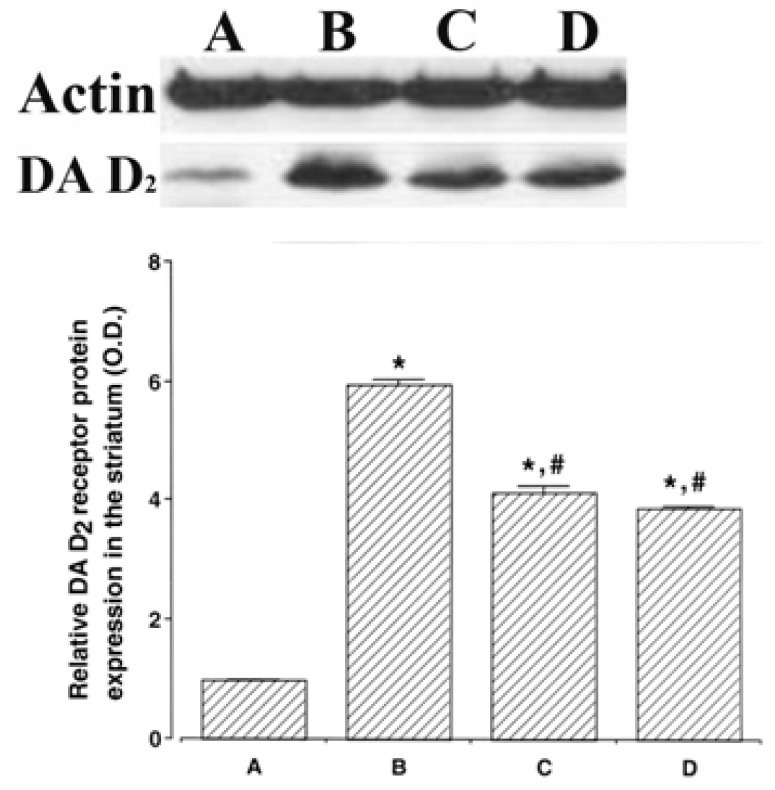
Dopamine D2 receptor (DA D2) expression levels in the striatum of spontaneous hypertensive rats. Actin (43 kDa) was used as the internal control. The results are presented as the means ± standard error of the mean. (A) Control group, (B) ADHD group, (C) ADHD & Exercise group, (D) ADHD & Methylphenidate-treated group. * represents P < 0.05 compared to Control group. # represents P < 0.05 compared to the ADHD group; O.D., optical density.
The dopamine D2 receptor expression levels in the striatum were higher in the ADHD group, as compared with the Control group (p < .05). By contrast, the dopamine D2 receptor expression levels in the ADHD group were inhibited in the ADHD & Exercise group and the ADHD & Methylphenidate-treated group (p <.05).
DISCUSSION
SHR is a valid and currently accepted model of ADHD [17]. The SHRs are known to display hyperactivity, impulsivity, poor sustained attention, and deficits in learning and memory processes in comparison with WKY rats. SHRs were used as the animal model of ADHD and showed hyperactivity, as compared to the control WKY rats (Fig. 1a).
Some studies have shown that regular physical exercise improves many areas of behavior in children with ADHD [18,19]. Archer and Richard suggested that exercise provides beneficial effects against anxiety, depression, hyperactivity, and poor impulse control [20]. The current data indicated that hyperactivity in ADHD rats was decreased by exercise (Fig. 1b). Exercise also ameliorated impulsivity in ADHD rats (Fig 2). The results of the present study showed that exercise alleviates behavioral symptoms of ADHD rats.
The neurotransmitter dopamine controls many functions including movement, cognition, mood and reward. Dopamine D2 receptors play an important role in regulating dopamine neuronal activity and the synthesis, release and uptake of dopamine. At the subcellular level, dopamine D2 receptor is distributed in both presynaptic and postsynaptic compartments, including dendrites and spines, and the axon terminal of both excitatory- and inhibitory-like synapses [21]. Activation of dopamine D2-like receptors decreases the excitability of dopamine neurons and release of dopamine [1].Hyperactivity in locomotion and extremely increased reward behavior with deletion of dopamine D2 receptor were reported in an experimental mouse model of ADHD [22]. Moreover, a meta-analysis study supported the association of impulsive-addictive-compulsive behavior with dopamine D2 receptor [23].
Physical activity activates the dopaminergic system and increases dopamine availability in the central nerve system [24]. Exercise also induces functional alteration of dopamine receptors in the nigrostriatal system that is related to behavioral responses [25]. The expression of the dopamine D2 receptor in substantia nigra and striatum were increased in the ADHD rats, as compared to control rats. However, exercise suppressed dopamine D2 receptor expression in ADHD rats (Fig. 3,4). The inhibitory effect of exercise on dopamine D2 receptor expression may be associated with the alleviation of symptoms in ADHD rats.
Recent study investigated the time-dependent effect of treadmill exercise on hyperactivity and dopamine expression in the striatum and substantia nigra of ADHD rats. Low-intensity treadmill exercise (8m/min) for 10, 30, and 60 min once a day, 5 times a week, for 4 weeks was performed individually. Treadmill exercise for 30 min most potently suppressed hyperactivity and also increased dopamine levels in ADHD rats. However, the treadmill exercise for 10 min showed no significant effects on hyperactivity and nigrostriatal dopamine activation [26]. The treadmill exercise protocol was according to the previous reported study [26]; the results showed that treadmill exercise suppressed hyperactivity and D2 receptor expression in ADHD rats.
In conclusion, we have shown that exercise alleviates ADHD-induced hyperactivity and impulsivity. The expression of dopamine D2 receptor in ADHD rats is decreased by exercise. Exercise was at least as effective as methylphenidate treatment in ADHD rats. These results suggested that exercise might be of therapeutic value in the treatment of ADHD.
REFERENCES
- 1.Wu J, Xiao H, Sun H, Zou L, Zhu L. Role of dopamine receptors in ADHD: A systematic meta-analysis. Mol Neurobiol. 2012;45(3):605–20. doi: 10.1007/s12035-012-8278-5. [DOI] [PubMed] [Google Scholar]
- 2.Robinson AM, Bucci DJ. Individual and combined effects of physical exercise and methylphenidate on orienting behavior and social interaction in spontaneously hypertensive rats. Behav Neurosci. 2014;128(6):703–12. doi: 10.1037/bne0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viggiano D, Vallone D, Sadile A. Dysfunctions in dopamine systems and ADHD: Evidence from animals and modeling. Neural Plast. 2004;11(1-2):97–114. doi: 10.1155/NP.2004.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowton E, Saunders C, Erreger K, Sakrikar D, Matthies HJ, Sen N, Jessen T, Colbran RJ, Caron MG, Javitch JA, Blakely RD, Galli A. Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. J Neurosci. 2010;30(17):6048–57. doi: 10.1523/JNEUROSCI.5094-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A, Couture J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD) Ann Pharmacother. 2014;48(2):209–25. doi: 10.1177/1060028013510699. [DOI] [PubMed] [Google Scholar]
- 6.Ford C. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baek S, Jun T, Kim K, Shin M, Kang S, Kim C. Effects of postnatal treadmill exercise on apoptotic neuronal cell death and cell proliferation of maternal-separated rat pups. Brain and Development. 2012;34(1):45–56. doi: 10.1016/j.braindev.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Cho H, Shin M, Song W, Jun T, Lim B, Kim Y, Kim C. Treadmill exercise alleviates short-term memory impairment in 6-hydroxydopamine-induced Parkinson’s rats. J Exerc Rehabil. 2013;9(3):354–61. doi: 10.12965/jer.130048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Heo H, Kim D, Ko I, Lee S, Kim S, Kim B, Kim T, Ji E, Kim J. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci Lett. 2011;504(1):35–9. doi: 10.1016/j.neulet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 10.Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: Relation to the speed of running. Brain Res Bull. 1994;35(1):41–9. doi: 10.1016/0361-9230(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 11.Sutoo D, Akiyama K. Regulation of brain function by exercise. Neurobiol Dis. 2003;13(1):1–14. doi: 10.1016/s0969-9961(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 12.Majorek M, Tüchelmann T, Heusser P. Therapeutic Eurythmy-movement therapy for children with attention deficit hyperactivity disorder (ADHD): A pilot study. Complementary therapies in nursing and midwifery. 2004;10(1):46–53. doi: 10.1016/S1353-6117(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 13.Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neuroscience & Biobehavioral Reviews. 2000;24(1):31–9. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins ME, Sharma M, Evans GC, Bucci DJ. Voluntary physical exercise alters attentional orienting and social behavior in a rat model of attention-deficit/hyperactivity disorder. Behav Neurosci. 2009;123(3):599–606. doi: 10.1037/a0015632. [DOI] [PubMed] [Google Scholar]
- 15.Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol. 1979;47(6):1278–83. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- 16.Seo J, Kim T, Kim C, Sung Y, Lee S. Treadmill exercise during pregnancy ameliorates posttraumatic stress disorderinduced anxietylike responses in maternal rats. Mol Med Rep. 2013;7(2):389–95. doi: 10.3892/mmr.2012.1197. [DOI] [PubMed] [Google Scholar]
- 17.Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Res Rev. 2003;42(1):1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- 18.Smith LC, Tamm L, Hughes CW, Bernstein IH. Separate and overlapping relationships of inattention and hyperactivity/impulsivity in children and adolescents with attention-deficit/hyperactivity disorder. ADHD Attention Deficit and Hyperactivity Disorders. 2013;5(1):9–20. doi: 10.1007/s12402-012-0091-5. [DOI] [PubMed] [Google Scholar]
- 19.Verret C, Guay MC, Berthiaume C, Gardiner P, Beliveau L. A physical activity program improves behavior and cognitive functions in children with ADHD: An exploratory study. J Atten Disord. 2012;16(1):71–80. doi: 10.1177/1087054710379735. [DOI] [PubMed] [Google Scholar]
- 20.Archer T, Kostrzewa RM. Physical exercise alleviates ADHD symptoms: Regional deficits and development trajectory. Neurotoxicity research. 2012;21(2):195–209. doi: 10.1007/s12640-011-9260-0. [DOI] [PubMed] [Google Scholar]
- 21.Negyessy L, Goldman-Rakic PS. Subcellular localization of the dopamine D2 receptor and coexistence with the calcium-binding protein neuronal calcium sensor-1 in the primate prefrontal cortex. J Comp Neurol. 2005;488(4):464–75. doi: 10.1002/cne.20601. [DOI] [PubMed] [Google Scholar]
- 22.Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388(6642):586–9. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- 23.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE. Dopamine D2 receptor gene variants: Association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics and Genomics. 1995;5(3):121–41. doi: 10.1097/00008571-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 24.O’dell S, Gross N, Fricks A, Casiano B, Nguyen T, Marshall J. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience. 2007;144(3):1141–51. doi: 10.1016/j.neuroscience.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 25.Dey S, Singh R. Modification of apomorphine-induced behaviour following chronic swim exercise in rats. Neuroreport. 1992;3(6):497–500. doi: 10.1097/00001756-199206000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Ji E, Kim C, Park J, Bahn G. Duration-dependence of the effect of treadmill exercise on hyperactivity in attention deficit hyperactivity disorder rats. J Exerc Rehabil. 2014;10(2):75–80. doi: 10.12965/jer.140107. [DOI] [PMC free article] [PubMed] [Google Scholar]