Abstract
Background Neisseria meningitidis serogroup C (NmC) outbreaks occur infrequently in the African meningitis belt; the most recent report of an outbreak of this serogroup was in Burkina Faso, 1979. Médecins sans Frontières (MSF) has been responding to outbreaks of meningitis in northwest Nigeria since 2007 with no reported cases of serogroup C from 2007-2012. MenAfrivac®, a serogroup A conjugate vaccine, was first used for mass vaccination in northwest Nigeria in late 2012. Reactive vaccination using polysaccharide ACYW135 vaccine was done by MSF in parts of the region in 2008 and 2009; no other vaccination campaigns are known to have occurred in the area during this period. We describe the general characteristics of an outbreak due to a novel strain of NmC in Sokoto State, Nigeria, in 2013, and a smaller outbreak in 2014 in the adjacent state, Kebbi. Methods Information on cases and deaths was collected using a standard line-list during each week of each meningitis outbreak in 2013 and 2014 in northwest Nigeria. Initial serogroup confirmation was by rapid Pastorex agglutination tests. Cerebrospinal fluid (CSF) samples from suspected meningitis patients were sent to the WHO Reference Laboratory in Oslo, where bacterial isolates, serogrouping, antimicrobial sensitivity testing, genotype characterisation and real-time PCR analysis were performed. Results In the most highly affected outbreak areas, all of the 856 and 333 clinically suspected meningitis cases were treated in 2013 and 2014, respectively. Overall attack (AR) and case fatality (CFR) rates were 673/100,000 population and 6.8% in 2013, and 165/100,000 and 10.5% in 2014. Both outbreaks affected small geographical areas of less than 150km2 and populations of less than 210,000, and occurred in neighbouring regions in two adjacent states in the successive years. Initial rapid testing identified NmC as the causative agent. Of the 21 and 17 CSF samples analysed in Oslo, NmC alone was confirmed in 11 and 10 samples in 2013 and 2014, respectively. Samples confirmed as NmC through bacterial culture had sequence type (ST)-10217. Conclusions These are the first recorded outbreaks of NmC in the region since 1979, and the sequence (ST)-10217 has not been identified anywhere else in the world. The outbreaks had similar characteristics to previously recorded NmC outbreaks. Outbreaks of NmC in 2 consecutive years in northern Nigeria indicate a possible emergence of this serogroup. Increased surveillance for multiple serogroups in the region is needed, along with consideration of vaccination with conjugate vaccines rather than for NmA alone.
Keywords: disease outbreak, infectious disease, meningococcal disease, Neisseria meningitidis, Nigeria
Background
In the African meningitis belt, and specifically within northern Nigeria, most meningitis outbreaks have been caused by N. meningitides serogroup A (NmA), including large (10-100 thousand cases) and widespread outbreaks 1 , 2 , 3 , 4. In the past 15 years, there have been an increasing number of large outbreaks caused by N. meningitidis serogroups W135 and X 5 , 6 , 7 , 8 , 9. Outbreaks due to Neisseria meningitidis serogroup C (NmC) have also occurred but were smaller and less frequent than NmA outbreaks 4. The last NmC outbreak in this region occurred in 1979 in Burkina Faso with 539 cases reported (attack rate (AR) 517/100,000) 10. Outbreaks caused by NmC in northern Nigeria are rare, with the last and only recorded outbreak in 1975 with no detailed report published 4. Other notable NmC outbreaks occurred in the 1970s in Sao Paulo, Brazil and Ho Chi Minh, Vietnam with 2005 (11/100,000 people) and 1015 (>20/100,000 people) cases respectively 4. In the USA, morbidity and mortality are higher among young adults in outbreaks caused by NmC compared with other serogroups 11.
Médecins sans Frontières (MSF) has conducted surveillance and response to cerebrospinal meningitis (CSM) outbreaks in northwest Nigeria since 2007. Meningitis outbreaks due to NmA in northwest Nigeria in 2008 and 2009 were recorded with 7601 and 9442 cases, respectively; MSF carried out reactive vaccination using polysaccharide ACYW135 vaccine in affected parts of the region in these years. An outbreak due to serogroup W135 occurred in 2010 with 2307 cases. From 2007-2012, MSF recorded no outbreak caused by NmC in the region. In December 2012, the most recent mass vaccination for meningitis in northwest Nigeria, conducted by the National Primary Health Care Development Agency (NPHCDA) of the Ministry of Health (MoH), the World Health Organization (WHO) and donor organizations, used MenAfrivac®, a serogroup A conjugate vaccine 12.To our knowledge, there has been no mass vaccination specifically targeting NmC alone in this region.
This paper describes the general characteristics of an outbreak due to a novel strain of NmC in Sokoto State, Nigeria in early 2013 and a smaller outbreak of the same strain in 2014 in the adjacent state, Kebbi, during which time no other serogroups were confirmed in the region.
Methods
Case definition. During this outbreak, the case definition used for CSM for those over 1 year of age was sudden onset fever and either neck stiffness or petechial rash. For infants under 1 year of age the case definition was sudden onset fever and either bulging fontanelle or petechial rash. Only cases adhering to this case definition were treated, had CSF samples taken, and were recorded as a suspected meningitis case.
Data collection. In the four northwestern states of Nigeria, meningitis surveillance is done by the MSF Surveillance Nurse through weekly proactive contact with all government disease notification officers. There is one notification officer for each Local Government Area in each state, and they are required to contact all health posts in their jurisdiction each week. The MSF Surveillance Nurse relays reports of meningitis cases to the MSF Emergency Response Unit for follow-up and confirmation using clinical and laboratory criteria. At MSF meningitis case-management sites, information for each case was recorded in a standardized line-list of core data. Maps of the outbreak area were created using data from case tracing. Affected population estimates were derived by combining the known population, as per the most recent national census, for each ward which had at least one case.
The aggregated data used for this paper were collected as part of routine activities which MSF has approval to conduct from the MOH. This work met the standards set by the independent MSF Ethics Review Board for retrospective analyses of routinely collected programmatic data 13.
Laboratory methods. Cerebral spinal fluid (CSF) samples were collected from all eligible suspected cases at the start of the outbreak and tested using the rapid Pastorex® latex agglutination kit. Pastorex test kits were kept in controlled, refrigerated storage, between 2 and 8 degrees Celsius. Cold chain procedures were maintained while transporting test kits to the field, in Gio’Style boxes with ice-packs. The field team conducted quality control tests on the kits with each usage, and returned the kits to refrigerated storage at the end of each day. The first 21 and 17 samples in 2013 and 2014, respectively, were inoculated into Trans-isolate media 14 and sent to the WHO Collaborating Centre for Reference and Research on Meningococci, Oslo, for confirmation. Bacterial identification was determinedby Gram staining, the oxidase reaction and standard biochemical tests. The strains were stored at –80°C in brain heart broth with 15% sterile glycerol or in Greaves solution. N. meningitidis strains were serogrouped by slide agglutination with commercial antisera (Remel, GA, USA) 15. Antimicrobial susceptibility testing was performed by determination of the minimal inhibitory concentrations (MIC) using Etest (AB Biodisk, Solna, Sweden). Isolates were tested for susceptibility to penicillin G, amoxicillin, ceftriaxone, ciprofloxacin, chloramphenicol, rifampin, tetracycline and sulphonamides, and classified using the breakpoints from the European Committee on Antimicrobial Susceptibility Testing 16.
Genotypic characterization: DNA from each strain was prepared by suspending bacteria in Tris-EDTA buffer (10 mM Tris-HCl and 1 mM EDTA), pH 8.0, heating at 95°C for 10 min, and followed by centrifugation at 16,000 x g for 5 min. The supernatant was used as DNA template for PCR. Multi-locus sequence typing (MLST) was performed as described on the MLST website 17. The DNA sequences were compared with those on the MLST website for determination of the allele numbers, STs, and clonal complexes of the isolates 18. Variation in the porA and fetA genes, coding for the outer membrane proteins PorA and FetA, respectively, was determined by DNA sequencing, as described previously 19 , 20 . New MLST alleles and STs were submitted to the MLST database 17 together with the strain serogroup and porA and fetA sequences. PCR analysis of the genes coding for the polysaccharide capsule was performed for genogroup determination of non-serogroupable isolates as described 21.
PCR analysis of culture negative specimens: DNA from Trans-isolate supernatants was purified using QiAmp DNA mini kit (Qiagen) and analysed by real-time PCR for species identification, followed by genogrouping if N. meningitidis was identified. Determination of the PorA variant was done by DNA sequencing of the porA gene using a nested porA-PCR 22.
Data analysis. Line-lists were entered into an MSF standardized database in Microsoft Excel. Quality checks on the data were done weekly. Epidemiological curves and frequency summaries of patient history and symptoms were generated in Excel.
Results
Case Numbers and Attack Rates, 2013. During the 20 weeks from February 9th until June 23rd, 2013, a total of 856 suspected cases of CSM presented for treatment at MSF or MoH treatment sites in Sokoto State (Table 1). The attack rate was 673 cases per 100,000 population in the affected wards of the state (Figure 1). Fifty-eight (58) deaths were recorded from treatment centres, giving a case fatality rate (CFR) of 6.8%. During the same period in 2013, some CSM cases were reported and treated by the MoH in Kebbi State, which borders Sokoto to the West. Detailed information on the cases from Kebbi State is not available.
Table 1: All suspected clinical cases and death numbers from the most affected state, with attack rates and case fatality rates for each year.
†Population figures are a combination of the population, as per most recent census, of all affected wards which had at least one case.
| Year | State most affected | Total population Affected† | Total number of cases | Attack Rate (per 100,000) | Total number of deaths | Case fatality rate (%) |
|---|---|---|---|---|---|---|
| 2013 | Sokoto | 127,097 | 856 | 673 | 58 | 6.8 |
| 2014 | Kebbi | 201,457 | 333 | 165 | 35 | 10.5 |
CSM cases presenting for treatment at MSF supported treatment centres in Sokoto state, 2013, by week of presentation.

Case Numbers and Attack Rates, 2014. In 2014, over the 8 weeks from March 14th until May3rd, 333 cases of suspected CSM from Kebbi State presented for treatment at MSF-supported sites. The overall attack rate during this outbreak was 165 cases per 100,000 population in the affected wards. The CFR was 10.5% (35 deaths). One suspected case also presented at a treatment site in Kebbi from the adjacent Sokoto State during this time.
Outbreak Demographics and Spread, 2013-2014. Of 856 cases in 2013, age and sex information were available for 826 (96%); 404 (49%) were female and 422 (51%) male. The highest proportions of cases were found in those aged 5-14 years (n=361; 44%) and 15-29 years (n=235; 29%). In 2014, the sex distribution was similar (53% male) to that in 2013, and similarly, those aged 5-29 years constituted about three-quarters of cases (57% [5-14 years]; 20% [15-29 years]). The 2013 outbreak was limited to a small geographical area, spreading gradually to 44 villages and remaining restricted to a region of 105km2 (Figure 2). The epidemic curve for the 2013 outbreak (Figure 1) shows a peak in the 9th epidemiological week, during which time the outbreak was mostly restricted to the two index villages with high attack rates. Case numbers in these villages then decreased, leading to the low attack rate seen in the 11th week. The increase in weeks 12 and 13 reflects presentations from areas outside the index villages. The gradual overall decline in cases after this period seems to be due to the subsequent serial rise and fall of cases in other villages. The 2014 outbreak was slightly more widespread, affecting 57 villages in a region of approximately 150km2.
Affected villages and outbreak spread in Sokoto State in 2013.
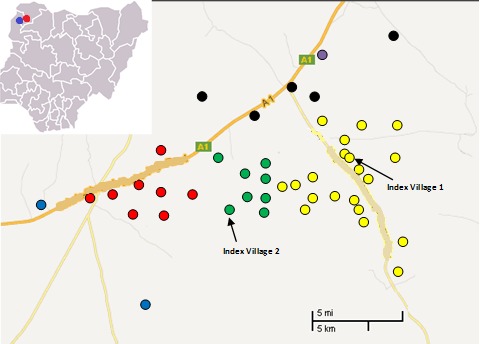
Each coloured dot represents a village which experienced at least one case in 2013, colour differs based on administrative ward. General location of outbreaks in Northwest Nigeria is indicated with a red dot (2013 outbreak) and a blue dot (2014 outbreak) on the full country map at top left.
Laboratory Results, 2013-2014. In the 2013 outbreak, out of the 856 clinically suspected cases, MSF tested 77 CSF samples with rapid Pastorex latex agglutination tests; 27 were positive for NmC, and 50 were negative. In 2014, out of the 333 clinically suspected cases, MSF tested 27 CSF samples by Pastorex test; 13 were positive for NmC and 14 were negative. 21 and 17 Trans-Isolate media inoculated with CSF from suspected meningitis patients were sent to the reference laboratory in Oslo in 2013 and 2014, respectively (Table 2). In total, 11 (52%) and 10 (59%) samples were confirmed as serogroup C in 2013 and 2014, respectively. Most were also confirmed as a new strain with sequencing of ST-10217 PorA type P1.21-15,16 and FetA type F1-7. No other serogroups were identified during testing of CSF samples from these outbreaks.
Table 2: Analyses of the CSF samples sent in Trans-Isolate medium to the Collaborating Centre for Reference and Research on Meningococci, Oslo.
RT-PCR = Real-Time Polymerase Chain Reaction
†For two samples the serogroup was not confirmed by RT-PCR due to low bacterial load, but the PorA type suggests that the same strain was involved.
| Year | Number of CSF samples | Number of samples positive for NmC in culture | PorA type | FetA type | Sequence type | Number culture-negative samples positive for NmC in RT-PCR | PorA type of RT-PCR positive samples |
|---|---|---|---|---|---|---|---|
| 2013 | 21 | 7 | 21-15,16 | F1-7 | 10217 | 6† | All 6 samples: 21-15,16 |
| 2014 | 17 | 5 | 21-15,16 | F1-7 | 10217 | 5 | 4 samples: 21-15,16 1 sample: 21-15,16-50 |
Discussion
These outbreaks were caused by a strain of NmC that has not been seen anywhere else in the world: sequence ST-10217 PorA type P1.21-15,16 and FetA type F1-7. As far as we know this is the first meningitis outbreak caused by NmC in northern Nigeria since 1975 and in the meningitis belt since 1979 4 , 10 , 23 . The seasonal pattern and presentation of these outbreaks, in the dry period during and following the Harmattan winds, did not differ much from those of other meningitis outbreaks caused by N. meningitidis in sub-Saharan Africa 24 , 25 , 26 The outbreaks were confined to relatively small areas, and did not have the ‘wild fire’ effect more typical of meningitis outbreaks caused by NmA 2 , 4. Age groups with the highest proportion of cases in these outbreaks were 5-14 years and 15-29 years, also typical of meningitis, with these ages possibly exposed to more risk factors for transmission such as overcrowding and active and passive smoke exposure 11 , 27 , 28 .
A relatively high percentage of Pastorex latex agglutination tests, carried out only on patients fitting the clinical case definition, had negative results during both the 2013 (64%) and 2014 (48%) outbreaks. This may have occurred for a number of reasons, including meningitis symptoms with non-bacterial cause, or self-medication with antibiotics or traditional medicines prior to presentation. As there is no other report of Pastorex testing being used in a real outbreak situation for NmC, it is uncertain whether these negative test result percentages are unusually high.
During the 2013 outbreak, the MSF control strategy consisted of active case finding and health promotion activities. Due to heightened security issues and anti-vaccination sentiments in the area, reactive vaccination was not done. It is not clear that health promotion activities significantly decreased outbreak spread, though it was observed that after initiation of this strategy, cases presented earlier to treatment centres and this could have contributed to lower mortality rates in the latter part of the outbreak. In 2014, the Kebbi state MoH, along with the WHO, attempted twice to apply for vaccines from the International Coordinating Group; however, neither request was granted. The state MoH later received approximately 20,000 doses of ACYW135 polysaccharide vaccine from the Federal MoH, which was used for reactive vaccination in some affected villages; details of vaccination strategy and outcomes were not available to MSF. Similar to the previous year, health promotion and active case finding was carried out by MSF teams during the 2014 outbreak. Control strategies employed in 2014 by MSF and the MoH did not clearly impact outbreak spread.
The 2014 NmC outbreak had fewer cases than the 2013 outbreak; however, since the outbreaks occurred in different regions and were controlled with different measures we cannot say this signifies a pattern of decrease. It is possible that these small, localized outbreaks will precede increasingly widespread occurrence of meningitis due to this serogroup (C) in the meningitis belt, as has been noted as a characteristic N. meningitidis outbreak pattern4 .
It is possible that the mass vaccination with a conjugate ‘A’ vaccine (MenAfriVac®) in this region a few months prior to the 2013 outbreak could have had an influence on the emergence of new strains or less commonly seen serogroups, such as NmC. Serogroup replacement following mass meningitis vaccination has been noted in west Africa; reports from Niger and Burkina Faso have indicated a significant increase in serogroup W prevalence in the years following campaigns with MenAfriVac® around 201029 , 30. Following a mass vaccination with MenAfriVac® in Chad in 2011/2012 it was seen that in one community serogroup A carriage decreased from 0.7 to 0.02%, while carriage of “other” serogroups (ie. not A, W, X) increased from 0.4 to 0.7%31. Because of the possibility of serogroup replacement following vaccination, enhanced surveillance systems in the region are a priority32 .
Conclusions and Recommendations
NmC outbreaks have emerged in northwest Nigeria in the past 2 years, and there is some evidence of serogroup replacement in the meningitis belt following recent mass vaccination with NmA conjugate vaccine. Meningitis case surveillance systems, for both serogroup and strain, should continue to be strengthened in this region to allow for early identification and proper control (such as vaccination for the appropriate serogroup) of outbreaks. If NmC outbreaks become more widespread in northern Nigeria or adjacent regions in the coming years, large-scale preventative action may be required; a key measure is to ensure availability of ACYW135 polysaccharide vaccine for reactive vaccination.
Competing Interests
The authors have declared that no competing interests exist.
Acknowledgments
Many thanks to the members of the Nigeria Emergency Response Unit of MSF, especially Na Dickson, Dorothy Wuyep, Titi Osamika, Jennifer Duncombe, Rik Vaassen, Dieudonne Kongolo Sango, and Salah Ibrahim Dongu'du for their contributions to case management and surveillance during the outbreaks. Our appreciation also goes to Joke Zeydner and Agatha Bestman, the medical coordinators, and Ivan Gayton and Michelle Chouinard the heads of mission, for their support during the 2013 and 2014 outbreaks respectively. We also would like to acknowledge the Sokoto and Kebbi state Ministry of Health teams for their support, especially the public health directors Shehu Tureta (Sokoto) and Sani Yusuf Argungu (Kebbi) for their review of this paper. We thank Sarah Venis (MSF UK) for editing assistance.
Biographies
MSc
I read medicine at the University of Benin, Benin City, Edo state, Nigeria and currently works as the Medical Team Leader of the Nigeria Emergency Response Unit (NERU) Project of Médecins sans Frontières (Doctors without Borders) - OCA Nigeria mission.
BSc.
Chief scientist at the Norwegian Institute of Public Health Head of the WHO Collaborating Centre for Reference and Research on Meningococci, Oslo, Norway Professor in International Health
MBBS, MPH, FWACP from the Sokoto state Ministry of Health, Sokoto, Nigeria
Funding Statement
This study was funded as part of MSF routine operations. The WHO Collaborating Centre for Reference and Research on Meningococci, Oslo, funded sample transport media and laboratory serogroup and strain tests.
Contributor Information
Anna Funk, Médecins sans Frontières, Sokoto, Nigeria.
Kennedy Uadiale, Nigeria Emergency Response Unit (NERU), Médecins sans Frontières, Sokoto, Nigeria.
Charity Kamau, Médecins sans Frontières, Amsterdam, Netherlands.
Dominique A. Caugant, WHO Collaborating Centre for Reference and Research on Meningococci, Norwegian Institute of Public Health, Oslo, Norway
Umar Ango, Sokoto State Ministry of Health, Sokoto, Nigeria.
Jane Greig, Manson Unit, Médecins Sans Frontières, London, United Kingdom.
References
- 1.Molesworth AM, Thomson MC, Connor SJ, Cresswell MP, Morse AP, Shears P, Hart CA, Cuevas LE. Where is the meningitis belt? Defining an area at risk of epidemic meningitis in Africa. Trans R Soc Trop Med Hyg. 2002 May-Jun;96(3):242-9. PubMed PMID:12174770. [DOI] [PubMed]
- 2.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009 Jun 24;27 Suppl 2:B51-63. PubMed PMID:19477562. [DOI] [PubMed]
- 3.Marc LaForce F, Ravenscroft N, Djingarey M, Viviani S. Epidemic meningitis due to Group A Neisseria meningitidis in the African meningitis belt: a persistent problem with an imminent solution. Vaccine. 2009 Jun 24;27 Suppl 2:B13-9. PubMed PMID:19477559. [DOI] [PubMed]
- 4.Control of Epidemic Meningococcal Disease, WHO Practical Guidelines, Second edition. 1998.
- 5.Boisier P, Nicolas P, Djibo S, Taha MK, Jeanne I, Maïnassara HB, Tenebray B, Kairo KK, Giorgini D, Chanteau S. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007 Mar 1;44(5):657-63. PubMed PMID:17278055. [DOI] [PubMed]
- 6.Gagneux SP, Hodgson A, Smith TA, Wirth T, Ehrhard I, Morelli G, Genton B, Binka FN, Achtman M, Pluschke G. Prospective study of a serogroup X Neisseria meningitidis outbreak in northern Ghana. J Infect Dis. 2002 Mar 1;185(5):618-26. PubMed PMID:11865418. [DOI] [PubMed]
- 7.Koumaré B, Ouedraogo-Traoré R, Sanou I, Yada AA, Sow I, Lusamba PS, Traoré E, Dabal M, Santamaria M, Hacen MM, Kaboré AB, Caugant DA. The first large epidemic of meningococcal disease caused by serogroup W135, Burkina Faso, 2002. Vaccine. 2007 Sep 3;25 Suppl 1:A37-41. PubMed PMID:17521783. [DOI] [PubMed]
- 8.Djibo S, Nicolas P, Alonso JM, Djibo A, Couret D, Riou JY, Chippaux JP. Outbreaks of serogroup X meningococcal meningitis in Niger 1995-2000. Trop Med Int Health. 2003 Dec;8(12):1118-23. PubMed PMID:14641847. [DOI] [PubMed]
- 9.Traoré Y, Njanpop-Lafourcade BM, Adjogble KL, Lourd M, Yaro S, Nacro B, Drabo A, Parent du Châtelet I, Mueller JE, Taha MK, Borrow R, Nicolas P, Alonso JM, Gessner BD. The rise and fall of epidemic Neisseria meningitidis serogroup W135 meningitis in Burkina Faso, 2002-2005. Clin Infect Dis. 2006 Oct 1;43(7):817-22. PubMed PMID:16941360. [DOI] [PubMed]
- 10.Broome CV, Rugh MA, Yada AA, Giat L, Giat H, Zeltner JM, Sanborn WR, Fraser DW. Epidemic group C meningococcal meningitis in Upper Volta, 1979. Bull World Health Organ. 1983;61(2):325-30. PubMed PMID:6345014. [PMC free article] [PubMed]
- 11.Harrison LH, Pass MA, Mendelsohn AB, Egri M, Rosenstein NE, Bustamante A, Razeq J, Roche JC. Invasive meningococcal disease in adolescents and young adults. JAMA. 2001 Aug 8;286(6):694-9. PubMed PMID:11495619. [DOI] [PubMed]
- 12.GAVI Alliance (Global Alliance for Vaccine and Immunization) website. Accessed on 11 Nov 2013
- 13.MSF Ethics Review Board (2013) Standard Operating Procedures. Geneva: MSF. Available: http://fieldresearch.msf.org/msf/handle/10144/294968. Accessed 2013 July 21
- 14.Ajello GW, Feeley JC, Hayes PS, Reingold AL, Bolan G, Broome CV, Phillips CJ. Trans-isolate medium: a new medium for primary culturing and transport of Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. J Clin Microbiol. 1984 Jul;20(1):55-8. PubMed PMID:6430956. [DOI] [PMC free article] [PubMed]
- 15.Craven DE, Frasch CE, Robbins JB, Feldman HA. Serogroup identification of Neisseria meningitidis: comparison of an antiserum agar method with bacterial slide agglutination. J Clin Microbiol. 1978 May;7(5):410-4. PubMed PMID:96123. [DOI] [PMC free article] [PubMed]
- 16.The European Committee on Antimicrobial Susceptibility Testing – EUCAST. Accessed on 17 June 2014
- 17.Neisseria Sequence Typing Home Page. Accessed on 17 June 2014
- 18.Katz LS, Bolen CR, Harcourt BH, Schmink S, Wang X, Kislyuk A, Taylor RT, Mayer LW, Jordan IK. Meningococcus genome informatics platform: a system for analyzing multilocus sequence typing data. Nucleic Acids Res. 2009 Jul;37(Web Server issue):W606-11. PubMed PMID:19468047. [DOI] [PMC free article] [PubMed]
- 19.Russell JE, Jolley KA, Feavers IM, Maiden MC, Suker J. PorA variable regions of Neisseria meningitidis. Emerg Infect Dis. 2004 Apr;10(4):674-8. PubMed PMID:15200858. [DOI] [PMC free article] [PubMed]
- 20.Thompson EA, Feavers IM, Maiden MC. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology. 2003 Jul;149(Pt 7):1849-58. PubMed PMID:12855736. [DOI] [PubMed]
- 21.Kristiansen PA, Diomandé F, Wei SC, Ouédraogo R, Sangaré L, Sanou I, Kandolo D, Kaboré P, Clark TA, Ouédraogo AS, Absatou KB, Ouédraogo CD, Hassan-King M, Thomas JD, Hatcher C, Djingarey M, Messonnier N, Préziosi MP, LaForce M, Caugant DA. Baseline meningococcal carriage in Burkina Faso before the introduction of a meningococcal serogroup A conjugate vaccine. Clin Vaccine Immunol. 2011 Mar;18(3):435-43. PubMed PMID:21228139. [DOI] [PMC free article] [PubMed]
- 22.Caugant DA, Høiby EA, Frøholm LO, Brandtzaeg P. Polymerase chain reaction for case ascertainment of meningococcal meningitis: application to the cerebrospinal fluids collected in the course of the Norwegian meningococcal serogroup B protection trial. Scand J Infect Dis. 1996;28(2):149-53. PubMed PMID:8792481. [DOI] [PubMed]
- 23.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007 Jun 30;369(9580):2196-210. PubMed PMID:17604802. [DOI] [PubMed]
- 24.Sultan B, Labadi K, Guégan JF, Janicot S. Climate drives the meningitis epidemics onset in west Africa. PLoS Med. 2005 Jan;2(1):e6. PubMed PMID:15696216. [DOI] [PMC free article] [PubMed]
- 25.Molesworth AM, Cuevas LE, Connor SJ, Morse AP, Thomson MC. Environmental risk and meningitis epidemics in Africa. Emerg Infect Dis. 2003 Oct;9(10):1287-93. PubMed PMID:14609465. [DOI] [PMC free article] [PubMed]
- 26.Palmgren H. Meningococcal disease and climate. Glob Health Action. 2009 Nov 11;2. PubMed PMID:20052424. [DOI] [PMC free article] [PubMed]
- 27.Tully J, Viner RM, Coen PG, Stuart JM, Zambon M, Peckham C, Booth C, Klein N, Kaczmarski E, Booy R. Risk and protective factors for meningococcal disease in adolescents: matched cohort study. BMJ. 2006 Feb 25;332(7539):445-50. PubMed PMID:16473859. [DOI] [PMC free article] [PubMed]
- 28.MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, Evans MR, Cann K, Baxter DN, Maiden MC, Stuart JM. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis. 2006 Jun;12(6):950-7. PubMed PMID:16707051. [DOI] [PMC free article] [PubMed]
- 29.Collard JM, Issaka B, Zaneidou M, Hugonnet S, Nicolas P, Taha MK, Greenwood B, Jusot JF. Epidemiological changes in meningococcal meningitis in Niger from 2008 to 2011 and the impact of vaccination. BMC Infect Dis. 2013 Dec 6;13:576. PubMed PMID:24313998. [DOI] [PMC free article] [PubMed]
- 30.MacNeil JR, Medah I, Koussoubé D, Novak RT, Cohn AC, Diomandé FV, Yelbeogo D, Kambou JL, Tarbangdo TF, Ouédraogo-Traoré R, Sangaré L, Hatcher C, Vuong J, Mayer LW, Djingarey MH, Clark TA, Messonnier NE. Neisseria meningitidis serogroup W, Burkina Faso, 2012. Emerg Infect Dis. 2014 Mar;20(3):394-9. PubMed PMID:24571805. [DOI] [PMC free article] [PubMed]
- 31.Daugla DM, Gami JP, Gamougam K, Naibei N, Mbainadji L, Narbé M, Toralta J, Kodbesse B, Ngadoua C, Coldiron ME, Fermon F, Page AL, Djingarey MH, Hugonnet S, Harrison OB, Rebbetts LS, Tekletsion Y, Watkins ER, Hill D, Caugant DA, Chandramohan D, Hassan-King M, Manigart O, Nascimento M, Woukeu A, Trotter C, Stuart JM, Maiden MC, Greenwood BM. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet. 2014 Jan 4;383(9911):40-7. PubMed PMID:24035220. [DOI] [PMC free article] [PubMed]
- 32.Altmann D, Aseffa A, Bash M, Basta N, Borrow R, Broome C, Caugant D, Clark T, Collard JM, Djingarey M, Goldblatt D, Greenwood B, Griffiths U, Hajjeh R, Hassan-King M, Hugonnet S, Kimball AM, LaForce M, MacLennan C, Maiden MC, Manigart O, Mayer L, Messonnier N, Moisi J, Moore K, Moto DD, Mueller J, Nascimento M, Obaro S, Ouedraogo R, Page AL, Perea W, Pluschke G, Preziosi MP, Sow S, Stephens D, Stuart J, Thomson M, Tiendrebeogo S, Trape JF, Vernet G. Priorities for research on meningococcal disease and the impact of serogroup A vaccination in the African meningitis belt. Vaccine. 2013 Mar 1;31(11):1453-7. PubMed PMID:23273967. [DOI] [PMC free article] [PubMed]


