Abstract
Many lines of evidence support that β-amyloid (Aβ) peptides play an important role in Alzheimer’s disease (AD), the most common cause of dementia. But despite much effort the molecular mechanisms of how Aβ contributes to AD remain unclear. While Aβ is generated from its precursor protein throughout life, the peptide is best known as the main component of amyloid plaques, the neuropathological hallmark of AD. Reduction in Aβ has been the major target of recent experimental therapies against AD. Unfortunately, human clinical trials targeting Aβ have not shown the hoped-for benefits. Thus, doubts have been growing about the role of Aβ as a therapeutic target. Here we review evidence supporting the involvement of Aβ in AD, highlight the importance of differentiating between various forms of Aβ, and suggest that a better understanding of Aβ’s precise pathophysiological role in the disease is important for correctly targeting it for potential future therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-014-0313-y) contains supplementary material, which is available to authorized users.
Key Words: Dementia, Alzheimer’s disease, amyloid precursor protein, amyloid, therapy
Evidence for the Role of β-amyloid in Alzheimer’s Disease
Pathologic, genetic, biologic, and biomarker evidence have supported an important role for the β-amyloid peptide (Aβ) in the development of Alzheimer’s disease (AD). Amyloid plaques composed primarily of aggregated Aβ and neurofibrillary tangles composed of microtubule-associated protein tau are neuropathological diagnostic criteria for AD. But, in contrast, to amyloid plaques, neurofibrillary tangles are less specific for AD, and are seen in a greater variety of neurodegenerative diseases, such as progressive supranuclear palsy, corticobasal degeneration, and subtypes of frontotemporal dementia. Mutations of the amyloid precursor protein (APP) occur in familial forms of AD. Moreover, mutations in presenilin 1 and 2, which are important proteases in the cleavage of Aβ from APP, are causes of familial AD. In contrast, familial mutations in tau are associated with symptoms and neurodegeneration that resemble frontotemporal dementia rather than AD. Biologic studies have shown that mutations in APP and presenilins lead to higher amounts of the disease-linked Aβ42 and/or other more aggregation prone forms of Aβ [1, 2]. Finally, biomarker studies on cerebrospinal fluid (CSF) show that the disease-associated Aβ42 peptides decline 1–2 decades prior to onset of symptoms in AD [3, 4]. For these reasons Aβ has become the prime suspect in the pathogenesis of AD.
Distinction Between Aβ and Amyloid
Aβ is a normal peptide generated throughout life, while amyloid plaques are a neuropathological hallmark of AD. It is remarkable that the normal function of APP, among the most studied proteins in science, remains unclear. A possible normal function of Aβ is even more uncertain. Nevertheless, Aβ production and secretion is stimulated by synaptic activity, the most unique and normal function of the nervous system. Thus, generation of the small Aβ peptide (up to 42 or 43 amino acids long) is not inherently toxic and might even have a physiological function, while amyloid plaques, composed of a multitude of highly aggregated Aβ fibrils, represents an abnormal pathological lesion. Electron microscopy analysis of postmortem brain shows that all forms of plaques, including diffuse plaques, are associated with neuropathology, particularly characterized by neuritic and synaptic dystrophies [5, 6]. Preplaque increased levels of Aβ correlate with AD-characteristic alterations in synapses, particularly evident on microscopy labeling the presynaptic protein synaptophysin in brain [7]. Preplaque aggregation of Aβ using an aggregation-specific antibody is directly associated with ultrastructural evidence of subcellular pathology [5]. However, it remains unclear what conformation soluble Aβ has in normal brain and what precise conformation(s) of Aβ aggregates are pathogenic. It is exceedingly difficult to define protein conformation in brain, as biochemical extraction from the brain and subsequent analysis can affect protein conformation. Current evidence suggests that Aβ in biological fluids is mainly monomeric [8], while nondenaturing studies to define Aβ in brain have shown it to run at a high molecular weight, although in what conformation is not clear [9].
The distinction between amyloid plaques and Aβ peptides is important because there is evidence that amyloid plaques are not a good measure of Aβ-induced brain damage in AD. For example, the Arctic familial mutation in APP leads to more aggregation-prone Aβ and AD dementia but shows no amyloid on positron emission tomography (PET) amyloid ligand brain scans, although histopathologically diffuse amyloid plaques are present [10]. The Osaka familial mutation in APP causes the generation of more aggregation-prone nonfibrillar Aβ in the setting of AD-like dementia but shows no amyloid plaques [11]. Transgenic mice harboring this mutation develop no amyloid plaques but do show Aβ aggregation as oligomers within neurons, which is associated with neurodegeneration and progressive cognitive decline [12].
Several lines of experimental evidence point to amyloid plaque-independent Aβ pathogenesis [6, 13, 14]. A comparison between Swedish mutant and wildtype (WT) human APP overexpressing transgenic mice with the same levels of APP showed that both had reductions in brain synaptophysin compared with WT mice, but only the Swedish mutant mice developed plaques. However, the APP mutant mice showed a greater loss of synaptophysin prior to plaques, which pointed to Aβ- rather than APP-dependent synapse damage [7]. Furthermore, induction of seizures leads to greater hippocampal cell loss and Aβ peptide elevation in preplaque APP mutant transgenic mice than in WT mice [15]. Finally, just the reduction of normal sensory input to the barrel cortex by unilateral whisker removal leads to synapse degeneration in APP mutant transgenic mice despite a reduction (rather than an increase) in amyloid plaques [16]. Thus, Aβ in a form other than amyloid plaques appears to be critical in inflicting damage to synapses and neurons. Moreover, transgenic mice engineered to secrete directly high amounts of Aβ not generated from APP readily form extensive plaques but do not show behavioral decline, arguing against plaque toxicity and also supporting that secreted Aβ may not be the primary toxic form of Aβ [17].
Experimental therapies further point to plaque-independent effects on cognitive function, as certain antibodies against Aβ can lead to improved behavioral function in AD transgenic mice, even in the absence of amyloid plaque removal [18]. Along these lines, in the aborted active vaccine clinical trial by ELAN/Wyeth patients studied at autopsy showed evidence consistent with plaque removal, despite a history of continued cognitive decline [19]. Thus, multiple lines of evidence indicate that amyloid plaques are not the main toxic Aβ entity. Consequently, it is important to be careful with terms and, at times, the terms amyloid and Aβ are erroneously used interchangeably. This is particularly noticeable in human brain imaging studies, where the introduction of PET ligand imaging of amyloid now allows for the identification of amyloid plaques in living people. The radioligands used for PET amyloid detection are derivatives of thioflavin T, which reacts to fibrillar aggregates of proteins, including fibrillar Aβ. In contrast, these thioflavin-based ligands do not detect soluble Aβ or even prefibrillar Aβ aggregates, including diffuse plaques. Nevertheless, negative PET amyloid scans are often referred to as having no ‘Aβ pathology’, whereas such negative scans should rather be described as having no ‘amyloid’ pathology.
Over the last decade prefibrillar oligomers of Aβ have been viewed as causative of Aβ-related damage in AD. A major challenge in the study of Aβ assemblies is that it is exceedingly difficult to determine the actual native structure of Aβ in the brain. Aβ aggregation can be studied in vitro using many techniques, although it is uncertain how well the experimental conditions of these studies can parallel those in the living brain. The increasing evidence for early intracellular accumulation and aggregation of Aβ within subcellular organelles (reviewed in [6]) indicates that new methods will need to be developed to model experimentally Aβ aggregation in living systems [20]. Endosomes are particularly prominent sites of such Aβ aggregation in neurons in AD [21]. These vesicles provide an environment that, based on in vitro aggregation studies, would be favorable for aggregation given their lower pH and higher metal content. In addition, endosomes allow for concentration of Aβ peptides in a limited space, which further favors aggregation. Recent work suggests that the major genetic risk factor for AD, apolipoprotein E ε4 (apoE4), which is well known to impact on amyloid pathology [22], can influence uptake and toxicity of Aβ [23]. Genetic evidence supports that vesicular trafficking components relating to endocytosis and endosomes are particularly important in AD, including sortilin-like receptor 1, bridging integrator 1, clusterin, CD2-associated protein, and phosphatidylinositol binding clathrin assembly protein, among others [24, 25]. In addition, genes related to lipid homeostasis, inflammation, and synapses are increasingly being associated with AD.
Experimental Therapeutics Targeting Aβ
It is possible that targeting Aβ will not turn out to be an effective therapeutic strategy for AD. Nevertheless, the failures of past clinical trials targeting Aβ do not mean that Aβ is an incorrect target. We do not yet understand the pathophysiology of Aβ in AD, while many arrows point to its involvement.
Major Anti-Aβ Therapeutic Directions
Secretase Inhibitors
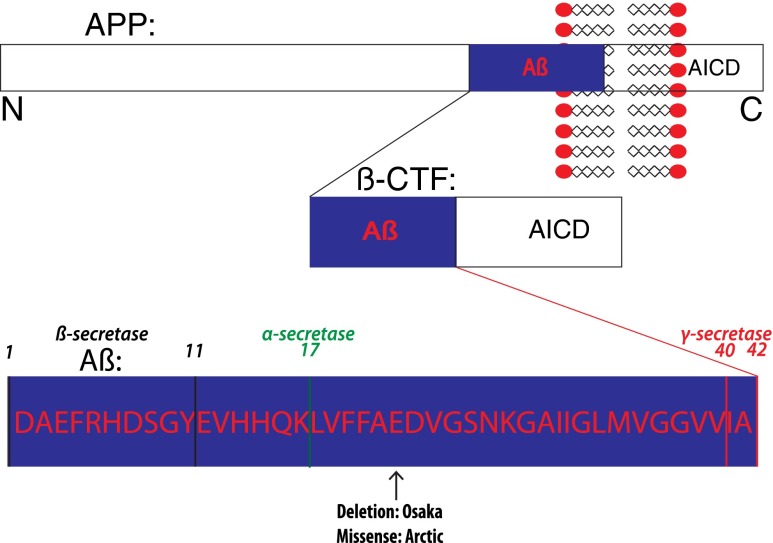
Aβ is generated from its precursor protein, APP, after cleavage first at the β and then the γ cleavage sites (Fig. 1). β-Secretase 1 (BACE1) is viewed as the major β-secretase, while presenilin is the main component of γ-secretase. Typically, the processing of APP is simplified in the literature, as a remarkable variety of Aβ peptides and other APP cleavage products exist. In addition, there are several splice variants of APP, with the 695 amino acid-long APP seen as the most important in neurons. Problems in targeting secretases are thought to hinge on these activities being important in a variety of other cleavages besides APP, such as cleavage of Notch by γ-secretase. In fact, a clinical trial with a γ-secretase inhibitor by Lilly led to accelerated cognitive decline in the treatment group (reviewed in [24]). It is now known that over 100 proteins are cleaved by γ-secretase, including a significant number of synaptic proteins [26]. Therapeutic strategies that modulate rather than inhibit the γ-secretase are under investigation, with the hope of developing a selective modulator targeting specifically APP γ-cleavage. However, it was shown that γ-secretase inhibitors do not induce spine deficits in APP knockout mice that are seen with such treatment in WT mice [27]. This finding suggests that it is specifically inhibition of APP γ-cleavage that might be problematic, possibly limiting the therapeutic value of γ-secretase inhibitors. Aberrant build up of the 99 amino acid C-terminal fragment (CTF) of APP (βCTF) as a result of inhibiting subsequent cleavage to Aβ may also be important for these detrimental effects of γ-secretase inhibition [28]. However, γ-secretase modulators that selectively reduce the Aβ42 to 40 ratio without inhibiting overall APP cleavage or increasing βCTFs are of particular therapeutic interest. While BACE1 is not known to cleave as many substrates, BACE1 knockout mice have deficits in myelination during brain development and show altered behavioral function [29, 30]. The first phase 2 clinical trial of a β-secretase inhibitor (LY2886721; Lilly) was halted because of hepatotoxicity, while ongoing clinical trials with other BACE inhibitors (MK-8931, Merck; AZD3293, Lilly/AstraZeneca) have shown target engagement, evident by Aβ reduction in the CSF of patients, without obvious detrimental effects noted.
Fig. 1.
A schema of amyloid precursor protein (APP) with its major cleavage sites and familial Alzheimer’s disease (AD) mutations mentioned in the text. Depicted are the amyloidgenic pathway of APP, cleavage sites on the Aβ peptide, and the Arctic and Osaka familial AD mutations. Although other mutations in β-amyloid (Aβ)/APP are not shown, it is important to note that all mutations in APP linked to familial AD are either within Aβ or at the β- or γ-cleavage sites. In the amyloidgenic pathway APP is cleaved by β-secretase to generate C-terminal fragment β (βCTF) and then further cleaved by γ-secretase to generate Aβ. In the nonamyloidgenic pathway, Aβ production is precluded as it is cleaved in the middle by α-secretase. AICD = APP intracellular domain
Stimulation of the Nonamyloidogenic Pathway
As α-cleavage precludes the generation of Aβ, promotion of this cleavage pathway is being explored as a therapy, with favorable results reported in APP mutant transgenic mice [31]. The ADAM (a disintegrin and metalloprotease) family of proteases is viewed as responsible for the shedding of APP at the cell surface via α-cleavage. However, neurons have relatively less α-cleavage compared with non-neuronal cells, where it is the major cleavage pathway [32, 33]. ADAM10 and ADAM17 are considered to be particularly important ADAMs for APP cleavage. Given the wide range and functions of these ADAMs as shedases throughout the body [34, 35], and the need to stimulate rather than inhibit these proteases, this line of therapy is challenging. In addition, despite α-cleavage precluding Aβ generation, such modulation of the α-pathway does not consistently reduce β-cleavage [36].
Aggregation Inhibitors
Given the abnormal aggregation of Aβ in AD there has been an extensive effort to pharmacologically block this process. This direction of therapy is most easily developed in in vitro systems where Aβ aggregation can be closely monitored using a variety of techniques. However, there have only been 2 aggregation inhibitors [3-amino-1-propanesulfonic acid (Neurochem Inc.) and scyllo-inositol (Elan)] that have made it to clinical trials, neither of which showed a clear benefit in patients with AD [37, 38]. While both were developed as aggregation inhibitors, their precise mechanisms of action were not fully clear. In fact, based on the observation that it lowers myoinositol in brain analogous to lithium, scyllo-inositol is now in a new clinical trial for bipolar disorder. It is difficult to model Aβ aggregation inhibitors and also challenging to translate such mainly in vitro results to a disease of the brain, which is further compounded by potentially having to target these compounds to the correct cellular and subcellular locations or by unanticipated side effects (e.g., with scyllo-inositol). Another direction of therapy is to sequester metal ions, which have been shown to enhance Aβ aggregation. Interestingly, Aβ contains a copper-binding site. Metal chelation therapy showed benefits in AD transgenic mouse models [39]. Thus, metal chelation (for copper and potentially also zinc) has been tried (Clioquinol and PBT2; Prana Biotechnology) in clinical trials for AD, although no obvious benefits were evident with either of these compounds.
Immunotherapy
Aβ immunotherapy emerged as an exciting new direction for therapy when it was shown that injection of Aβ into APP mutant transgenic mice led to an antibody-mediated immune response to the injected Aβ that both cleared amyloid plaque pathology and improved behavior [40]. Multiple subsequent studies confirmed and extended these results, showing that passive immunotherapy was also effective in AD transgenic mouse models. The human AN1792 active vaccine trial provided the first significant setback to this promising therapeutic direction [19]. The trial had to be halted when about 6 % of patients developed brain inflammation evident on magnetic resonance imaging, now termed amyloid-related imaging abnormalities. As noted above, postmortem examination of a subset of patients who died showed that the treatment resulted in the removal of amyloid plaques, although the patients had shown continued cognitive decline [19]. Nevertheless, immunotherapy remains of considerable interest and closer analysis of the data from one clinical trial after it was halted for lack of efficacy (Solanezumab; Eli Lilly), suggested some stabilization in those with milder cognitive deficits (see [24]). Consequently, the A4 study will evaluate the effect of Aβ immunotherapy in cognitively healthy elderly individuals exhibiting amyloid pathology as evident by PET imaging.
The mechanism(s) of action of Aβ immunotherapy is not clear. A leading possibility is Aβ antibody-mediated microglia induced phagocytosis and degradation of amyloid plaques and Aβ oligomers, although the fact that Fab fragments alone were protective questions this most straightforward explanation [41]. Another leading hypothesis was the “sink hypothesis”, whereby higher levels of Aβ antibodies in the peripheral circulation drive Aβ across the blood–brain barrier out of the brain [42]. However, a study that treated mice with a radiolabeled Aβ antibody showed that the antibody preferentially made its way into the brain, thereby also questioning this hypothesis [43]. Moreover, Aβ levels in blood are not consistently seen to rise following Aβ immunotherapy. An alternative hypothesis is that protection occurs by Aβ antibodies reducing intraneuronal Aβ, which might be hindered by sequestration of antibodies by plaques in the setting of human AD [44]. Finally, Aβ antibodies are further hypothesized to bind to Aβ monomers or oligomers and thereby block subsequent aggregation and/or conformational change to the most toxic form of Aβ [45], which might, as in the preceding hypothesis, be hindered by sequestration of antibodies to plaques.
Promoting Aβ Degradation and Clearance
Several proteases are known to degrade Aβ, including in particular neprilysin and insulin degrading enzyme . Inhibition of either of these augments amyloid pathology and worsens behavior in mouse models of β-amyloidosis [46]. Thus, augmentation of Aβ degrading proteases is a viable therapeutic direction Interestingly, neprilysin levels fall in aging rodent brains, particularly in synaptic terminals, and APP via the APP intracellular domain regulates gene expression of neprilysin [47–50]. Furthermore, increasing evidence indicates that cellular degradation systems such as the endosome–lysosome, autophagy, and ubiquitin–proteasome systems are impaired in neurodegenerative diseases of aging and the stimulation of these pathways is also under investigation for AD therapy [51].
Modulation of Synapses
It is increasingly becoming apparent that synapses are central sites of pathogenesis in neurodegenerative diseases of aging [52]. Many lines of evidence have shown that Aβ targets synapses [14, 21, 53] and that, in turn, synaptic stimulation can modulate APP cleavage and levels of Aβ [16, 54–56]. Synaptic stimulation has been shown to enhance Aβ generation and secretion, while reducing intracellular Aβ. Chronic synaptic activity is considered to promote AD pathology, as areas of the default network of the brain that are most active at rest, that is most of the time, are particularly prone to the development of AD pathology [57]. However, experimental evidence points to detrimental effects of both synaptic hyper- and hypoactivity in the setting of elevated Aβ. For example, in APP mutant AD transgenic mice induction of seizures (hyperactivity) leads to more loss of neurons, while reduced synaptic activity (hypoactivity) leads to greater loss of synapses [15, 16]. These results are consistent with an altered biology as a result of elevated Aβ, which is detrimental when synaptic activity is modulated either way. Use of benzodiazepines, presumably by reducing synaptic activity, has been linked with an increased incidence in developing AD and experimentally damages synapses in AD transgenic models [16, 58].
Of note, both of the approved classes of medications for AD, cholinesterase inhibitors and N-methyl-D-aspartate receptor modulators, target synapses, though these therapies are generally considered not to affect the underlying disease process. Recent developments in targeting synapses in therapy include modulation of serotonin receptor subtypes [e.g., the serotonin 6(5-HT6) receptor antagonist Lu AE58054 (Lundbeck)] or muscarinic glutamate receptors.
An ongoing effort has been made to determine how secreted extracellular Aβ interacts with synapses. Various potential Aβ receptors have been described (including lipoprotein receptors, prion protein, α7 nicotinic acetylcholine receptor, N-methyl-D-aspartate receptor, metabotropic glutamate receptor 5, and neuroligin, among many others), although as yet no consensus has developed in the field on which of these is most specific and important. A considerable amount of evidence points to the importance of APP, as neurons devoid of APP are protected from extracellular Aβ toxicity [59, 60]. A challenge for studies on Aβ interactions is that Aβ is hydrophobic and prone to interaction with other proteins, and that the use of biological methods to assess interactions, such as knockout of a potential Aβ receptor, can lead to other biological effects (e.g., synapse dysfunction) that can have detrimental effects which complicate interpretation. Nonetheless, if a specific receptor(s) could be found, modulation of this interaction would be a logical therapeutic direction.
Other Aβ-modifying Therapies
Above are major directions of ongoing experimental therapies targeting Aβ, but there are many other directions. These include pharmacological reduction of APP [61], modulating signaling pathways involved in Aβ pathogenesis, or targeting tau, which has been shown to be important in Aβ-induced damage. Among signaling kinases being targeted, inhibition of mammalian target of rapamycin (mTOR) by rapamycin has been shown to be effective in prolonging life from lower organisms up to rodents [62], and was further shown to improve behavior and reduce Aβ in transgenic mouse models of AD [63, 64]. This and other work have led to an increasing interest in autophagy in the field of neurodegeneration, as rapamycin is known to induce autophagy. However, accumulation of multilamellar autophagic vacuoles in dystrophic neurites is also a neuropathological hallmark of AD and other neurodegenerative diseases, and it has been suggested that autophagy is involved in Aβ production and secretion [65–67]. Finally, mTOR is a central signaling kinase that plays multiple important physiological roles, such as in synaptic plasticity, which could make mTOR a challenging therapeutic target in the elderly.
Caveats to Current Aβ-directed Experimental Therapies
Potential Normal Function of Aβ
It has been generally presumed that Aβ is an unfortunate toxic byproduct of APP metabolism and thus that its therapeutic reduction should not be harmful. Yet, this assumption may be erroneous. Although there is no clear-cut proof for a physiologically normal function of Aβ, experimental work is increasingly suggesting this [68, 69]. However, a polymorphism in APP present in up to 1 % of Scandinavians, which leads to a reduction in Aβ generation by 20 % in those with one allele, was reported to not only protect against AD, but also against normal age-related cognitive decline [70]. This polymorphism could suggest that Aβ is not essential, although, without information on a potential polymorphism or mutation that precludes Aβ production, there remains insufficient evidence to rule out a physiological role for Aβ.
Oversimplification in Dementia Diagnosis
Another important caveat for Aβ-directed therapies for AD is that clinical diagnosis of dementia is often simplified in order to provide a diagnostic label of the patient and to be able to start symptomatic treatment. The high proportion of patients subsequently determined not to have AD enrolled in AD clinical trials has been a topic of major interest. Ongoing and future studies can limit this issue by incorporating CSF biomarker and/or amyloid imaging. Moreover, while AD pathology is the leading cause of dementia, the border between AD pathology and normal aging is not clear-cut. To varying extents, AD pathology accompanies normal aging. Furthermore, patients with dementia rarely only have a single pathology. Atherosclerosis accompanies normal aging and affects the cerebral vasculature to varying degrees. α-Synuclein pathology, which develops most prominently in Parkinson’s disease and Lewy body dementia, also occurs with aging. Clinical diagnosis in dementia usually leads to 1 major disease diagnosis, while typically there is mixed pathology. For example, up to 50 % of patients with Parkinson’s disease with dementia have sufficient plaques and tangles for a secondary diagnosis of AD [71]. It is important to keep in mind that elderly patients with such mixed pathologies might not fare as well when targeting only one such pathology. Thus, treatments that show benefit in genetically homogenous and relatively young experimental mice that are mostly designed to develop a single pathology may not translate well to elderly patients with mixed pathologies.
Experimental Animals
The use of experimental mice is often blamed as a reason for lack of translation of experimental therapies to humans, and it is important to understand the limitations of transgenic rodent models, although several clinical trials for AD therapy never provided animal data, such as in the case of latrepirdine (Dimebon; Medivation, Inc.). Transgenic models of AD typically overexpress mutant human AD-linked genes. In contrast, humans with AD (even those with triplication of APP or in Down syndrome with trisomy of chromosome 21) do not overexpress APP to the extent of common transgenic mouse models of AD. Thus, therapies that inhibit APP transcription or translation might be particularly effective in the setting of APP overexpression in transgenic mice but less so in typical human AD. Further, it is possible that a therapy directed at familial AD mutations does not extend to those without the mutation, that is cases with “sporadic” AD.
Defining the Aβ/APP Pathogenic Species
Although evidence points strongly to Aβ42, there remains the possibility that other APP fragments, such as APP CTFs, may be of importance in AD. Mutations in APP that elevate β-cleavage also raise levels of βCTFs. Moreover, APP mutations that increase Aβ aggregation, such as the Arctic and Osaka mutations, could also lead to a greater propensity for the aggregation of βCTFs. In addition, presenilin mutations that raise the ratio of Aβ42 to Aβ40 are also known to have reduced γ-cleavage activity, which would elevate βCTFs.
There has been a major debate in the field on the relative roles of intra- versus extracellular Aβ [52]. Increasing evidence points to a complex prion-like relationship between these different pools of Aβ. The term “prion-like” should not imply infectivity [72], but rather the ability of toxic protein conformations to be passed from one cell to another, something that is becoming a common theme among aggregation-prone proteins and peptides linked to neurodegenerative diseases [73–75]. If there is a prion-like intercellular spread of Aβ then blocking that transmission is a possible therapeutic target. Recently, evidence for different “strains” of Aβ have received increasing attention. For example, Aβ-containing brain tissue derived from postmortem tissue from 2 different patients with AD were shown to aggregate differently in vitro [76].
Our insufficient understanding of APP might also hinder our approach to Aβ/APP-mediated therapy for AD. APP is evolutionarily conserved across species, abundantly expressed throughout the body, and has, as yet, still poorly defined physiological functions [77, 78], including at synapses, a better understanding of which might have a major effect on Aβ directed therapies.
Aβ Treatments Fail Because They are Started too Late
This is currently a common view in the field that mostly lacks specific evidence. While the pathological cascade for AD is now thought to begin much earlier than had been considered in the past, there is no clear reason why a slowly progressive disease that gradually spreads through the brain could not be halted if the pathogenic process was interrupted. Certainly, no one would argue that way regarding atherosclerosis or diabetes. In fact, inducible mouse models of mutant APP overexpression have shown marked improvement in behavior upon turning off of the APP mutant transgene [79, 80]. This supports that Aβ pathology can be halted and even, at least partially, reversed, although neurodegeneration that has already taken place would, of course, not be corrected. For Aβ immunotherapy there is even a specific rationale for why early intervention might be better. If, indeed, amyloid plaques are more tombstones than a source of toxic Aβ, then sequestration of therapeutic antibodies to Aβ-containing plaques could lessen the amount of antibody available for the more toxic soluble species of Aβ42. Early Aβ immunotherapy that precedes plaques should then be more efficacious.
Future Therapeutic Directions
Aging is the most important risk factor for AD and related neurodegenerative diseases of aging. Progress in the biology of aging is leading to experimental therapies for AD that target molecular changes that occur during aging, such as modulation of the insulin/phosphoinositide 3-kinase/protein kinase B/mTOR-related pathways [81]. Mitochondrial dysfunction and oxidative stress are considered to be particularly important in aging [82], and remain targets for AD therapy. Research is gradually elucidating how the most important genetic risk factor, apoE4, is involved in AD pathogenesis, which should lead to more experimental therapies targeting apoE4. Tau is a growing target for experimental AD therapy and, increasingly, tau is being shown to be important for the pathogenic effects of Aβ [83]. Rather than prematurely rejecting the involvement of Aβ that has been linked by multiple lines of evidence to AD, the AD research field needs to unravel the complex biochemical pathways involved in the disease, as well as focusing more effort on understanding the normal role of APP, Aβ, and the many other proteins linked genetically and pathologically to AD. For example, the interactions between apoE and Aβ or tau and Aβ could be particularly important therapeutic targets. Personalized combination therapies that take into account individual aspects of disease in a given patient may be the therapy for dementia in the future.
Electronic supplementary material
(PDF 1224 kb)
Acknowledgments
We are grateful for funding support from MultiPark, the Swedish Research Council, and the European Research Council.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Contributor Information
Gunnar K. Gouras, Email: gunnar.gouras@med.lu.se
Oskar Hansson, Email: oskar.hansson@med.lu.se.
References
- 1.Chávez-Gutiérrez L, Bammens L, Benilova I, et al. The mechanism of γ-secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31:2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and Proteolytic processing of APP. Cold Spring Harb Perspect Med. 2012;2:a006270–a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchhave P, Minthon L, Zetterberg H, Wallin ÅK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- 4.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi RH. Oligomerization of Alzheimer's—amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Intraneuronal β-amyloid accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathol. 2010;119:523–541. doi: 10.1007/s00401-010-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mucke L, Masliah E, Yu G-Q, et al. High-level neuronal expression of Aβ1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong S, Ostaszewski BL, Yang T, et al. Soluble Ab oligomers are rapidly sequestered from brain ISF in vivo and bind GM1 ganglioside on cellular membranes. Neuron. 2014;82:1–12. doi: 10.1016/j.neuron.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upadhaya AR, Lungrin I, Yamaguchi H, Fändrich M, Thal DR. High-molecular weight Aβ oligomers and protofibrils are the predominant Aβ species in the native soluble protein fraction of the AD brain. J Cell Mol Med. 2012;16:287–295. doi: 10.1111/j.1582-4934.2011.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schöll M, Wall A, Thordardottir S, et al. Low PiB PET retention in presence of pathologic CSF biomarkers in Arctic APP mutation carriers. Neurology. 2012;79:229–236. doi: 10.1212/WNL.0b013e31825fdf18. [DOI] [PubMed] [Google Scholar]
- 11.Shimada H, Ataka S, Tomiyama T, Takechi H, Mori H, Miki T. Clinical course of patients with familial early-onset Alzheimer’s disease potentially lacking senile plaques bearing the E693Δ mutation in amyloid precursor protein. Dement Geriatr Cogn Disord. 2011;32:45–54. doi: 10.1159/000330017. [DOI] [PubMed] [Google Scholar]
- 12.Tomiyama T, Matsuyama S, Iso H, et al. A mouse model of amyloid oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J Neurosci. 2010;30:4845–4856. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer's disease and its models. Neuroscience. 2013;251:51–65. doi: 10.1016/j.neuroscience.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 14.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohajeri MH, Saini K, Schultz JG, et al. Passive immunization against beta -amyloid peptide protects central nervous system (CNS) neurons from increased vulnerability associated with an Alzheimer's disease-causing mutation. J Biol Chem. 2002;277:33012–33017. doi: 10.1074/jbc.M203193200. [DOI] [PubMed] [Google Scholar]
- 16.Tampellini D, Capetillo-Zarate E, Dumont M, et al. Effects of synaptic modulation on beta-amyloid, synaptophysin, and memory performance in Alzheimer's disease transgenic mice. J Neurosci. 2010;30:14299–14304. doi: 10.1523/JNEUROSCI.3383-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Chakrabarty P, Hanna A, et al. Normal cognition in transgenic BRI2-Aβ mice. Mol Neurodegeneration. 2013;8:15. doi: 10.1186/1750-1326-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodart J-C, Bales KR, Gannon KS, et al. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 19.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 20.Esbjörner EK, Chan F, Rees E, et al. Direct observations of amyloid β self-assembly in live cells provide insights into differences in the kinetics of Ab(1–40) and Ab(1–42) aggregation. Chem Biol. 2014;21:732–742. doi: 10.1016/j.chembiol.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi RH, Milner TA, Li F, et al. Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/S0002-9440(10)64463-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtzman DM, Herz J, Bu G. Apolipoprotein E and Apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312–a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuszczyk MA, Sanchez S, Pankiewicz J, et al. Blocking the interaction between apolipoprotein E and Aβ reduces intraneuronal accumulation of Aβ and inhibits synaptic degeneration. Am J Pathol. 2013;182:1750–1768. doi: 10.1016/j.ajpath.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy J, Bogdanovic N, Winblad B, et al. Pathways to Alzheimer's disease. J Inter Med. 2014;275:296–303. doi: 10.1111/joim.12192. [DOI] [PubMed] [Google Scholar]
- 25.Small S, Gandy S. Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Restituito S, Khatri L, Ninan I, et al. Synaptic autoregulation by metalloproteases and γ-secretase. J Neurosci. 2011;31:12083–12093. doi: 10.1523/JNEUROSCI.2513-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bittner T, Fuhrmann M, Burgold S, et al. Gamma-secretase inhibition reduces spine density in vivo via an amyloid precursor protein-dependent pathway. J Neurosci. 2009;29:10405–10409. doi: 10.1523/JNEUROSCI.2288-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saura CA, Chen G, Malkani S, et al. Conditional inactivation of presenilin 1 prevents amyloid accumulation and temporarily rescues contextual and spatial working memory impairments in amyloid precursor protein transgenic mice. J Neurosci. 2005;25:6755–6764. doi: 10.1523/JNEUROSCI.1247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison SM, Harper AJ, Hawkins J, et al. BACE1 (β-secretase) transgenic and knockout mice: identification of neurochemical deficits and behavioral changes. Mol Cell Neurosci. 2003;24:646–655. doi: 10.1016/S1044-7431(03)00227-6. [DOI] [PubMed] [Google Scholar]
- 30.Ohno M, Sametsky EA, Younkin LH, et al. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer's disease. Neuron. 2004;41:27–33. doi: 10.1016/S0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 31.Postina R, Schroeder A, Dewachter I, et al. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gouras GK, Xu H, Jovanovic JN, et al. Generation and regulation of beta-amyloid peptide variants by neurons. J Neurochem. 1998;71:1920–1925. doi: 10.1046/j.1471-4159.1998.71051920.x. [DOI] [PubMed] [Google Scholar]
- 33.DeBoer SR, Dolios G, Wang R, Sisodia SS. Differential release of β-amyloid from dendrite- versus axon-targeted APP. J Neurosci. 2014;34:12313–12327. doi: 10.1523/JNEUROSCI.2255-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Gall SM, Bobé P, Reiss K, et al. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor alpha, L-selectin, and tumor necrosis factor alpha. Mol Biol Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musardo S, Marcello E, Gardoni F, Di Luca M. ADAM10 in synaptic physiology and pathology. Neurodegener Dis. 2014;13:72–74. doi: 10.1159/000354233. [DOI] [PubMed] [Google Scholar]
- 36.Dobrowolska JA, Michener MS, Wu G, et al. CNS amyloid-β, soluble APP-α and -β kinetics during BACE inhibition. J Neurosci. 2014;34:8336–8346. doi: 10.1523/JNEUROSCI.0540-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aisen PS, Gauthier S, Ferris SH, et al. Tramiprosate in mild-to-moderate Alzheimer's disease—a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study) Arch Med Sci. 2011;7:102–111. doi: 10.5114/aoms.2011.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salloway S, Sperling R, Keren R, et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011;77:1253–1262. doi: 10.1212/WNL.0b013e3182309fa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adlard PA, Bica L, White AR, et al. Metal ionophore treatment restores dendritic spine density and synaptic protein levels in a mouse model of Alzheimer's disease. PLoS ONE. 2011;6:e17669. doi: 10.1371/journal.pone.0017669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 41.Bacskai BJ, Kajdasz ST, McLellan ME, et al. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeMattos RB. Brain to plasma amyloid-beta efflux: a Measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 43.Yamada K, Yabuki C, Seubert P, et al. Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. J Neurosci. 2009;29:11393–11398. doi: 10.1523/JNEUROSCI.2021-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tampellini D, Magrané J, Takahashi RH, et al. Internalized antibodies to the Abeta domain of APP reduce neuronal Abeta and protect against synaptic alterations. J Biol Chem. 2007;282:18895–18906. doi: 10.1074/jbc.M700373200. [DOI] [PubMed] [Google Scholar]
- 45.Morgan D. Immunotherapy for Alzheimer’s disease. J Intern Med. 2010;269:54–63. doi: 10.1111/j.1365-2796.2010.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nalivaeva NN, Belyaev ND, Kerridge C, Turner AJ. Amyloid-clearing proteins and their epigenetic regulation as a therapeutic target in Alzheimer's disease. Front Aging Neurosci. 2014;6:235. doi: 10.3389/fnagi.2014.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pardossi-Piquard R, Petit A, Kawarai T, et al. Presenilin-dependent transcriptional control of the Abeta-degrading enzyme neprilysin by intracellular domains of betaAPP and APLP. Neuron. 2005;46:541–554. doi: 10.1016/j.neuron.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Hama E, Saido TC. Etiology of sporadic Alzheimer's disease: somatostatin, neprilysin, and amyloid beta peptide. Med Hypotheses. 2005;65:498–500. doi: 10.1016/j.mehy.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 49.Belyaev ND, Nalivaeva NN, Makova NZ, Turner AJ. Neprilysin gene expression requires binding of the amyloid precursor protein intracellular domain to its promoter: implications for Alzheimer disease. EMBO Rep. 2009;10:94–100. doi: 10.1038/embor.2008.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimm MOW, Mett J, Stahlmann CP, Haupenthal VJ, Zimmer VC, Hartmann T. Neprilysin and Aβ clearance: impact of the APP intracellular domain in NEP regulation and implications in Alzheimer's disease. Front Aging Neurosci. 2013;5:98. doi: 10.3389/fnagi.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ihara Y, Morishima-Kawashima M, Nixon R. The ubiquitin-proteasome system and the autophagic-lysosomal system in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006361–a006361. doi: 10.1101/cshperspect.a006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gouras GK. Convergence of synapses, endosomes, and prions in the biology of neurodegenerative diseases. Int J Cell Biol. 2013;2013:1–6. doi: 10.1155/2013/141083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lacor PN, Buniel MC, Chang L, et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 55.Kamenetz F, Tomita T, Hsieh H, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/S0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 56.Tesseur I, Pimenova AA, Lo AC, et al. Chronic 5-HT4 receptor activation decreases Aβ production and deposition in hAPP/PS1 mice. Neurobiol Aging. 2013;34:1779–1789. doi: 10.1016/j.neurobiolaging.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 57.Myers N, Pasquini L, Gottler J, et al. Within-patient correspondence of amyloid- and intrinsic network connectivity in Alzheimer's disease. Brain. 2014;137:2052–2064. doi: 10.1093/brain/awu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer's disease: case-control study. BMJ. 2014;349:5205–5205. doi: 10.1136/bmj.g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaked GM, Kummer MP, Lu DC, Galvan V, Bredesen DE, Koo EH. Abeta induces cell death by direct interaction with its cognate extracellular domain on APP (APP 597-624) FASEB J. 2006;20:1254–1256. doi: 10.1096/fj.05-5032fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tampellini D, Rahman N, Gallo EF, et al. Synaptic activity reduces intraneuronal Abeta, promotes APP transport to synapses, and protects against Abeta-related synaptic alterations. J Neurosci. 2009;29:9704–9713. doi: 10.1523/JNEUROSCI.2292-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asuni AA, Guridi M, Pankiewicz JE, Sanchez S, Sadowski MJ. Modulation of amyloid precursor protein expression reduces β-amyloid deposition in a mouse model. Ann Neurol. 2014;75:684–699. doi: 10.1002/ana.24149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS ONE. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nixon RA, Wegiel J, Kumar A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 66.Yu WH, Cuervo AM, Kumar A, et al. Macroautophagy—a novel β-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nilsson P, Loganathan K, Sekiguchi M, et al. Aβ secretion and plaque formation depend on autophagy. Cell Rep. 2013;5:61–69. doi: 10.1016/j.celrep.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 68.Puzzo D, Privitera L, Fa M, et al. Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann Neurol. 2011;69:819–830. doi: 10.1002/ana.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fogel H, Frere S, Segev O, et al. APP homodimers transducean amyloid-β-mediated increasein release probability at excitatory synapses. Cell Rep. 2014;7:1560–1576. doi: 10.1016/j.celrep.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 70.Jonsson T, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 71.Irwin DJ, Lee VMY, Trojanowski JQ. Parkinson's disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14:626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Irwin DJ, Abrams JY, Schonberger LB, et al. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol. 2013;70:462–468. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 75.Nussbaum JM, Seward ME, Bloom GS. Alzheimer disease: a tale of two prions. Prion. 2013;7:14–19. doi: 10.4161/pri.22118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nhan HS, Chiang K, Koo EH. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol 2014 Oct 7 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 78.Dawkins E, Small DH. Insights into the physiological function of the β-amyloid precursor protein: beyond Alzheimer's disease. J Neurochem. 2014;129:756–769. doi: 10.1111/jnc.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Born HA, Kim J-Y, Savjani RR, et al. Genetic suppression of transgenic APP rescues hypersynchronous network activity in a mouse model of Alzeimer's disease. J Neurosci. 2014;34:3826–3840. doi: 10.1523/JNEUROSCI.5171-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Melnikova T, Fromholt S, Kim H, et al. Reversible pathologic and cognitive phenotypes in an inducible model of Alzheimer-amyloidosis. J Neurosci. 2013;33:3765–3779. doi: 10.1523/JNEUROSCI.4251-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Neill C, Kiely AP, Coakley MF, Manning S, Long-Smith CM. Insulin and IGF-1 signalling: longevity, protein homoeostasis and Alzheimer's disease. Biochem Soc Trans. 2012;40:721–727. doi: 10.1042/BST20120080. [DOI] [PubMed] [Google Scholar]
- 82.Dumont M, Lin MT, Beal MF. Mitochondria and antioxidant targeted therapeutic strategies for Alzheimer's disease. J Alzheimers Dis. 2010;20(Suppl. 2):S633–S643. doi: 10.3233/JAD-2010-100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roberson ED, Scearce-Levie K, Palop JJ, et al. Reducing endogenous tau ameliorates amyloid-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)