Abstract
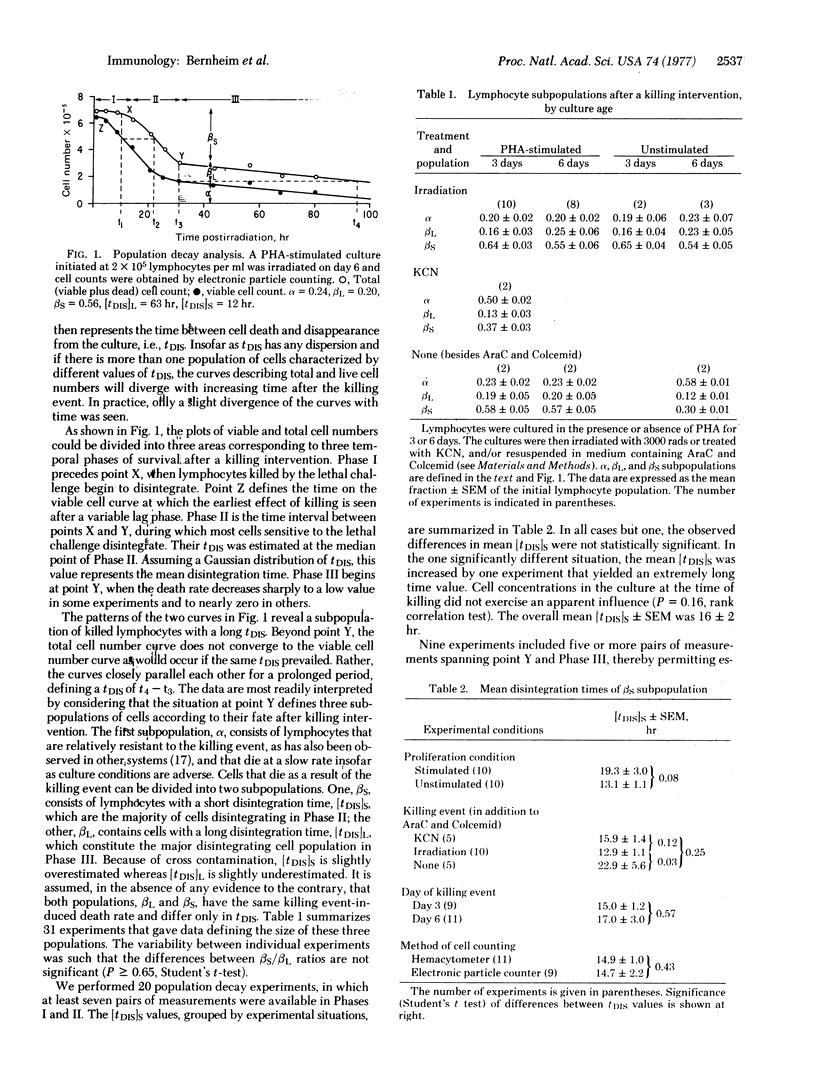
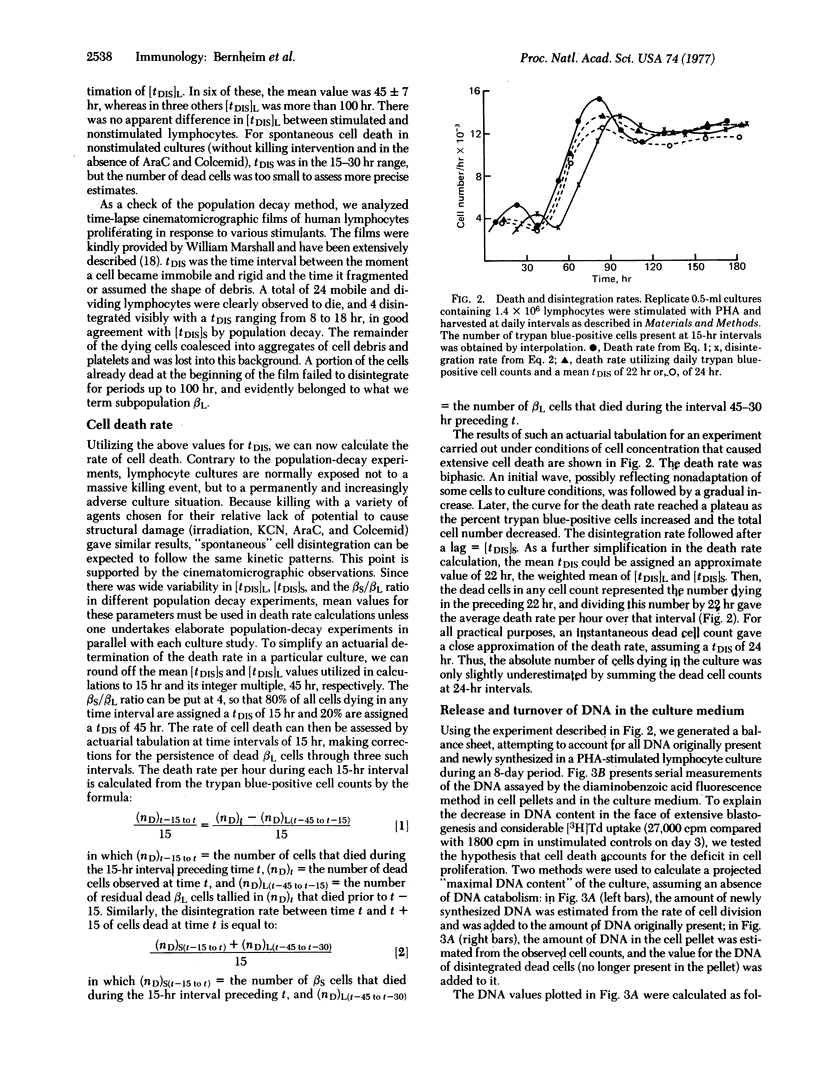
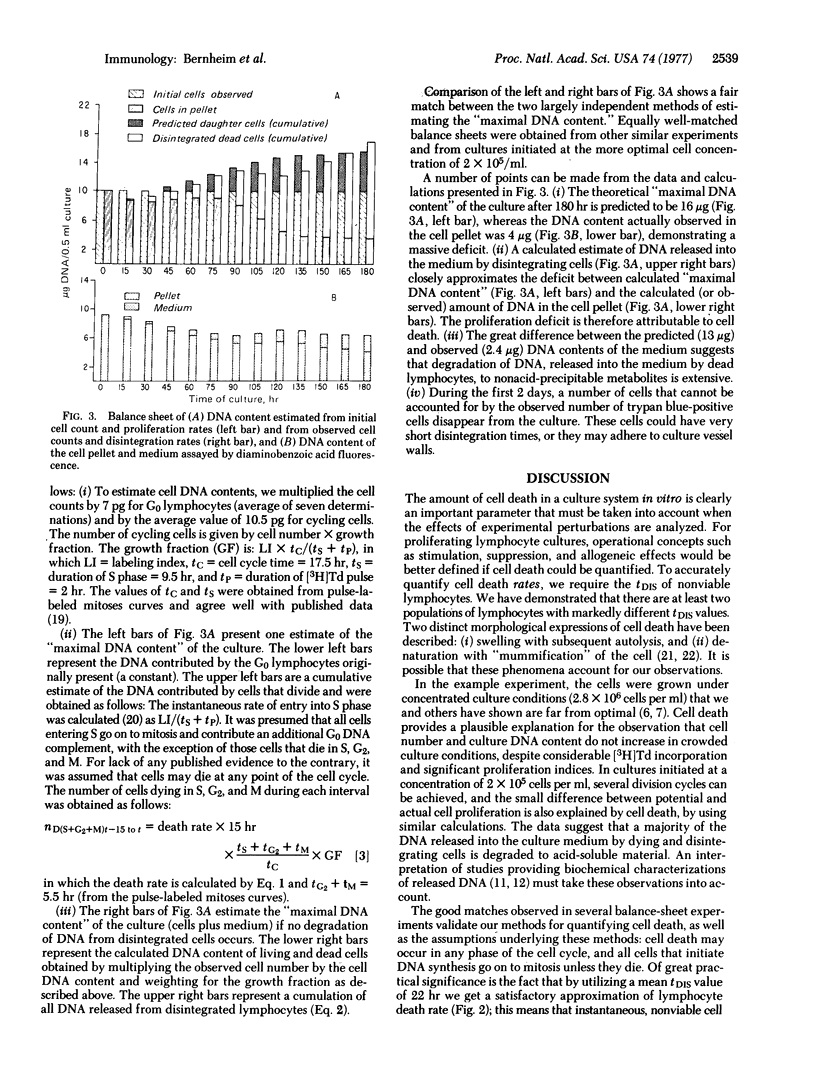
In order to quantitate lymphocyte proliferative responses, we explored the role of cell death in the kinetics of phytohemagglutinin-stimulated cultures. Unless the disintegration time (tDIS) of nonviable lymphocytes in culture is known, the rate of cell death cannot be calculated. To obtain tDIS, we determined the time interval between total and viable cell population decay after various killing events. Two subpopulations of lymphocytes were observed, the major (80%) with a mean (+/-SEM) tDIS of 16+/-2 hr and the minor (20%) with a tDIS of 45+/-7 hr. Kinetic balance sheets were constructed predicting total culture DNA content (cells plus medium), as calculated both from proliferation rates and from observed death and disintegration rates. In an experiment characterized by extensive cell death, the two tallies were well-matched when the above data were utilized. The large discrepancy between predicted and observed DNA contents of the medium indicates that the DNA of disintegrated lymphocytes is extensively degraded. We conclude that cell death explains proliferation deficits in stimulated lymphocyte cultures. Our approach to quantitation of cell death may have general applicability to kinetic studies of cultured cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders E. M., McAdam K. P., Anders R. F. Cell-mediated immunity in amyloidosis secondary to lepromatous leprosy. Clin Exp Immunol. 1977 Jan;27(1):111–117. [PMC free article] [PubMed] [Google Scholar]
- Durie B. G., Salmon S. E. High speed scintillation autoradiography. Science. 1975 Dec 12;190(4219):1093–1095. doi: 10.1126/science.1188385. [DOI] [PubMed] [Google Scholar]
- Eurenius K., McIntyre O. R. Cell dynamics in phytohemagglutinin (PHA) stimulated guinea-pig lymph node lymphocyte cultures. Int Arch Allergy Appl Immunol. 1970;37(4):393–408. doi: 10.1159/000230802. [DOI] [PubMed] [Google Scholar]
- Forsdyke D. R. Quantitiative nucleic acid changes during phytohaemagglutinin-induced lymphocyte transformation in vitro. Dependence of the response on phytohaemagglutinin-serum rati. Biochem J. 1967 Nov;105(2):679–684. doi: 10.1042/bj1050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Lohrmann H. P., Graw C. M., Graw R. G., Jr Stimulated lymphocyte cultures: responder recruitment and cell cycle kinetics. J Exp Med. 1974 May 1;139(5):1037–1048. doi: 10.1084/jem.139.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., LA GATTUTA M., THOMPSON T. E. Cellular death and necrosis: chemical, physical and morphologic changes in rat liver. Virchows Arch Pathol Anat Physiol Klin Med. 1960;333:421–465. doi: 10.1007/BF00955327. [DOI] [PubMed] [Google Scholar]
- Marshall W. H., Valentine F. T., Lawrence H. S. Cellular immunity in vitro. Clonal proliferation of antigen-stimulated lymphocytes. J Exp Med. 1969 Aug 1;130(2):327–343. doi: 10.1084/jem.130.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R. J., Smith R. G., Gallo R. C. DNA-metabolizing enzymes in normal human lymphoid cells. VI. Induction of DNA polymerases alpha, beta, and gamma following stimulation with phytohemagglutinin. Blood. 1975 Oct;46(4):509–518. [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C., Daniele R. P., Winger L. A. Kinetics of human lymphocyte proliferation: proportion of cells responsive to phytohemagglutinin and correlation with E rosette formation. J Reticuloendothel Soc. 1975 Jan;17(1):47–56. [PubMed] [Google Scholar]
- Rogers J. C., Boldt D., Kornfeld S., Skinner A., Valeri C. R. Excretion of deoxyribonucleic acid by lymphocytes stimulated with phytohemagglutinin or antigen. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1685–1689. doi: 10.1073/pnas.69.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C. Characterization of DNA excreted from phytohemagglutinin-stimulated lymphocytes. J Exp Med. 1976 May 1;143(5):1249–1264. doi: 10.1084/jem.143.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C. Identification of an intracellular precursor to DNA excreted by human lymphocytes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3211–3215. doi: 10.1073/pnas.73.9.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma D. S., Zubroff J. Synthesis and fragmentation of DNA in phytohaemagglutinin-stimulated human peripheral blood lymphocytes. Immunol Commun. 1973;2(3):277–285. doi: 10.3109/08820137309022799. [DOI] [PubMed] [Google Scholar]
- Schellekens P. T., Eijsvoogel V. P. Lymphocyte transformation in vitro. I. Tissue culture conditions and quantitative measurements. Clin Exp Immunol. 1968 Jul;3(6):571–584. [PMC free article] [PubMed] [Google Scholar]
- Stewart C. C., Cramer S. F., Steward P. G. The response of human peripheral blood lymphocytes to phytohemagglutinin: determination of cell numbers. Cell Immunol. 1975 Apr;16(2):237–250. doi: 10.1016/0008-8749(75)90115-x. [DOI] [PubMed] [Google Scholar]
- TOLMACH L. J. Growth patterns in x-irradiated HeLa cells. Ann N Y Acad Sci. 1961 Nov 13;95:743–757. doi: 10.1111/j.1749-6632.1961.tb50074.x. [DOI] [PubMed] [Google Scholar]