Abstract
Antipsychotic drugs (APs) are of great benefit in several psychiatric disorders, but they can be associated with various adverse effects, including seizures. To investigate the effects of chronic antipsychotic treatment on seizure susceptibility in genetically epilepsy-prone rats, some APs were administered for 7 weeks, and seizure susceptibility (audiogenic seizures) was evaluated once a week during treatment and for 5 weeks after drug withdrawal. Furthermore, acute and subchronic (5-day treatment) effects were also measured. Rats received haloperidol (0.2–1.0 mg/kg), clozapine (1–5 mg/kg), risperidone (0.03–0.50 mg/kg), quetiapine (2–10 mg/kg), aripriprazole (0.2–1.0 mg/kg), and olanzapine (0.13–0.66 mg/kg), and tested according to treatment duration. Acute administration of APs had no effect on seizures, whereas, after regular treatment, aripiprazole reduced seizure severity; haloperidol had no effects and all other APs increased seizure severity. In chronically treated rats, clozapine showed the most marked proconvulsant effects, followed by risperidone and olanzapine. Quetiapine and haloperidol had only modest effects, and aripiprazole was anticonvulsant. Finally, the proconvulsant effects lasted at least 2–3 weeks after treatment suspension; for aripiprazole, a proconvulsant rebound effect was observed. Taken together, these results indicate and confirm that APs might have the potential to increase the severity of audiogenic seizures but that aripiprazole may exert anticonvulsant effects. The use of APs in patients, particularly in patients with epilepsy, should be monitored for seizure occurrence, including during the time after cessation of therapy. Further studies will determine whether aripiprazole really has a potential as an anticonvulsant drug and might also be clinically relevant for epileptic patients with psychiatric comorbidities.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-014-0318-6) contains supplementary material, which is available to authorized users.
Keywords: Audiogenic seizures, Clozapine, Aripiprazole, Olanzapine, Quetiapine, Haloperidol
Introduction
Antipsychotic drugs (APs) can be of great benefit in several psychiatric disorders, including schizophrenia and bipolar disorder, although they are all associated with different adverse effects such as oversedation, neuroleptic malignant syndrome, extrapyramidal symptoms, tardive dyskinesia, anticholinergic symptoms, and seizures [1–6]. Most neuroleptics can lower seizure threshold and increase the chance of seizure occurrence [3, 7–9]. Furthermore, a strong link exists between epilepsy and psychiatric disorders; it is known that such comorbidity exists; many epileptic patients have psychiatric disorders; and, conversely, depressed patients have a higher risk of becoming epileptic [10–14].
Chlorpromazine, a first-generation AP, appears to be associated with the greatest risk of seizure induction; in contrast, haloperidol, fluphenazine, pimozide, and trifluoperazine are associated with a lower risk of seizures. Clozapine and olanzapine are the second-generation antipsychotics most frequently associated with seizures, while risperidone appears to have a relatively low seizure induction risk [9]. Previous reports indicated that, among the conventional APs, the clinical incidence of seizures during treatment with chlorpromazine was 1.25 % (10/800 patients) [15], whereas haloperidol rarely induces clinical seizure [16]. Furthermore, the clinical incidence of seizures during treatment with atypical APs was 2.89 % (41/1418 patients) for clozapine, 2.34 % (28/1196 patients) for zotepine, 0.88 % (22/2500 patients) for olanzapine, 0.75 % (18/2387 patients) for quetiapine, and 0.35 % (9/2607 patients) for risperidone [3, 7–9]. However, the above data are largely based on studies that were not adequately controlled [3, 7–9, 15, 16].
Some years ago, Kumlien and Lundberg [17] have surveyed reports of suspected seizures from 1968 to February 2006 and have reported cases of adverse drug reactions for each psychotropic drug. Of a total of 71,471 convulsive events, the APs most frequently associated with convulsive adverse drug reactions were clozapine (9.00 %), chlorprothiexene (8.89 %), and quetiapine (5.90 %). However, fluphenazine, haloperidol, pimozide, and risperidone exhibited a relatively low risk [17, 18].
Chronic treatment with clozapine has been shown to induce epileptic seizures consistent with kindling in rats [19]. Also, antipsychotic treatments during ethanol withdrawal may worsen audiogenic seizures, whereas risperidone, quetiapine, and ziprasidone are effective on audiogenic seizures during ethanol withdrawal syndrome [20]. Neither clozapine nor olanzapine treatments affected the incidence and the latency of audiogenic seizures in ethanol-dependent rats [21, 22]. Recently, we have reported that aripiprazole, a new atypical AP, was able to reduce absence seizures with positive modulatory actions on depression and anxiety in WAG/Rij rats, an animal model of epilepsy and depression comorbidity [23].
Genetically epilepsy-prone rats (GEPRs) represent an established animal model to study the pathophysiology of seizures and to screen potential new antiepileptic drugs [24–31]. In this strain, epilepsy is genetically determined, even though the exact mechanisms underlying the development of the disorder remain incompletely understood [24, 27]. A nonspecific propensity for generalized seizures, regardless of the eliciting stimuli, enhances the value of this strain of rats as a model in the study of gene-linked generalized epilepsy. GEPRs are a useful model of convulsive epilepsy; the rats exhibit generalized tonic–clonic seizures in response to certain stimuli (i.e., sound and hyperthermia), with a lower threshold and more intense seizure response to a given stimulus (i.e., electrical or chemical) than other strains [24, 26, 30, 32]. Various neurotransmitters have been involved in the genesis of seizures in this animal model, and particular attention has been focused on both gamma-aminobutyric acid (GABA) and glutamate [24, 33, 34]. Additionally, drugs decreasing noradrenergic or serotonergic transmission increase convulsion intensity in GEPRs, while drugs increasing serotonergic or noradrenergic function decrease convulsion intensity [27, 35–37]. Finally, noradrenergic deficiencies exist in GEPRs that have experienced multiple sound-induced seizures, as well as in GEPRs that have been protected from seizure-provoking stimuli [27, 38].
There is a lack of information about the effects of the exposure of chronic APs on genetic animal models of epilepsy. The aim of the present study was to investigate and compare possible AP-induced alterations in the development of seizure susceptibility in a genetic rat model of audiogenic (convulsive) seizures (GEPRs).
Methods
Animals
GEPRs, a strain derived from Sprague–Dawley (SD) rats, were selected in our breeding stock (Pharmacology Unit, Department of Health Sciences, University of Catanzaro, Italy) from a colony originally supplied by Professor B.S. Meldrum (University of London, UK). SD rats of 1 month of age were purchased from Harlan Italy (Correzzana, Milan, Italy). In order to select the rats for experiments, only male GEPRs were tested 3 times at weekly intervals between 6 and 8 weeks of age, and only animals that showed a stage 2–3 (GEPR-3s) or 8–9 (GEPR-9s) audiogenic seizure in all 3 exposures to sound stimulation were divided into respective groups and used for these experiments (for details, see the audiogenic stimulation protocol paragraph and Table 1) [39]. The GEPRs that showed no audiogenic seizures in all 3 exposures to sound stimulation were considered as group GEPR-0. In addition, SD rats were tested 3 times at weekly intervals between 6 and 8 weeks of age in order to exclude responsive animals.
Table 1.
Experimental protocol scheme
| Rats of the following strains/substrains were used in this protocol: | |
| Sprague–Dawley rats (nonepileptic rats; n = 112) | |
| GEPR-0s (genetically prone epilepsy rats that have no manifest tonic and/or clonic seizure; n = 112) | |
| GEPR-3s (rats with clonic seizures; n = 112) | |
| GEPR-9s (rats with tonic/clonic seizures; n = 120) | |
| Every experimental group consisted of 8 animals undergoing different treatment durations as follows: | |
| Acute treatment | Rats were tested 30 min after the intraperitoneal (i.p.) administration of all drugs |
| Subchronic treatment | Rats were administered i.p. for 5 consecutive days and tested for audiogenic seizures on day 5, 30 min after the last administration |
| Chronic drug treatment | Rats started oral treatment at ~ P70 (10 weeks of age) and were kept on drug for 7 additional weeks; drug treatment was then suspended Rats were tested for audiogenic seizures every week during the 7 weeks of treatment and for 5 more weeks after drug suspension in order to measure also withdrawal effects |
| Drugs used were tested at the following doses: | |
| haloperidol (0.2 and 1.0 mg/kg) | |
| clozapine (1 and 5 mg/kg) | |
| risperidone (0.13 and 0.66 mg/kg) | |
| quetiapine (2 and 10 mg/kg) | |
| aripriprazole (0.2, 0.5, and 1.0 mg/kg) | |
| olanzapine (0.13 and 0.66 mg/kg) | |
| ketotifen fumarate (3 mg/kg) | |
All drugs at all doses were tested in all experimental paradigms with 8 animals in every drug/dose group plus a vehicle control group for every rat strain/substrain
Only male rats of all strains and subgroups were used and housed 3 or 4 per cage under stable conditions of humidity (60 ± 5 %) and temperature (21 ± 2 °C), and were kept under a reversed light/dark (12/12 h) cycle (light on at 19:00). The latter were given free access to food (Harlan Teklad rodent diet) and tap water until the time of experiments. Each experimental group in this protocol included 8 rats. Procedures involving animals and their care were conducted in conformity with the international and national law and policies (European Union Directive 2010/63/EU for animal experiments; Animal Research: Reporting of In Vivo Experiments guidelines; and the Basel declaration, including the 3R concept). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Acute Treatment Procedure
For intraperitoneal (i.p.) administration, haloperidol and clozapine (Sigma-Aldrich, Milan, Italy), risperidone (Janssen Cilag, Milan, Italy), quetiapine (Astra Zeneca, Milan, Italy), and (Abilify, Otsuka Pharmaceutical Italy S.r.l., Milan, Italy) were dissolved in 0.1 % acetic acid, while olanzapine (Eli Lilly, Florence, Italy) and ketotifen fumarate (Biofutura Farmaceutica, Rome, Italy) were dissolved in sterile saline. Haloperidol (0.2 and 1.0 mg/kg), clozapine (1 and 5 mg/kg), risperidone (0.03, 0.13, and 0.50 mg/kg), quetiapine (2 and 10 mg/kg), aripiprazole (0.2, 0.5, and 1.0 mg/kg), olanzapine (0.13 and 0.66 mg/kg), and ketotifen fumarate (3 mg/kg) were injected i.p. for acute treatment at a volume of 1 ml/kg body weight. Control animals received equivalent volumes of vehicle at the respective times before the test. Rats were always tested 30 min after drugs or vehicle administration (see Table 1 for experimental scheme). Doses of the drugs were selected from our preliminary experiments and previous studies [20, 40–42]. As higher doses of most of antipsychotics and other drugs used in our preliminary studies and in previous reports caused sedation and/or impairment of motor coordination [43], such doses were not used.
Chronic and Sub-chronic Treatment Procedure
For chronic or subchronic treatment, all drugs were orally administered at the doses above described (see “Acute Treatment Procedure” section) dissolving adequate samples of each drug in 120 ml of drinking water (e.g., clozapine 1 mg/kg: 1 mg in 120 ml of water). Dosage was calculated on the basis of the knowledge that rats drink, on average, 10–12 ml/100 g/day; the volume drunk was also checked weekly [44]. Drug solutions were freshly prepared, replaced 2 or 3 times a week, and bottles were wrapped in silver foil to exclude light [45, 46].
Chronic drug treatment protocol
Rats (n = 8 animals for each group and each dose) started treatment at ~ P70 (10 weeks of age) and were kept on the drug for 7 additional weeks; treatment was then stopped and animals were normally housed for 5 more weeks in order to evaluate possible withdrawal effects.
For subchronic treatment
All animals (n = 8 per group) received orally administered drug for 5 consecutive days at the same doses used for chronic treatment and tested 30 min after the last administration. Control animals (n = 8) were kept under standard conditions and tested in the same time window of the corresponding treated groups (see Table 1 for experimental scheme). During this period, animals were weighed weekly every Monday between 9:00 and 11:00. Furthermore, particular attention was paid to the possible appearance of any obvious drug induced side-effects [47].
Audiogenic Stimulation Protocol
At 10 weeks of age, rats of every GEPRs subgroup (GEPR-0s, GEPR-3s, and GEPR-9s) were randomly assigned to a single drug dose (n = 8 for each dose). Similarly, SD rats were assigned to each drug. Rats were then weekly tested for audiogenic seizures by exposing them to a mixed frequency sound of 12–16 kHz, 109 dB intensity under a hemispheric Plexiglas dome (diameter of 58 cm). Individual animals were placed into the dome box for habituation at least 2 min before sound stimulation. Auditory stimulation was applied for 1 min. A full seizure response consisted of 1 or 2 running phases, followed by a convulsion (clonus of forelimbs, hindlimbs, head, pinnae, vibrissae, and tail) and tonic extension to give a score of 9 [48]. In particular, the audiogenic seizure response was assessed on the following scale, as previously described [27, 39]: 0 = no response; 1 = running only; 2 = 2 running phases, followed by a clonic convulsion (clonus of forelimbs, hindlimbs, head, pinnae, vibrissae, and tail); 3 = 1 running phase, followed by a clonic convulsion (clonus of forelimbs, hindlimbs, head, pinnae, vibrissae, and tail); 4 = 2 running phases followed by tonus of neck, trunk, and forelimb, and hindlimb clonus; 5 = 1 running phase followed by tonus of neck, trunk, and forelimb, and hindlimb clonus; 6 = 2 running phases followed by nearly complete tonic extension except hindfeet; 7 = 1 running phase followed by nearly complete tonic extension except hindfeet; 8 = 2 running phases followed by complete tonic extension; and 9 = 1 running phase followed by complete tonic extension. The maximum response was recorded for each animal.
Statistical Analysis
All statistical procedures were performed using SPSS 15.0.0 (IBM, Armonk, NY, USA). Comparison between acutely injected and subchronically treated groups of rats was accomplished using one-way analysis of variance followed by Dunnett’s post hoc analysis comparing every drug dose group with its own control rat group; for example clozapine (1 mg/kg) GEPR-3s group versus control GEPR-3s group. Data from chronic treatments were first grouped by strain subgroups (i.e., SD, GEPR-0s, GEPR-3s, and GEPR-9s) and then divided by week of treatment. Such divided data were then compared by one-way analysis of variance followed by Tukey’s post hoc test being treatment the only variable. For each GEPR, maximum response to auditory stimuli was recorded. A p-value ≤ 0.05 was considered significant for every test. Considering that a large number of comparisons might, in theory, engender false positive results (type 1 errors) in pharmacological research, we have reduced, at the minimum, the number of comparisons in our statistics. Furthermore, it is unlikely that false positive results are reported in our study for 2 reasons: 1) we use a relatively small sample size of mice—a situation that minimizes the occurrence of false positive findings (it is well known that p-value associated with a fixed effect is reduced as the sample size increases and vice versa); 2) the biological plausibility and the statistical consistency of study results [49].
Results
Effects of Acute Intraperitoneal Administration of Antipsychotic Drugs on Audiogenic Seizures
In order to evaluate the possible effects of acute administration of APs on audiogenic seizures in GEPRs, we used 3 subgroups of rats: the first group had no seizures (GEPR-0); the second group manifested the clonic component only (GEPR-3); and the third group comprised rats who had a full seizure response that culminated in a complete tonic extension (GEPR-9). These responses were identified in the 3 previous screening tests carried out between 6 and 8 weeks of the rat’s life.
Single i.p. administration of haloperidol (0.2 and 1.0 mg/kg), clozapine (1 and 5 mg/kg), risperidone (0.03, 0.13, and 0.50 mg/kg), quetiapine (2 and 10 mg/kg), aripiprazole (0.2, 0.5, and 1.0 mg/kg), or olanzapine (0.13 and 0.66 mg/kg) induced no significant changes in seizure score severity in GEPR-9s, GEPR-3s and GEPR-0s (data not shown).
No significant effect on the latency time from audiogenic stimulus, onset to the initiation of wild running or clonus in GEPR-3s, and tonus in GEPR-9s after systemic administration of the above-reported APs was observed (data not shown). All control animals (SD rats or GEPRs vehicle-treated) did not manifest significant changes in audiogenic seizures score following auditory stimulation, as previously described [50, 51].
Effects of Daily Antipsychotic Treatment on Severity of Audiogenic Seizures
Oral administration of haloperidol (0.2 and 1.0 mg/kg/day), clozapine (1 and 5 mg/kg/day), risperidone (0.03, 0.13, and 0.50 mg/kg/day), quetiapine (2 and 10 mg/kg/day), aripiprazole (0.2, 0.5, and 1.0 mg/kg/day), and olanzapine (0.13 and 0.66 mg/kg/day) was carried out for 5 days, and each group of rats and its vehicle control group received auditory stimulus once after 5 days.
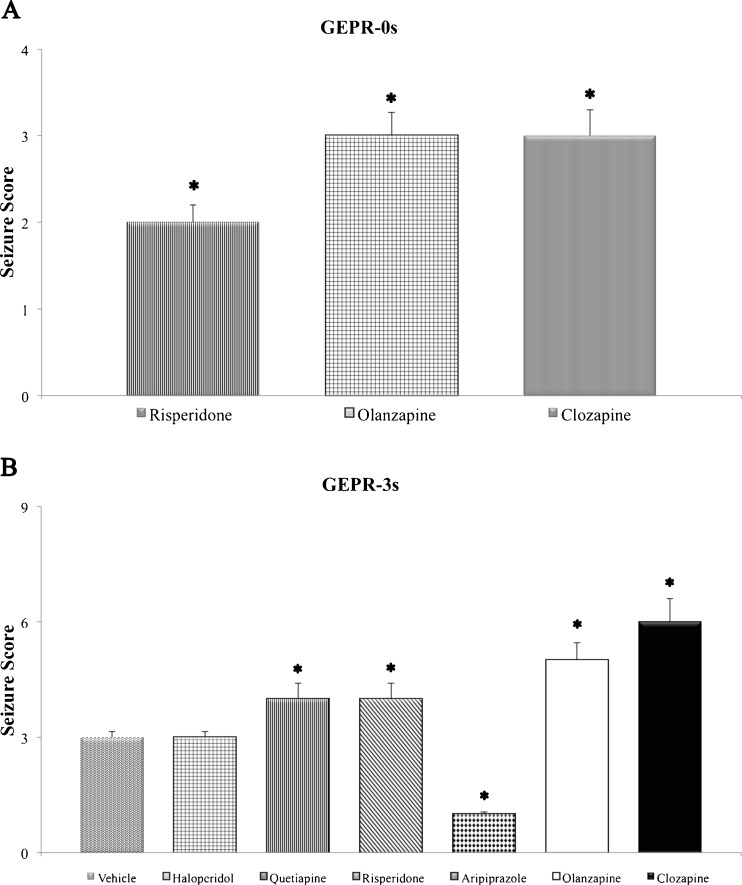
The oral subchronic treatment with APs for 5 days in SD rats did not induce the appearance of audiogenic seizures following auditory stimulation in comparison with the SD control group, as previously described (data not shown) [26, 51, 52]. In GEPR-0s, haloperidol, quetiapine, and aripiprazole did not induce any change compared with the control GEPR-0 group at all doses used, whereas clozapine, risperidone, and olanzapine were able to induce the appearance of clonic phase of the audiogenic seizures (score 2–3) at the highest doses used (Fig. 1a).
Fig. 1.
Effects of subchronic treatment with antipsychotic drugs on seizure severity in genetically epilepsy-prone rats (GEPRs). (a) Seizure score in GEPR-0s not displaying seizure in control conditions; (b) seizure score in GEPR-3s only displaying clonic phase in control conditions. Doses reported were the highest used in the study (haloperidol = 1 mg/kg; quetiapine = 10 mg/kg; risperidone = 0.5 mg/kg; aripiprazole = 1 mg/kg; clozapine = 5 mg/kg; olanzapine = 0.66 mg/kg). *Significantly (p < 0.05) different from respective controls (analysis of variance). Data are means ± SEM (n = 8 for each experimental group)
In GEPR-3s, subchronic treatment with aripiprazole reduced the incidence of clonus at a dose of 1 mg/kg, while haloperidol, at all doses used, induced no significant changes in seizure severity. In contrast, all other drugs significantly increased seizure score at the highest doses with the appearance in some cases of tonic phase in GEPR-3 rats (Fig. 1b). No significant changes in the incidence of audiogenic seizures after 5 days of treatment with APs were observed in GEPR-9s, with the exception of aripiprazole, which was able to reduce significantly the incidence and severity of seizures.
Effects of Chronic AP Treatment on Severity of Audiogenic Seizures
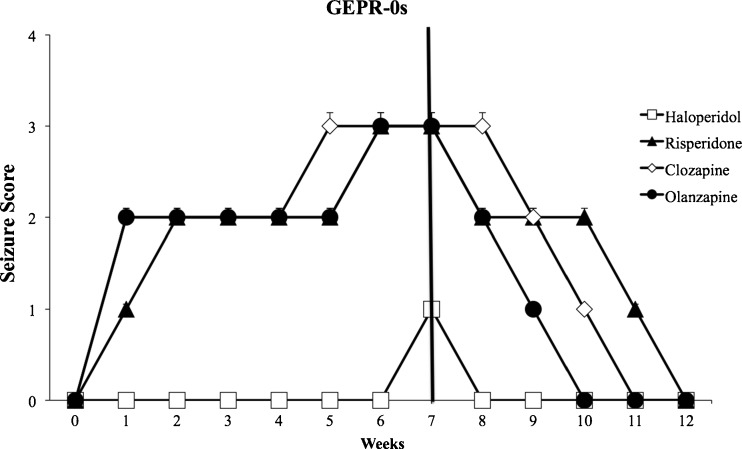
Chronic treatment in all groups and for every drug did not have any effect on rat weight (data not shown). In GEPR-0s, chronic administration of clozapine, risperidone, and olanzapine, at the highest doses, was able to induce the appearance of clonus after the first week of treatment, and significantly increased seizure score severity (p < 0.05), reaching a peak between the sixth and seventh week of treatment (Fig. 2). The effects of all low doses of antipsychotics are not reported as no marked changes in seizure score were observed. Chronic treatment with haloperidol was able to induce the appearance of clonus only on the last (week 7) week of treatment, whereas there were no significant changes on onset of audiogenic seizures with quetiapine and aripiprazole (Fig. 2).
Fig. 2.
Effects of chronic (7 weeks) antipsychotic treatment in genetically epilepsy-prone rats (GEPR)-0 s on seizure score. Note that 5 weeks after drug suspension, the black bar indicates treatment suspension. Risperidone, clozapine, and olanzapine significantly increased seizure score severity (p < 0.05) after the first week of treatment, reaching a peak between weeks 6 and 7; after withdrawal, this significant increase lasted for 3, 4, and 5 weeks for olanzapine, clozapine, and risperidone, respectively. Haloperidol had significant (p < 0.05) effects only during week 7 of treatment. Doses reported were the highest used in the study (haloperidol = 1 mg/kg; risperidone = 0.5 mg/kg; clozapine = 5 mg/kg; olanzapine = 0.66 mg/kg). Data are means ± SEM (n = 8 for each experimental group)
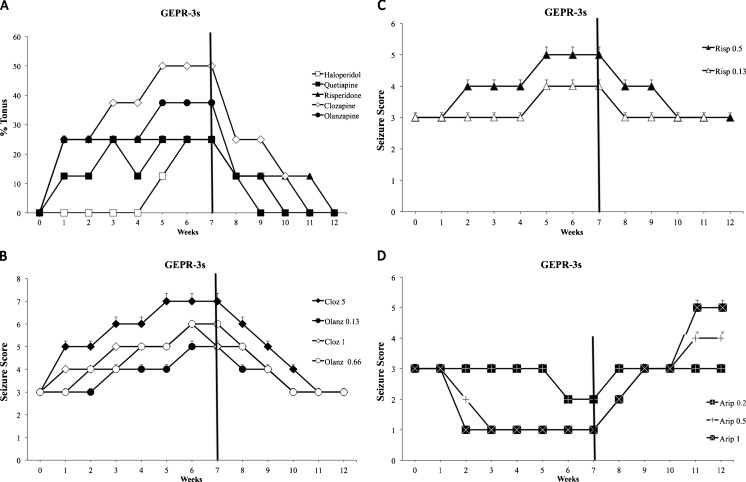
Repeated treatment with clozapine, risperidone, quetiapine, and olanzapine caused a significant (p < 0.05) increase in audiogenic seizure severity after the first week in GEPR-3s (Fig. 3a–c); this was maintained at least up to 3 weeks after drug cessation. In particular, the highest doses of these APs induced a significant increase in seizure score severity (up to 5–7) in GEPR-3s between weeks 5 and 7 of treatment. Haloperidol, at a dose of 1 mg/kg, produced a significant worsening (p < 0.05) of audiogenic seizure score, inducing the appearance of tonic phase (overall score 4–5) only between weeks 5 and 7 in GEPR-3s (Fig. 3a). In contrast, chronic treatment with aripiprazole (0.5 and 1.0 mg/kg) produced a significant (p < 0.05) reduction in seizure score severity; these effects were already evident in the second week of treatment and persisted for all 7 weeks of treatment and 1 further week after drug cessation (Fig. 3d). Aripiprazole significantly increased the latency time from stimulus onset to the initiation of wild running or clonus in GEPR-3s (data not shown).
Fig. 3.
Effects of chronic (7 weeks) antipsychotic treatment in genetically epilepsy-prone (GEPR)-3 s rats (clonus only) on seizure severity. Black bar indicates treatment suspension. Doses reported were the highest used in the study (haloperidol = 1 mg/kg; quetiapine = 10 mg/kg; risperidone = 0.5 mg/kg; clozapine = 5 mg/kg; olanzapine = 0.66 mg/kg), if not otherwise specified. (a) Effects of drugs on tonus incidence (% of rats with tonus). (b) Proconvulsant effects of clozapine (Cloz) and olanzapine (Olanz) at different doses; clozapine (1 and 5 mg/kg) significantly (p < 0.05) increased seizure score after the first week of treatment, returning to baseline levels 4–5 weeks after withdrawal. Olanzapine (0.13 and 0.66 mg/kg) significantly (p < 0.05) increased seizure score after the second week of treatment and returned to baseline 3 weeks after withdrawal. Note the strongest proconvulsant effects of clozapine 5 mg/kg. (c) Proconvulsant effects of risperidone (Risp) at different doses; risperidone (0.5 mg/kg) significantly (p < 0.05) increased seizure score in week 2 of treatment, returning to baseline levels 3 weeks after withdrawal, whereas at the dose of 0.13 mg/kg, risperidone significantly (p < 0.05) increased seizure score only between weeks 5 and 7 of treatment, with no withdrawal effects. (d) Effects of various aripiprazole (Arip) doses on seizure score; aripiprazole (0.5 and 1 mg/kg) significantly (p < 0.05) reduced seizure score after the first week up to the second week after withdrawal. Aripiprazole (0.2 mg/kg) significantly (p < 0.05) reduced seizure score only during weeks 6 and 7 of treatment with no withdrawal effects. Note the significant (p < 0.05) proconvulsant effects of aripiprazole (0.5 and 1 mg/kg) after the third week after withdrawal. Data are means ± SEM (n = 8 for each experimental group)
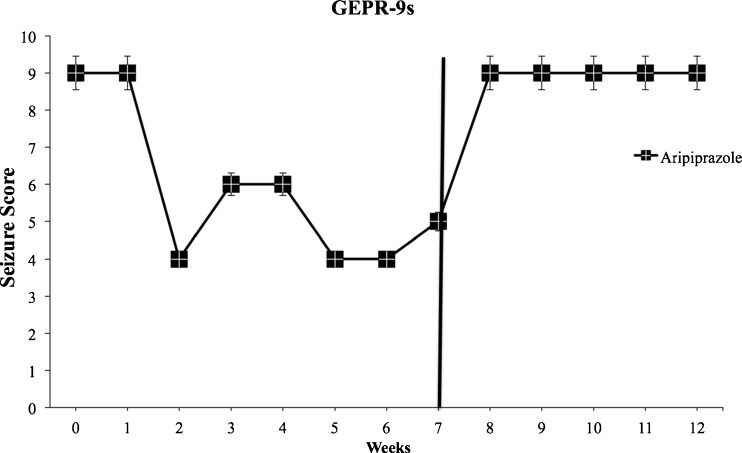
No significant changes in the incidence of audiogenic seizures after chronic administration of APs were observed in GEPR-9 rats compared with the GEPR-9 control group. In GEPR-9 rats, only chronic treatment with aripiprazole (1 mg/kg/day) was able to protect against audiogenic seizures, reducing seizure score severity, and increasing the onset time of wild running or clonus starting from the second week of treatment and lasting up to the end of drug treatment. Therefore, aripiprazole shows clear anticonvulsant properties in this animal model (Fig. 4). Chronic treatment with the same doses of APs produced no significant proconvulsant effects in SD rats.
Fig. 4.
Significant (p < 0.05) anticonvulsant effects of chronic (7 weeks) oral treatment with aripiprazole (1 mg/kg) in genetically epilepsy-prone (GEPR)-9 s from the second week without withdrawal effects. Black bar indicates treatment suspension. Data are means ± SEM (n = 8 for each experimental group)
Effects of the Withdrawal of APs on Audiogenic Seizure Susceptibility in GEPRs
In order to evaluate possible withdrawal effects, severity scores of audiogenic seizures in GEPRs were also determined for 5 weeks after the cessation of APs. The severity of audiogenic seizures returned to baseline levels during withdrawal of clozapine, risperidone, and olanzapine treatment in GEPR-0s after 3–5 weeks (Fig. 2). In GEPR-3 rats, for all the drugs increasing seizure severity, audiogenic seizure score returned to baseline levels (score = 3) after about 4 weeks (Fig. 3a). The severity of seizure score returned to baseline after the first haloperidol withdrawal week in GEPR-0s (Fig. 2), and after the third withdrawal week in GEPR-3s (Fig. 3a). In GEPR-3 rats, withdrawal of quetiapine induced a return of severity score of audiogenic seizures to baseline after 2 weeks (Fig. 3a).
In addition, aripiprazole withdrawal in both GEPR-3 and GEPR-9 groups induced a complete recovery of the seizure response after 1 week of withdrawal (Figs. 3d and 4). Of note, 3 weeks after suspension, a rebound effect was observed with the appearance of tonus in some previously treated GEPR-3 rats (Fig. 3d).
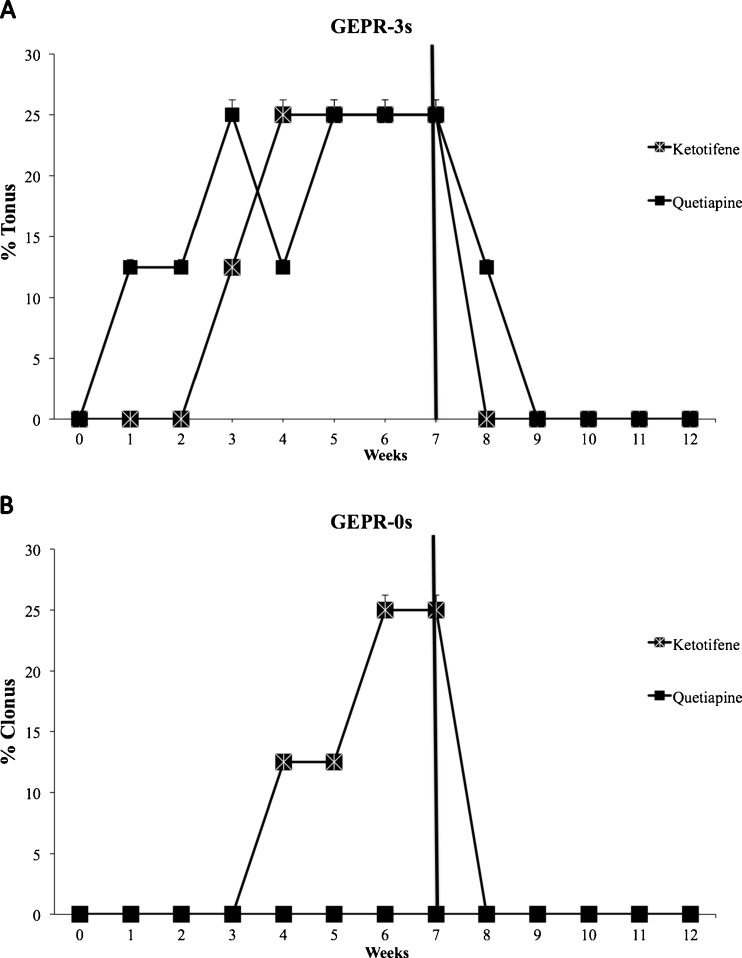
Effects of Acute, Subchronic, and Chronic Ketotifen Fumarate Treatment on Severity of Audiogenic Seizures in GEPR Groups
In order to evaluate the influence of H1 histamine antagonism on seizure severity and use it as a comparator for the APs used (see “Discussion”), single i.p. administration of ketotifen fumarate (3 mg/kg) was carried out. Acute administration and subchronic ketotifen fumarate treatment produced no significant changes in seizure severity scores in GEPR-9s, GEPR-3s, and GEPR-0s (data not shown). Oral chronic administration of ketotifen fumarate (3 mg/kg/day) was carried out for 7 weeks, and rats received auditory stimuli once every week, as described above for APs. Ketotifen fumarate produced a significant increase in seizure severity score in GEPR-3s after the third week of treatment, while no significant changes in the incidence of audiogenic seizures after ketotifen fumarate administration in GEPR-9s were observed. Such changes were of minor severity compared with those observed after clozapine and some atypical antipsychotics (Fig. 5a). In GEPR-0 rats, chronic ketotifen fumarate treatment induced the appearance of clonic phase (score 3) at the week 5–6 of treatment. In addition, the effects of chronic ketotifen fumarate treatment on audiogenic seizures were determined for 5 weeks after drug withdrawal (Fig. 5b). The severity of audiogenic seizures returned to control levels for both GEPR-0s and GEPR-3s after the first week.
Fig. 5.
Effects of ketotifen fumarate (3 mg/kg; Ketotifene; H1 histaminergic antagonist) and quetiapine (10 mg/kg) on seizure scores in genetically epilepsy-prone rats (GEPR)-3 s (panel a; % of rats with tonus) and GEPR-0s (panel b; % of rats with clonus) rats. Black bar indicates treatment suspension. Data are means ± SEM (n = 8 for each experimental group)
Discussion
General Considerations on the Effect of APs on Seizures
It is generally accepted that APs can lower seizure threshold or provoke seizures [3, 8, 17]. Despite the fact that APs show efficacy in the treatment of psychosis, several differences exist in their mechanisms of action, which might differentially contribute to their effects on seizures. The present study aimed at modeling some clinical scenarios with treatment with APs: 1) rats belonging to a genetically prone epilepsy strain that have no manifest tonic and/or clonic seizure (first treatment group or GEPR-0s; people with a low seizure threshold but without previous clinical manifestation); 2) rats that have clonic seizures (second treatment group or GEPR-3s; epileptic patients); 3) rats that have manifest tonic–clonic seizures (third treatment group or GEPR-9s; epileptic patients); 4) rats that have no manifest tonic and/or clonic seizure and not belonging to genetically prone epilepsy strain currently taking their antipsychotic medication (SD control group; analogous to humans without a propensity for seizures).
Our results demonstrate that chronic treatment with some atypical antipsychotics enhances seizure susceptibility in GEPRs and that such increased seizure susceptibility is long-lasting after drug withdrawal, indicating that APs might induce plastic changes in brain that could facilitate hyperexcitability. In contrast, haloperidol, a typical AP, had only minimal proconvulsant effects, while aripiprazole showed a clear anticonvulsant action although, upon withdrawal, a rebound proconvulsant effect was noted.
More specifically, no proconvulsant effects were observed in SD rats (not seizure prone). Such a result might support the absence of effects on brain excitability in rats without seizure predisposition or at least not sufficient to increase seizure susceptibility. However, it must be considered that in our experimental protocol only reflex audiogenic seizures were tested and therefore the effect of APs treatment on other seizure types (spontaneous or induced) is not known. In GEPRs: 1) haloperidol, in general, did not significantly affect audiogenic seizure occurrence with increasing severity only at the end of chronic treatment (after 5–6 weeks of administration); 2) clozapine showed the most marked proconvulsant effects; 3) risperidone and olanzapine were both proconvulsant but less so than clozapine; 4) quetiapine showed only modest proconvulsant properties; 5) aripiprazole has anticonvulsant properties; 6) risperidone, in comparison with other APs, maintained its proconvulsant effects after withdrawal for some weeks, more than other compounds, whereas aripiprazole had a proconvulsant rebound effect.
Despite the action of APs on several neurotransmitter receptor subtypes, none of the drugs tested in this animal model showed acute effects on seizures. However, chronic administration of APs is known to alter the regional density of several neurotransmitter receptors in the central nervous system, including those for dopamine, serotonin, acetylcholine, histamine, and glutamate, that may contribute to the observed proconvulsant effects of some antipsychotics [42, 53, 54]. These changes, in particular upregulation of dopamine receptors, have been suggested as the basis for some of the adverse effects that occur with long-term antipsychotic therapy [55]. The effects of APs and their known affinities for several receptor subtypes are listed in Table 2.
Table 2.
Typical and atypical antipsychotic drugs: mechanisms of action and relative affinities to central nervous system neurotransmitter receptors
| Receptor subtypes | Quetiapine (A) | Risperidone (A) | Aripiprazole (A) | Olanzapine (A) | Clozapine (A) | Haloperidol (T) | Ref. |
|---|---|---|---|---|---|---|---|
| D2 | AT + |
AT +++ |
PA ++++ |
AT ++ |
AT + |
AT +++ |
[56, 57] |
| 5-HT1A | PA + |
AT + |
PA +++ |
AT (*) |
PA + |
AT (*) |
[56, 58–61] |
| 5-HT2A | AT + |
AT ++++ |
AT +++ |
AT +++ |
AT +++ |
AT ++ |
[60, 62, 63] |
| 5-HT2C | IA (*) |
IA ++ |
PA ++ |
IA +++ |
IA ++ |
AT (*) |
[58, 60, 64] |
| 5-HT7 | AT + |
AT +++ |
PA +++ |
AT + |
AT ++ |
AT + |
[60, 64, 65] |
| α1A | AT ++ |
AT +++ |
AT ++ |
AT + |
AT +++ |
AT ++ |
[64, 66] |
| α2A | AT* | AT + |
AT ++ |
AT + |
AT + |
AT* | [58, 66, 67] |
| α2B | AT + |
AT + |
AT + |
AT ++ |
AT ++ |
AT + |
[58, 66, 67] |
| α2C | AT ++ |
AT +++ |
AT ++ |
AT ++ |
AT ++ |
AT + |
[58, 66–68] |
| M1 | AT + |
AT* | AT* | AT ++ |
AT ++ |
AT* | [69] |
| M3 | AT* | AT* | AT* | AT + |
AT ++ |
AT* | [69] |
| H1 | AT ++ |
AT ++ |
AT ++ |
AT +++ |
AT +++ |
AT* | [70] |
A = atypical antipsychotic drug; T = typical antipsychotic drug; 5-HT = serotonin receptors; D = dopamine receptors; AT = antagonist; PA = partial agonist; IA = inverse agonist; α = noradrenaline receptors; M = acetylcholine receptors; H = histamine receptors; ; + = weak binding affinity (100 nM < Ki <1000 nM); ++ = moderate binding affinity (10 nM < Ki <100 nM); +++ = strong binding affinity (1 nM < Ki <10 nM); ++++ = very strong binding affinity (Ki <1 nM)
*Negligible binding affinity (Ki >1000 nM)
Indeed, the proconvulsant effects of clozapine, risperidone, quetiapine, and olanzapine could be explained, in part, via their interactions with serotonergic and/or dopaminergic receptor systems, even though other mechanisms also possessed by such drugs could account for the observed proconvulsant action. Several APs are reported to affect GABAergic neurotransmission; in particular, some of them, including clozapine, inhibit GABA response on the GABAA receptor–chloride channel complex. The inhibitory effect of some APs on GABA-induced chloride currents was ranked as follows: clozapine>zotepine>chlorpromazine>olanzapine>risperidone [71]. This rank is in agreement with our results; however, some of the APs used in our study were not previously evaluated. Finally, all tested APs block noradrenergic α2 receptors, which is also known to increase audiogenic seizure susceptibility [72], and, most noticeably, clozapine, risperidone, and olanzapine, which are the most proconvulsant APs of this group, differ from the others by their ability to act as inverse agonist on 5-hydroxytryptamine (HT) 2c receptors, which has been previously suggested to play a role in epilepsy and particularly in audiogenic seizures [73, 74].
The effects of each drug are considered below.
Haloperidol
Haloperidol, which possesses antagonist activity at D2 dopamine receptors, demonstrated a very modest proconvulsant activity in our model, and this might suggest that dopaminergic neurotransmission at D2 receptors is not involved in the proconvulsant effects observed in GEPR strain after chronic atypical antipsychotic treatment (also supported by the highest proconvulsant effects during clozapine treatment, which, among the APs used, is the one with the lowest affinity for D2 receptors). Furthermore, haloperidol binds with a low affinity to the adrenergic (α1 and α2) and histaminergic H1 receptors [75, 76]. We investigated the effects of haloperidol on audiogenic seizure in epileptic rats, demonstrating that this drug did not significantly increase seizure incidence and/or severity (Figs. 1b, 2, 3a). Our data are in agreement with that observed in human therapy where haloperidol is one of the agents with the least seizure-induction activity among antipsychotics [8].
Clozapine
Clozapine was the AP with the highest significant proconvulsant effect on GEPRs, and these effects persisted for 3–4 weeks during withdrawal in chronically treated rats, suggesting that its effects are long-lasting over its elimination [77]. Clozapine is a multireceptor acting AP; it has antagonistic activity on dopaminergic receptors, with a higher affinity at the D1 and D4 receptors than at the D2 receptors, also binding to the extra-striatal D3 receptors. Clozapine has also antagonistic activity at the 5-HT1A, 5-HT2A, 5-HT2C, and 5-HT3 receptors [75]. Our results are in agreement with previous reports indicating that clozapine is the atypical AP that more frequently is associated with seizures [17], or involving seizures/lowered seizure threshold associated with its use [78, 79]. The incidence of audiogenic seizures was significantly increased by clozapine chronic treatment in all subgroups of GEPRs (Figs. 1a, b; 2; 3a, b). At odds are the 5-HT2 and/or 5-HT3 antagonistic properties of clozapine, which were indicated to be responsible for its inhibitory effects on audiogenic seizures in ethanol withdrawal syndrome (EWS) in rats [80]. The observed discrepancy between audiogenic seizures in GEPRs and those in EWS rats may be due to differences between the 2 strains and the likely different mechanisms involved in the pathogenesis of ethanol withdrawal-induced audiogenic seizures [81–83].
Serotonergic transmission has been suggested to be involved in seizures in GEPRs; in particular, a previous study showed that the severity of audiogenic seizures was decreased in a dose-dependent fashion by fluoxetine, a selective serotonin reuptake inhibitor [84].
Clozapine displays strong affinity for several dopamine receptor subtypes and it has been proposed that such changes have selectivity for mesolimbic dopamine receptors, which might account for its proconvulsant effects [41]. However, clozapine is an antagonist with a high affinity at α2-adrenoceptors [66, 85]; this mechanism may also be responsible for proconvulsant effects on audiogenic seizures in GEPRs, as previously described for other compounds acting on α2-adrenoceptors [86].
Risperidone
Risperidone has a high affinity for the 5-HT2A receptors, with a D2 receptor affinity similar to most typical APs. Compared with typical APs, it also binds with a lower affinity at the adrenergic (α1 and α2) and H1 histaminergic receptors [75, 76]. We found that risperidone increases the incidence and the severity of the audiogenic seizures (Figs. 1a, b; 2; 3a, c). The latter data are partially in contrast with that observed in human therapy where risperidone is an agent with the lowest seizure induction activity among APs [8]. However, a previous clinical study indicated that risperidone overdose is able to induce seizures [87].
Quetiapine and Ketotifen Fumarate
Quetiapine, another atypical AP, has similar receptor-binding properties to clozapine, but with relatively lower affinity for all receptors and nearly no affinity for muscarinic and 5-HT2c receptors, and a higher relative affinity for H1 and α1 adrenergic receptors [76]. Quetiapine also increased the severity of audiogenic seizures, but we observed that such effects disappeared within 2 weeks of withdrawal (Figs. 1b; 3a; 5a, b). This proconvulsant effect observed in GEPR strain was opposite to the anticonvulsant properties in EWS rats reported by Celikyurt et al. [20].
Considering that the main difference between quetiapine and the other APs studied is represented by its strong relative anti-H1 activity and that H1 antagonists are known to be proconvulsant [88, 89]; we evaluated the effects of ketotifen fumarate in order to establish whether a link between quetiapine effects and such a mechanism of action would be responsible to the observed lower proconvulsant effects compared with other APs. We found that ketotifen fumarate was also proconvulsant; therefore, we can conclude that other mechanisms are involved in the lower proconvulsant activity of quetiapine. Furthermore, the proconvulsant effect of ketotifen fumarate due to H1 receptor block also supports the idea that such a mechanism can contribute to the proconvulsant effects of all APs tested.
Aripiprazole
Aripiprazole was the only AP with anticonvulsant properties in this genetic model of audiogenic epilepsy (Figs. 1b; 3d; 4). Aripiprazole is a partial D2 agonist and an antagonist/partial agonist of 5-HT2 receptors [90]. Aripiprazole has only 0.1 % seizure-inducing potential, much lower than other APs [91, 92]. The effects of aripiprazole were observed at highest doses characterized by occupancy of >90 % of D2 receptors [90]. The effects of aripiprazole might be owing to its partial agonistic effects on D2 and 5-HT2c receptors.
Olanzapine
We have shown worsened audiogenic seizures during olanzapine chronic treatment, and such effects were observed for 3–4 weeks, even after withdrawal (Figs. 1a, b; 2; 3a,b).
Olanzapine has a high binding ratio for 5-HT2A, D2, D4, and H1 receptors. Similarly to clozapine, olanzapine is an antagonist of dopamine (D1–D4) and 5-HT2A receptors [93]. As doses of olanzapine >2 mg/kg caused sedative effects in rats [43], doses of 0.13 and 0.66 mg/kg of olanzapine were used in the present study. We observed that olanzapine enhances the severity of audiogenic seizures, and this effect may be explained by its serotonin 5-HT2, dopamine D2, and histamine H1 receptor antagonistic activity. Our results are in agreement with other previous reports in which olanzapine treatment was associated with seizures or lowered seizure threshold [9, 94, 95]. The difference observed in comparison with clozapine might be due to the dose used and higher doses might have similar effects to those observed during clozapine treatment.
Conclusions
In summary, these results confirm that APs might have potential in increasing the severity of audiogenic seizures but, more interestingly, that aripiprazole alone exerts anticonvulsant effects. The low incidence of seizures related to the use of aripiprazole in patients, together with our results, seems promising. However, further studies in other animal models of epilepsy are required to confirm this action.
APs showed proconvulsant effects only in seizure-prone animals and after 5 days or more of treatment. This might indicate that: 1) APs may need an already established predisposition toward hyperexcitability in order to promote seizures, even though it cannot be excluded that seizure thresholds for other seizures types might have been lowered and not observed; 2) acute treatment seems to have no effects and therefore it is very likely that most of our results might be due to plastic changes occurring during longer periods of treatment, which are, in any case, reversible when stopping treatment. In conclusion, the use of APs in patients, and particularly in patients with epilepsy, should be strictly monitored for the occurrence of seizures; however, attention should also be paid to the withdrawal of APs. Further studies will determine whether aripiprazole really has potential as an anticonvulsant drug and be clinically relevant for patients with epilepsy with psychiatric comorbidities such as psychosis and mood disorders.
Electronic supplementary material
(PDF 499 kb)
Acknowledgments
The European Commission, the European Social Fund, and the Calabria Region Government are gratefully acknowledged for their support with student grants. All such organizations only provided economical support without interfering with the content of the present article.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Disclosure/conflict of interest
None of the authors has any conflict of interest to disclose in relation to this work. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Marks RC, Luchins DJ. Antipsychotic medications and seizures. Psychiatr Med. 1991;9:37–52. [PubMed] [Google Scholar]
- 2.Trifiro G, Spina E. Age-related changes in pharmacodynamics: focus on drugs acting on central nervous and cardiovascular systems. Curr Drug Metab. 2011;12:611–620. doi: 10.2174/138920011796504473. [DOI] [PubMed] [Google Scholar]
- 3.Arana GW. An overview of side effects caused by typical antipsychotics. J Clin Psychiatry. 2000;61(Suppl. 8):5–11. [PubMed] [Google Scholar]
- 4.Gareri P, De Fazio P, Stilo M, Ferreri G, De Sarro G. Conventional and atypical antipsychotics in the elderly: A review. Clin Drug Investig. 2003;23:287–322. doi: 10.2165/00044011-200323050-00001. [DOI] [PubMed] [Google Scholar]
- 5.Gareri P, De Fazio P, De Fazio S, et al. Adverse effects of atypical antipsychotics in the elderly: A review. Drugs Aging. 2006;23:937–956. doi: 10.2165/00002512-200623120-00002. [DOI] [PubMed] [Google Scholar]
- 6.Gareri P, De Fazio P, Russo E, Marigliano N, De Fazio S, De Sarro G. The safety of clozapine in the elderly. Expert Opin Drug Saf. 2008;7:525–538. doi: 10.1517/14740338.7.5.525. [DOI] [PubMed] [Google Scholar]
- 7.Alldredge BK. Seizure risk associated with psychotropic drugs: clinical and pharmacokinetic considerations. Neurology. 1999;53:S68–75. [PubMed] [Google Scholar]
- 8.Lertxundi U, Hernandez R, Medrano J, Domingo-Echaburu S, Garcia M, Aguirre C. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22:141–143. doi: 10.1016/j.seizure.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Hedges D, Jeppson K, Whitehead P. Antipsychotic medication and seizures: a review. Drugs Today (Barc) 2003;39:551–557. doi: 10.1358/dot.2003.39.7.799445. [DOI] [PubMed] [Google Scholar]
- 10.Kanner AM. Can neurobiological pathogenic mechanisms of depression facilitate the development of seizure disorders? Lancet Neurol. 2012;11:1093–1102. doi: 10.1016/S1474-4422(12)70201-6. [DOI] [PubMed] [Google Scholar]
- 11.Kanner AM, Schachter SC, Barry JJ, et al. Depression and epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav. 2012;24:156–168. doi: 10.1016/j.yebeh.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Kanner AM. Is depression a risk factor of worse response to therapy in epilepsy? Epilepsy Curr. 2011;11:50–51. doi: 10.5698/1535-7511-11.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanner AM. Depression and epilepsy: A bidirectional relation? Epilepsia. 2011;52(Suppl. 1):21–27. doi: 10.1111/j.1528-1167.2010.02907.x. [DOI] [PubMed] [Google Scholar]
- 14.Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59:35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- 15.Lomas J, Boardman RH, Markowe M. Complications of chlorpromazine therapy in 800 mental-hospital patients. Lancet. 1955;268:1144–1147. doi: 10.1016/S0140-6736(55)90642-5. [DOI] [PubMed] [Google Scholar]
- 16.Casey DE. The relationship of pharmacology to side effects. J Clin Psychiatry. 1997;58(Suppl. 10):55–62. [PubMed] [Google Scholar]
- 17.Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19:69–73. doi: 10.1016/j.seizure.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Pisani F, Oteri G, Costa C, Di Raimondo G, Di Perri R. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25:91–110. doi: 10.2165/00002018-200225020-00004. [DOI] [PubMed] [Google Scholar]
- 19.Stevens JR, Denney D, Szot P. Kindling with clozapine: behavioral and molecular consequences. Epilepsy Res. 1996;26:295–304. doi: 10.1016/S0920-1211(96)00061-7. [DOI] [PubMed] [Google Scholar]
- 20.Celikyurt IK, Kayir H, Ulak G, Erden FB, Ulusoy GK, Uzbay TI. Effects of risperidone, quetiapine and ziprasidone on ethanol withdrawal syndrome in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:528–536. doi: 10.1016/j.pnpbp.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Kayir H, Uzbay T. Effects of clozapine on ethanol withdrawal syndrome in rats. Alcohol Alcohol. 2008;43:619–625. doi: 10.1093/alcalc/agn052. [DOI] [PubMed] [Google Scholar]
- 22.Unsalan N, Saglam E, Kayir H, Uzbay T. Effects of olanzapine on ethanol withdrawal syndrome in rats. Eur J Pharmacol. 2008;579:208–214. doi: 10.1016/j.ejphar.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Russo E, Citraro R, Davoli A, Gallelli L, Di Paola ED, De Sarro G. Ameliorating effects of aripiprazole on cognitive functions and depressive-like behavior in a genetic rat model of absence epilepsy and mild-depression comorbidity. Neuropharmacology. 2013;64:371–379. doi: 10.1016/j.neuropharm.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Faingold CL. Neuronal networks in the genetically epilepsy-prone rat. Adv Neurol. 1999;79:311–321. [PubMed] [Google Scholar]
- 25.De Sarro G, De Sarro A, Ammendola D, Patel S. Lack of development of tolerance to anticonvulsant effects of two excitatory amino acid antagonists, CGP [corrected] 37849 and CGP 39551 in genetically epilepsy-prone rats. Brain Res. 1996;734:91–97. doi: 10.1016/0006-8993(96)00616-6. [DOI] [PubMed] [Google Scholar]
- 26.De Sarro A, Grasso S, Zappala M, Nava F, De Sarro G. Convulsant effects of some xanthine derivatives in genetically epilepsy-prone rats. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:48–55. doi: 10.1007/PL00005027. [DOI] [PubMed] [Google Scholar]
- 27.Dailey JW, Reigel CE, Mishra PK, Jobe PC. Neurobiology of seizure predisposition in the genetically epilepsy-prone rat. Epilepsy Res. 1989;3:3–17. doi: 10.1016/0920-1211(89)90063-6. [DOI] [PubMed] [Google Scholar]
- 28.Mishra PK, Dailey JW, Reigel CE, Jobe PC. Audiogenic convulsions in moderate seizure genetically epilepsy-prone rats (GEPR-3s) Epilepsy Res. 1989;3:191–198. doi: 10.1016/0920-1211(89)90023-5. [DOI] [PubMed] [Google Scholar]
- 29.Reigel CE, Jobe PC, Dailey JW, Savage DD. Ontogeny of sound-induced seizures in the genetically epilepsy-prone rat. Epilepsy Res. 1989;4:63–71. doi: 10.1016/0920-1211(89)90059-4. [DOI] [PubMed] [Google Scholar]
- 30.Reigel CE, Dailey JW, Jobe PC. The genetically epilepsy-prone rat: an overview of seizure-prone characteristics and responsiveness to anticonvulsant drugs. Life Sci. 1986;39:763–774. doi: 10.1016/0024-3205(86)90454-6. [DOI] [PubMed] [Google Scholar]
- 31.Dailey JW, Jobe PC. Anticonvulsant drugs and the genetically epilepsy-prone rat. Fed Proc. 1985;44:2640–2644. [PubMed] [Google Scholar]
- 32.De Sarro GB, De Sarro A. Anticonvulsant properties of non-competitive antagonists of the N-methyl-D-aspartate receptor in genetically epilepsy-prone rats: comparison with CPPene. Neuropharmacology. 1993;32:51–58. doi: 10.1016/0028-3908(93)90129-Q. [DOI] [PubMed] [Google Scholar]
- 33.De Sarro A, De Sarro GB. Responsiveness of genetically epilepsy-prone rats to aminophylline-induced seizures and interactions with quinolones. Neuropharmacology. 1991;30:169–176. doi: 10.1016/0028-3908(91)90200-U. [DOI] [PubMed] [Google Scholar]
- 34.Jobe PC, Picchioni AL, Chin L. Role of brain norepinephrine in audiogenic seizure in the rat. J Pharmacol Exp Ther. 1973;184:1–10. [PubMed] [Google Scholar]
- 35.Jobe PC, Mishra PK, Ludvig N, Dailey JW. Scope and contribution of genetic models to an understanding of the epilepsies. Crit Rev Neurobiol. 1991;6:183–220. [PubMed] [Google Scholar]
- 36.Citraro R, Scicchitano F, De Fazio S, et al. Preclinical activity profile of alpha-lactoalbumin, a whey protein rich in tryptophan, in rodent models of seizures and epilepsy. Epilepsy Res. 2011;95:60–69. doi: 10.1016/j.eplepsyres.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Mishra PK, Dailey JW, Reigel CE, Jobe PC. Brain norepinephrine and convulsions in the genetically epilepsy-prone rat: Sex-dependent responses to Ro 4-1284 treatment. Life Sci. 1988;42:1131–1137. doi: 10.1016/0024-3205(88)90607-8. [DOI] [PubMed] [Google Scholar]
- 38.Jobe PC, Dailey JW, Reigel CE. Noradrenergic and serotonergic determinants of seizure susceptibility and severity in genetically epilepsy-prone rats. Life Sci. 1986;39:775–782. doi: 10.1016/0024-3205(86)90455-8. [DOI] [PubMed] [Google Scholar]
- 39.Gareri P, Condorelli D, Belluardo N, et al. Anticonvulsant effects of carbenoxolone in genetically epilepsy prone rats (GEPRs) Neuropharmacology. 2004;47:1205–1216. doi: 10.1016/j.neuropharm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Russo E, Citraro R, Scicchitano F, et al. Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia. 2011;52:1341–1350. doi: 10.1111/j.1528-1167.2011.03112.x. [DOI] [PubMed] [Google Scholar]
- 41.Minabe Y, Watanabe K, Nishimura T, Ashby CR., Jr Acute and chronic administration of clozapine produces greater proconvulsant actions than haloperidol on focal hippocampal seizures in freely moving rats. Synapse. 1998;29:272–278. doi: 10.1002/(SICI)1098-2396(199807)29:3<272::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 42.Tarazi FI, Baldessarini RJ, Kula NS, Zhang K. Long-term effects of olanzapine, risperidone, and quetiapine on ionotropic glutamate receptor types: implications for antipsychotic drug treatment. J Pharmacol Exp Ther. 2003;306:1145–1151. doi: 10.1124/jpet.103.052597. [DOI] [PubMed] [Google Scholar]
- 43.Ortega-Alvaro A, Gibert-Rahola J, Mico JA. Influence of chronic treatment with olanzapine, clozapine and scopolamine on performance of a learned 8-arm radial maze task in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:104–111. doi: 10.1016/j.pnpbp.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Russo E, Citraro R, Scicchitano F, et al. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia. 2010;51:1560–1569. doi: 10.1111/j.1528-1167.2009.02400.x. [DOI] [PubMed] [Google Scholar]
- 45.Russo E, Citraro R, Scicchitano F, et al. Vigabatrin has antiepileptogenic and antidepressant effects in an animal model of epilepsy and depression comorbidity. Behav Brain Res. 2011;225:373–376. doi: 10.1016/j.bbr.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 46.Russo E, Citraro R, Donato G, et al. mTOR inhibition modulates epileptogenesis, seizures and depressive behavior in a genetic rat model of absence epilepsy. Neuropharmacology. 2013;69:25–36. doi: 10.1016/j.neuropharm.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Citraro R, Chimirri S, Aiello R, et al. Protective effects of some statins on epileptogenesis and depressive-like behavior in WAG/Rij rats, a genetic animal model of absence epilepsy. Epilepsia. 2014;55:1284–1291. doi: 10.1111/epi.12686. [DOI] [PubMed] [Google Scholar]
- 48.Jobe PC, Dailey JW. Neurobiology of seizure predisposition in the genetically epilepsy prone rat. Proc West Pharmacol Soc. 1991;34:223–225. [PubMed] [Google Scholar]
- 49.Russo E, Donato di Paola E, Gareri P, et al. Pharmacodynamic potentiation of antiepileptic drugs’ effects by some HMG-CoA reductase inhibitors against audiogenic seizures in DBA/2 mice. Pharmacol Res 2013;70:1–12. [DOI] [PubMed]
- 50.De Sarro G, Trimarchi GR, Federico F, De Sarro A. Anticonvulsant activity of some calcium antagonists in genetically epilepsy prone rats. Epilepsy Res Suppl. 1991;3:49–55. [PubMed] [Google Scholar]
- 51.Russo E, Citraro R, De Fazio S, Torcasio G, De Sarro G, Di Paola ED. Effects of ethanol on the development of genetically determined epilepsies in rats. Int J Dev Neurosci. 2008;26:739–744. doi: 10.1016/j.ijdevneu.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Gareri P, Cotroneo A, Lacava R, et al. Comparison of the efficacy of new and conventional antipsychotic drugs in the treatment of behavioral and psychological symptoms of dementia (BPSD). Arch Gerontol Geriatr Suppl 2004:207–215. [DOI] [PubMed]
- 53.Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on serotonin 1A, 2A and 2C receptors in rat forebrain regions. Psychopharmacology (Berl) 2002;161:263–270. doi: 10.1007/s00213-002-1016-3. [DOI] [PubMed] [Google Scholar]
- 54.Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmacother. 1999;33:554–556. doi: 10.1345/aph.17385. [DOI] [PubMed] [Google Scholar]
- 55.Tarazi FI, Florijn WJ, Creese I. Differential regulation of dopamine receptors after chronic typical and atypical antipsychotic drug treatment. Neuroscience. 1997;78:985–996. doi: 10.1016/S0306-4522(96)00631-8. [DOI] [PubMed] [Google Scholar]
- 56.Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M. Dopamine D2 and serotonin 5-HT1A receptors mediate the actions of aripiprazole in mesocortical and mesoaccumbens transmission. Neuropharmacology. 2012;62:765–774. doi: 10.1016/j.neuropharm.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 57.Strange PG. Antipsychotic drugs: Importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacol Rev. 2001;53:119–133. [PubMed] [Google Scholar]
- 58.Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 59.McIntyre RS, Soczynska JK, Woldeyohannes HO, Alsuwaidan M, Konarski JZ. A preclinical and clinical rationale for quetiapine in mood syndromes. Expert Opin Pharmacother. 2007;8:1211–1219. doi: 10.1517/14656566.8.9.1211. [DOI] [PubMed] [Google Scholar]
- 60.Meltzer HY, Massey BW. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr Opin Pharmacol. 2011;11:59–67. doi: 10.1016/j.coph.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Lerond J, Lothe A, Ryvlin P, et al. Effects of aripiprazole, risperidone, and olanzapine on 5-HT1A receptors in patients with schizophrenia. J Clin Psychopharmacol. 2013;33:84–89. doi: 10.1097/JCP.0b013e31827b97a6. [DOI] [PubMed] [Google Scholar]
- 62.Love RC, Nelson MW. Pharmacology and clinical experience with risperidone. Expert Opin Pharmacother. 2000;1:1441–1453. doi: 10.1517/14656566.1.7.1441. [DOI] [PubMed] [Google Scholar]
- 63.Yadav PN, Kroeze WK, Farrell MS, Roth BL. Antagonist functional selectivity: 5-HT2A serotonin receptor antagonists differentially regulate 5-HT2A receptor protein level in vivo. J Pharmacol Exp Ther. 2011;339:99–105. doi: 10.1124/jpet.111.183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shapiro DA, Renock S, Arrington E, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 65.Teitler M, Toohey N, Knight JA, Klein MT, Smith C. Clozapine and other competitive antagonists reactivate risperidone-inactivated h5-HT7 receptors: radioligand binding and functional evidence for GPCR homodimer protomer interactions. Psychopharmacology (Berl) 2010;212:687–697. doi: 10.1007/s00213-010-2001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Svensson TH. Alpha-adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1145–1158. doi: 10.1016/j.pnpbp.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Brosda J, Jantschak F, Pertz HH. alpha2-Adrenoceptors are targets for antipsychotic drugs. Psychopharmacology (Berl) 2014;231:801–812. doi: 10.1007/s00213-014-3459-8. [DOI] [PubMed] [Google Scholar]
- 68.Kalkman HO, Loetscher E. alpha2C-Adrenoceptor blockade by clozapine and other antipsychotic drugs. Eur J Pharmacol. 2003;462:33–40. doi: 10.1016/S0014-2999(03)01308-6. [DOI] [PubMed] [Google Scholar]
- 69.Bymaster FP, Felder CC, Tzavara E, Nomikos GG, Calligaro DO, McKinzie DL. Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1125–1143. doi: 10.1016/j.pnpbp.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 70.He M, Deng C, Huang XF. The role of hypothalamic H1 receptor antagonism in antipsychotic-induced weight gain. CNS Drugs. 2013;27:423–434. doi: 10.1007/s40263-013-0062-1. [DOI] [PubMed] [Google Scholar]
- 71.Yokota K, Tatebayashi H, Matsuo T, et al. The effects of neuroleptics on the GABA-induced Cl- current in rat dorsal root ganglion neurons: differences between some neuroleptics. Br J Pharmacol. 2002;135:1547–1555. doi: 10.1038/sj.bjp.0704608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan QS, Dailey JW, Steenbergen JL, Jobe PC. Anticonvulsant effect of enhancement of noradrenergic transmission in the superior colliculus in genetically epilepsy-prone rats (GEPRs): a microinjection study. Brain Res. 1998;780:199–209. doi: 10.1016/S0006-8993(97)01139-6. [DOI] [PubMed] [Google Scholar]
- 73.Brennan TJ, Seeley WW, Kilgard M, Schreiner CE, Tecott LH. Sound-induced seizures in serotonin 5-HT2c receptor mutant mice. Nat Genet. 1997;16:387–390. doi: 10.1038/ng0897-387. [DOI] [PubMed] [Google Scholar]
- 74.Applegate CD, Tecott LH. Global increases in seizure susceptibility in mice lacking 5-HT2C receptors: a behavioral analysis. Exp Neurol. 1998;154:522–530. doi: 10.1006/exnr.1998.6901. [DOI] [PubMed] [Google Scholar]
- 75.Richtand NM, Welge JA, Logue AD, Keck PE, Jr, Strakowski SM, McNamara RK. Dopamine and serotonin receptor binding and antipsychotic efficacy. Neuropsychopharmacology. 2007;32:1715–1726. doi: 10.1038/sj.npp.1301305. [DOI] [PubMed] [Google Scholar]
- 76.Uzbay IT. New pharmacological approaches to the treatment of schizophrenia. Turk Psikiyatri Derg. 2009;20:175–182. [PubMed] [Google Scholar]
- 77.Baldessarini RJ, Centorrino F, Flood JG, Volpicelli SA, Huston-Lyons D, Cohen BM. Tissue concentrations of clozapine and its metabolites in the rat. Neuropsychopharmacology. 1993;9:117–124. doi: 10.1038/npp.1993.50. [DOI] [PubMed] [Google Scholar]
- 78.Langosch JM, Trimble MR. Epilepsy, psychosis and clozapine. Hum Psychopharmacol. 2002;17:115–119. doi: 10.1002/hup.375. [DOI] [PubMed] [Google Scholar]
- 79.White DM, Van Cott AC. Clozapine (Clozaril), seizures, and EEG abnormalities. Am J Electroneurodiagnostic Technol. 2007;47:190–197. [PubMed] [Google Scholar]
- 80.Uzbay TI. Atypical antipsychotic drugs and ethanol withdrawal syndrome: a review. Alcohol Alcohol. 2012;47:33–41. doi: 10.1093/alcalc/agr142. [DOI] [PubMed] [Google Scholar]
- 81.Trzaskowska E, Krzascik P, Staniszewska A, Pucilowski O, Kostowski W. On the relative importance of D-1 vs. D-2 dopaminergic receptors in the control of audiogenic seizures in ethanol withdrawn rats. Drug Alcohol Depend. 1989;24:265–267. doi: 10.1016/0376-8716(89)90066-5. [DOI] [PubMed] [Google Scholar]
- 82.Uzbay IT, Usanmaz SE, Tapanyigit EE, Aynacioglu S, Akarsu ES. Dopaminergic and serotonergic alterations in the rat brain during ethanol withdrawal: association with behavioral signs. Drug Alcohol Depend. 1998;53:39–47. doi: 10.1016/S0376-8716(98)00102-1. [DOI] [PubMed] [Google Scholar]
- 83.Yu L, Fisher H, Wagner GC. Monoaminergic changes associated with audiogenic seizures in ethanol-dependent rats. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:1379–1392. doi: 10.1016/S0278-5846(00)00133-0. [DOI] [PubMed] [Google Scholar]
- 84.Yan QS, Jobe PC, Cheong JH, Ko KH, Dailey JW. Role of serotonin in the anticonvulsant effect of fluoxetine in genetically epilepsy-prone rats. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:149–152. doi: 10.1007/BF00241089. [DOI] [PubMed] [Google Scholar]
- 85.Kalkman HO, Neumann V, Hoyer D, Tricklebank MD. The role of alpha2-adrenoceptor antagonism in the anti-cataleptic properties of the atypical neuroleptic agent, clozapine, in the rat. Br J Pharmacol. 1998;124:1550–1556. doi: 10.1038/sj.bjp.0701975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yan QS, Jobe PC, Dailey JW. Noradrenergic mechanisms for the anticonvulsant effects of desipramine and yohimbine in genetically epilepsy-prone rats: studies with microdialysis. Brain Res. 1993;610:24–31. doi: 10.1016/0006-8993(93)91212-B. [DOI] [PubMed] [Google Scholar]
- 87.Acri AA, Henretig FM. Effects of risperidone in overdose. Am J Emerg Med. 1998;16:498–501. doi: 10.1016/S0735-6757(98)90001-8. [DOI] [PubMed] [Google Scholar]
- 88.Gerald MC, Richter NA. Studies on the effects of histaminergic agents on seizure susceptibility in mice. Psychopharmacologia. 1976;46:277–282. doi: 10.1007/BF00421114. [DOI] [PubMed] [Google Scholar]
- 89.Sturman G, Freeman P, Quinn L. Histamine H1-antagonists potentiate seizures in the EL (epilepsy-like) mouse model of temporal lobe epilepsy. Inflamm Res. 2001;50((Suppl.) 2):S80–S81. doi: 10.1007/PL00022416. [DOI] [PubMed] [Google Scholar]
- 90.Natesan S, Reckless GE, Nobrega JN, Fletcher PJ, Kapur S. Dissociation between in vivo occupancy and functional antagonism of dopamine D2 receptors: comparing aripiprazole to other antipsychotics in animal models. Neuropsychopharmacology. 2006;31:1854–1863. doi: 10.1038/sj.npp.1300983. [DOI] [PubMed] [Google Scholar]
- 91.Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67:995–996. doi: 10.4088/JCP.v67n0619b. [DOI] [PubMed] [Google Scholar]
- 92.Lin KH, Chen YJ, Lin YT, et al. Serious generalized tonic-clonic seizures induced by aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:231–232. doi: 10.1016/j.pnpbp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 93.Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- 94.Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178:85–86. doi: 10.1192/bjp.178.1.85-a. [DOI] [PubMed] [Google Scholar]
- 95.Camacho A, Garcia-Navarro M, Martinez B, Villarejo A, Pomares E. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28:145–147. doi: 10.1097/01.wnf.0000165351.10841.fa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 499 kb)