Abstract
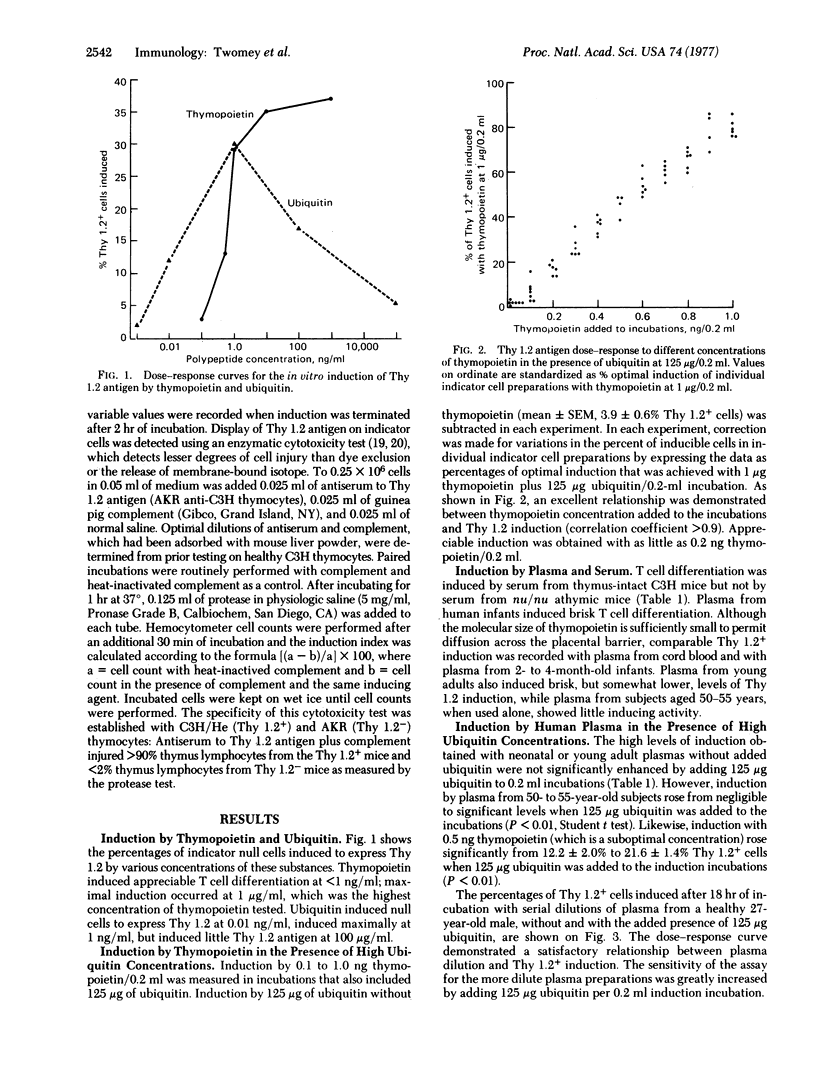
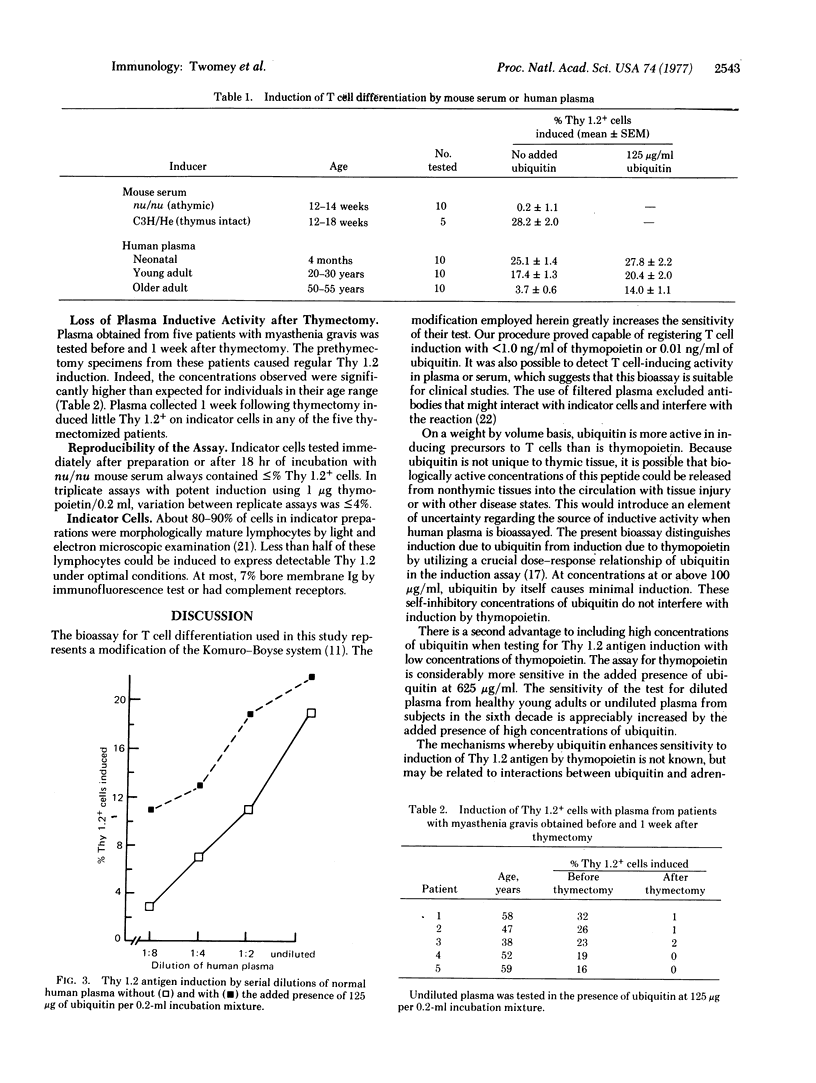
Thymopoietin is a thymic hormone that induces differentiation of thymocytes from precursor cells which arise in hemopoietic tissues. This paper describes a sensitive in vitro assay for the induction of Thy 1.2 antigen on null lymphocytes from germ-free athymic (nu/nu) mice. The sensitivity and specificity of the bioassay were increased by adding high concentrations of ubiquitin (a nonspecific inducer) to the induction incubations. The bioassay was sufficiently sensitive to detect thymopoietin at less than 0.25 ng/ml. A dose-response relationship was shown between thymopoietin concentration and the percentage of cells induced to express Thy 1.2 antigen. When normal human plasma was assayed, induction was registered with activity corresponding to thymopoietin at greater than 1 ng/ml in plasma from infants or young adults. Activities in the thymopoietin range of 0.25 ng/ml were registered with plasma from healthy subjects over 50 years of age. Thymectomy was followed by loss of this inductive activity from the plasma. This bioassay permits clinical studies on T (thymus-derived) cell inducers released by the human thymus into the circulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astaldi A., Astaldi G. C., Schellekens P. T., Eijswoogel V. P. Thymic factor in human sera demonstrable by a cyclic AMP assay. Nature. 1976 Apr 22;260(5553):713–715. doi: 10.1038/260713a0. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Papiernik M., Levasseur P., Dardenne M., Barois A., Le Brigand H. Evidence for a serum-factor secreted by the human thymus. Lancet. 1972 Nov 18;2(7786):1056–1058. doi: 10.1016/s0140-6736(72)92339-2. [DOI] [PubMed] [Google Scholar]
- Basch R. S., Goldstein G. Induction of T-cell differentiation in vitro by thymin, a purified polypeptide hormone of the thymus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1474–1478. doi: 10.1073/pnas.71.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A., Gilmour D. G., Goldstein G. Lymphocyte-differentiating hormone of bursa of fabricius. Science. 1976 Jul 23;193(4250):319–321. doi: 10.1126/science.180600. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke K. A., van Noord M. J., van Bekkum D. W. Attempts at morphological identification of the hemopoietic stem cell in rodents and primates. Exp Hematol. 1973;1(1):36–45. [PubMed] [Google Scholar]
- Gershwin M. E., Steinberg A. D., Woody J. N., Ahmed A. Studies of thymic factors. I. Evaluation of the mouse rosette assay for thymic hormone. J Immunol. 1975 Nov;115(5):1444–1448. [PubMed] [Google Scholar]
- Goldstein G., Hofmann W. W. Endocrine function of the thymus affecting neuromuscular transmission. Clin Exp Immunol. 1969 Feb;4(2):181–189. [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. Isolation of bovine thymin: a polypeptide hormone of the thymus. Nature. 1974 Jan 4;247(5435):11–14. doi: 10.1038/247011a0. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Mackay I. R. The thymus in systemic lupus erythematosus: a quantitative histopathological analysis and comparison with stress involution. Br Med J. 1967 May 20;2(5550):475–478. doi: 10.1136/bmj.2.5550.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. The isolation of thymopoietin (thymin). Ann N Y Acad Sci. 1975 Feb 28;249:177–185. doi: 10.1111/j.1749-6632.1975.tb29067.x. [DOI] [PubMed] [Google Scholar]
- Incefy G. S., L'Esperance P., Good R. A. In vitro differentiation of human marrow cells into T lymphocytes by thymic extracts using the rosette technique. Clin Exp Immunol. 1975 Mar;19(3):475–483. [PMC free article] [PubMed] [Google Scholar]
- Komuro K., Boyse E. A. In-vitro demonstration of thymic hormone in the mouse by conversion of precursor cells into lymphocytes. Lancet. 1973 Apr 7;1(7806):740–743. doi: 10.1016/s0140-6736(73)92127-2. [DOI] [PubMed] [Google Scholar]
- PETERSON R. D., COOPER M. D., GOOD R. A. THE PATHOGENESIS OF IMMUNOLOGIC DEFICIENCY DISEASES. Am J Med. 1965 Apr;38:579–604. doi: 10.1016/0002-9343(65)90135-x. [DOI] [PubMed] [Google Scholar]
- Pachciarz J. A., Teague P. O. Age-associated involution of cellular immune function. I. Accelerated decline of mitogen reactivity of spleen cells in adult thymectomized mice. J Immunol. 1976 Apr;116(4):982–988. [PubMed] [Google Scholar]
- Scheid M. P., Goldstein G., Hammerling U., Boyse E. A. Lymphocyte differentiation from precursor cells in vitro. Ann N Y Acad Sci. 1975 Feb 28;249:531–540. doi: 10.1111/j.1749-6632.1975.tb29102.x. [DOI] [PubMed] [Google Scholar]
- Scheid M. P., Hoffmann M. K., Komuro K., Hämmerling U., Abbott J., Boyse E. A., Cohen G. H., Hooper J. A., Schulof R. S., Goldstein A. L. Differentiation of T cells induced by preparations from thymus and by nonthymic agents. J Exp Med. 1973 Oct 1;138(4):1027–1032. doi: 10.1084/jem.138.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger D. H., Goldstein G., Niall H. D. The complete amino acid sequence of ubiquitin, an adenylate cyclase stimulating polypeptide probably universal in living cells. Biochemistry. 1975 May 20;14(10):2214–2218. doi: 10.1021/bi00681a026. [DOI] [PubMed] [Google Scholar]
- Schlesinger D. H., Goldstein G. The amino acid sequence of thymopoietin II. Cell. 1975 Aug;5(4):361–365. doi: 10.1016/0092-8674(75)90054-9. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Wernet P., Dickler H. B., Fu S. M., Kunkel H. G. B lymphocytes lacking surface IG in patients with immune deficiency: initiation of IG synthesis in culture in cells of a patient with thymoma. Birth Defects Orig Artic Ser. 1975;11(1):40–44. [PubMed] [Google Scholar]
- Stewart C. C., Goldstein S. The direct determination of cytolytic antibody titer and specificity. J Lab Clin Med. 1974 Sep;84(3):425–437. [PubMed] [Google Scholar]
- Stewart C. C., Ingram M. A method for counting phytohemagglutinin-stimulated lymphocytes. Blood. 1967 Apr;29(4 Suppl):628–639. [PubMed] [Google Scholar]
- Trizio D., Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974 Oct;113(4):1093–1097. [PubMed] [Google Scholar]
- Twomey J. J., Waddell C. C., Krantz S., O'reilly R., L'esperance P., Good R. A. Chronic mucocutaneous candidiasis with macrophage dysfunction, a plasma inhibitor, and co-existent aplastic anemia. J Lab Clin Med. 1975 Jun;85(6):968–977. [PubMed] [Google Scholar]
- Walford R. L. Immunologic theory of aging: current status. Fed Proc. 1974 Sep;33(9):2020–2027. [PubMed] [Google Scholar]


