Abstract
Three-dimensional (3D) printing has recently expanded in popularity, and become the cutting edge of tissue engineering research. A growing emphasis from clinicians on patient-specific care, coupled with an increasing knowledge of cellular and biomaterial interaction, has led researchers to explore new methods that enable the greatest possible control over the arrangement of cells and bioactive nanomaterials in defined scaffold geometries. In this light, the cutting edge technology of 3D printing also enables researchers to more effectively compose multi-material and cell-laden scaffolds with less effort. In this review, we explore the current state of 3D printing with a focus on printing of nanomaterials and their effect on various complex tissue regeneration applications.
Introduction
Both scientists and engineers have worked independently to elucidate the mechanisms behind physiological functions and biochemical processes in order to gain a greater understanding of the human body on a micron, or cellular, scale, and even at a nano level. These distinct approaches to human biology overlap harmoniously in the field of tissue engineering. Tissue engineers take a problem-solving oriented methodology found in engineering and apply it to biological situations for the augmentation and innovation of health and healing. They believe that the innate mechanisms of the human body and cellular biology can be unlocked to solve key medical challenges such as poor healing capacity of specific tissue injury (e.g., cartilage and nerves, among other tissues) or the lack of available donors for organ transplants.1,2 Current treatment options for damaged tissue with poor healing capacity or delicate structures are nonideal, and often involve painful surgeries and long recovery times without offering a complete restoration of the tissue's function. Over the past few decades, the field of tissue engineering has expanded rapidly, and researchers have proposed a variety of unique approaches to many problems. Much of the most promising work involves harnessing the body's adult stem cell population3–5 to repair and regenerate tissues. In tissue engineering, the use of stem cells relies on matching an appropriate, controllable cellular environment and stem cell species. It is well known that both the micro and nano environments which stem cells are exposed to play a crucial role in stem cell fate, and controlling these environments may provide a key to successfully engineer novel systems for successful tissue regeneration. In classical scaffold-based tissue engineering, researchers seek to create biologically inspired constructs that mimic natural tissue structure and function. The goal is to enable healthy and rapid restoration, regeneration, and/or maintenance of the implanted construct, while simultaneously promoting integration of natural tissue with the tissue engineered implant.6,7 To achieve truly biomimetic constructs, complex properties, such as extracellular matrix (ECM) feature size and composition, appropriate chemical gradients, varied mechanical properties, and specific morphologies for the engineered construct to integrate well in situ should be understood and accurately recreated.8–10 Incorporating all of these parameters in a single, implantable construct is very difficult, and requires researchers to search for novel biomaterials and advanced three-dimensional (3D) manufacturing techniques to formulate a viable solution.
Current progress in the field of scaffold-based tissue engineering provides us with several key characteristics that should be concurrently employed for successful tissue emulation and regeneration: (1) Possess sufficient mechanical strength and material degradation rate; (2) be able to modulate a 3D cellular microenvironment; (3) encourage cellular adhesion, proliferation, and tissue formation; and (4) enable adequate nutrient and waste exchange.6,7,11 Regarding 3D scaffold fabrication techniques, traditional approaches include phase separation,12 freeze drying,13,14 porogen leaching,15,16 and electrospinning.17,18 Each method has distinct advantages and disadvantages that researchers work with to create viable biological constructs. Many of them may offer limited control over scaffold geometry, pore size and distribution, pore interconnectivity, as well as internal channel construction. The random, spontaneously generated, and disconnected pores may significantly decrease nutrient transportation, cell migration, and survival, especially in the center of the scaffold. One of the most novel ways to combine highly ordered scaffold microarchitecture and biomimicry to combat these problems is through the modality of 3D printing. Three-dimensional printing can be used to deposit cells and biomaterials in a 3D matrix for the purposes of tissue regeneration. It offers great precision and control of the internal architecture and outer shape of a scaffold, and can fabricate complicated structures that closely mirror the architecture of biological tissue.19
Although the future of the field is promising, current 3D printing technologies for tissue regeneration are still hindered by the lack of advanced biomaterials that can recapitulate the complexity of native structures, as well as integrate with native tissue/organs. Cells exhibit optimal behavior in materials with nano-sized features, as human tissue ECM is naturally a nanocomposite.20 Biomimetic nanomaterials, which are designed to resemble cellular microenvironment components and regulate cell behavior, are currently having a profound impact on the field of tissue and organ regeneration.21 Increasing a material's biomimetic characteristics can be done by modulating a number of factors, including increasing surface area, addition of nanoroughness, modifying surface chemistry, and so on. Researchers are continually searching for better nanomaterials to best control stem cell behaviors. Most nanomaterials support stem cell growth and differentiation by replicating the properties of natural ECM. For example, bone ECM contains bundles of collagen and ceramics, such as nanocrystalline hydroxyapatite (nHA).22 Research conducted with scaffolds containing materials such as collagen I,23 nHA,24–26 and tricalcium phosphate (TCP)27 have been shown to improve bone formation for a number of different cell types.28 Cartilage has been shown to respond well to nanostructured scaffolds.18,21,29 Neural scaffolds are generally augmented to increase their mechanical strength and their electrical conductivity, primarily through carbon-based nanomaterials such as carbon nanotubes30–32 and graphene.33,34 The emphasis on the use of nanomaterials has begun to play a significant role synergistically with the recent boom in 3D printing. Traditionally, only a small subset of biomaterials can be used in 3D printing and a few of them exhibit nano features. However, new research has begun to focus on the creation of 3D printable nanomaterials that enable researchers to harness both the biomimetic qualities and enhanced physical/mechanical/electrical/chemical properties for improved tissue and organ regeneration.
In the next few sections, we will briefly overview the current state of 3D printing techniques and discuss their applications for several important modalities of tissue engineering, particularly musculoskeletal, neural, vascular tissue, and organ regeneration. In addition, we will place special emphasis on nanomaterials used in conjunction with 3D printing for complex tissue and organ regeneration applications.
Three-Dimensional Printing Techniques for Tissue Engineering: The Fundamental Principles
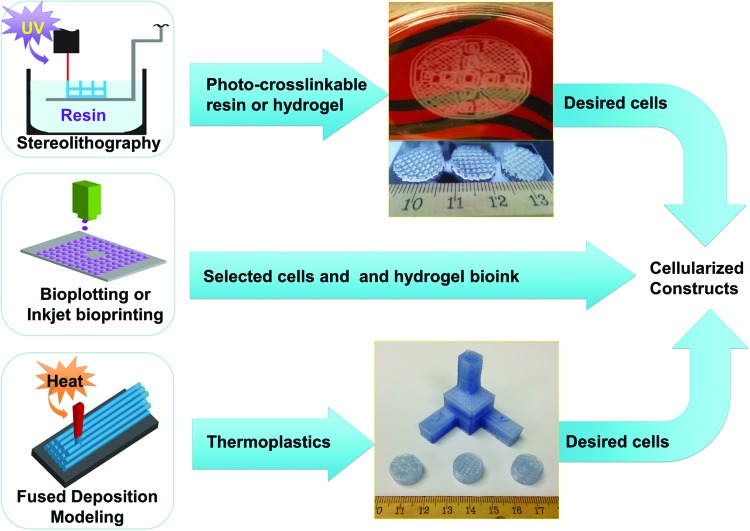
Three-dimensional printing is an emerging technique in the tissue engineering field. The unique control offered by designed scaffolds opens up additional avenues for tissue engineers to take advantage of that were until recently not realistic. Much of this versatility comes from the basis of most 3D printing for tissue engineering on techniques and technology perfected by industrial 3D rapid prototyping and additive manufacturing. In any form of 3D printing, the desired structure is precisely designed using computer aided design (CAD) software. The 3D design is then passed to a slicing program that parses the solid object into a stack of thin, axial cross sections. The sections are then sent to the printer, and each respective 2D cross section is reproduced in order, starting from the bottom and printing up along the Z axis. Three-dimensional printing for tissue engineering applications can take one of two forms, with and without incorporated living cells printed directly into the construct (several examples are illustrated in Fig. 1 and Table 1). By 3D printing a scaffold without cells, researchers can take advantage of 3D additive manufacturing techniques requiring high temperatures or other volatile environments that would be harmful to cell populations. Alternatively, one could instead print both cells and biomaterials simultaneously. This creates a hybrid biological-synthetic scaffold that can have suitable mechanical and cytocompatibility properties while simultaneously arranging multiple cell types in tissue-specific positions. However, modalities that attempt to do this are much more complex and possess other limitations which will be discussed next.
FIG. 1.
Several 3D printing modalities discussed in this article with examples of the resulting scaffolds. The stereolithography printed scaffold is PEG-DA, and the FDM printed scaffold is poly-lactic acid. 3D, three dimensional; FDM, fused-deposition modeling; PEG-DA, poly(ethylene glycol) diacrylate. Color images available online at www.liebertpub.com/teb
Table 1.
Several Examples of 3D Printing for Various Tissue and Organ Regeneration
| Tissue types | Printing methods | Printing materials | Description | References |
|---|---|---|---|---|
| Bone | FDM | Polymer and ceramic | PCL/CaP was printed into a 3D scaffold and seeded with MSC hydrogel. The scaffold can support MSC attachment and osteogenic differentiation | 52 |
| SLS | TCP | Microwaves were used in a novel device to sinter particles into a biomimetic, porous scaffold that increased bone formation. | 61 | |
| Cartilage and osteochondral tissue | Inkjet Bioprinting | Collagen-fibrin hydrogel | In conjunction with electrospun PCL, cartilage scaffolds were fabricated that supported the development of collagen-like structures both in vitro and in vivo. | 64 |
| Bioplotting | Alginate hydrogel | Two layers of an osteochondral scaffold were fabricated, and evaluated both in vitro and in vivo. The scaffold developed distinctly different ECM morphologies in the corresponding bone and cartiage layers. | 67 | |
| FDM | Poly-lactic acid polymer and collagen | An osteochondral construct conjugated with collagen was created to enhance MSC growth and differentiation. | 68 | |
| Neural | Inkjet Bioprinting | Fibrin hydrogel | NT2 neural cells were printed between layers of 3D fibrin hydrogel to create a neural mat. The cells adhered well, proliferated, and began to extend neuritis after 12 days of culture. | 73 |
| SL | Hyaluronic acid hydrogel | Biomimetic nerve conduits were fabricated and found to support neuronal growth and axonal extension in vitro. | 75 | |
| Vascular | Bioplotting | Hyaluronan-gelatin hydrogel | Cellular constructs were fabricated that formed a vascular construct with excellent cellular viability. Aortic root sinus cells and aortic valve leaflet cells were printed in the same construct, in biomimetic form. | 84 |
| Complex tissue and organ | Bioplotting | Alginate spheroids | Stem cells were printed in an organ mimetic fashion. Cell viability was high, and spheroids fused in time to a continuous geometry. | 91 |
| Inkjet Bioprinting | Calcium chloride/sodium alginate hydrogel | 3 cell types were printed concurrently into a single scaffold. All cell types maintained viability and proliferative capacity, as well as phenotypic expression and physiological function. In vivo, vascularization was observed. | 90 |
3D, three dimensional; ECM, extracellular matrix; FDM, fused-deposition modeling; MSC, mesenchymal stem cell; PCL, poly caprolactone; SL, stereolithography; SLS, selective laser sintering; TCP, tricalcium phosphate.
Inkjet bioprinting
Inkjet bioprinting (Fig. 1) uses a modified consumer grade inkjet printer to deposit cells and biomaterial, dubbed “bioink,” onto a substrate, dubbed “biopaper.” Advantages of this 3D printing include high resolution, and the potential to print different cell types together in the same construct, but most bioinks have poor mechanical strength,35 and the small nozzle size of inkjet bioprinters currently in production are too small to permit cell printing without causing damage to the cells.36 Regardless of this, it has been shown to be the fastest and the most economical path to biofabricate useful tissues or even organs for human use.
Although most inkjet bioprinting is live cell printing, some researchers have taken the concept and developed a method to create rigid scaffolds using an inkjet bioprinter-like process. It combines the concept of depositing layers of hard biomaterial beads that will be fused into a 3D construct with liquid binder dispensed from an inkjet bioprinter-like deposition system. The print head selectively deposits an adhesive material that binds the loose beads together in the designed geometry. This process works with a number of materials, is reasonably inexpensive compared with other modalities, and avoids damaging integrated nanomaterials, growth factors, or peptides.19,37
Leveraging inkjet technology is not only promising, but also faces multiple challenges for complex tissue regeneration. For instance, it is very difficult to create 3D complex tissue structures using this modality. Most significant in this author's opinion is the tiny volume dispensed per printing drop. Even older inkjet printers with high drop volume employ less than 10 pL of solution per drop, requiring a working concentration of at minimum 5 million cells per mL38 to maximize the possibility that one cell is deposited per drop of bioink. Where older inkjet printer cartridges had small volume reservoirs that could be more easily accessed and used for tissue engineering applications, modern printer cartridges use volumes of ink in excess of 15 mL, which in conjunction with a very high required cell density further limits potential application of the technology. In addition, inkjet printers are designed to work with low viscosity materials, again limiting the choice of biomaterials usable for creation of a lasting cellularized construct. Despite all of the challenges mentioned earlier, inkjet bioprinting is very useful for depositing cells with relative precision. In addition, if depositing layers of cells into a mold, as in “scaffoldless” tissue engineering, it can demonstrate high promise.38 In integrating 3D printing and nanomaterials for complex tissue and organ regeneration section, we will discuss current research progress in tissue regeneration utilizing inkjet bioprinting.
Bioplotting
Bioplotting is another 3D printing method that has garnered great interest in the world of tissue engineering and biofabrication. Bioplotting refers to the process of extruding either tubes or spheroids of material from a syringe, layered on top of each other and cured through the addition of radiation, a chemical reaction, or solidification occurring over time. Bioplotting modalities often have several syringes containing multiple cell types to enable easy integration of multiple tissue types in the final construct. It is currently one of the easiest and most popular methods for the creation of co-cultured scaffolds and tissue/organ-like constructs due to the possibility of including multiple syringes on bioprinters.39 It requires the use of relatively viscous biomaterials that can at least briefly support themselves, and, in addition, be extruded from a needle and syringe, heavily limiting the material choice, material properties, and maximum resolution.39
Bioplotting eliminates many of the drawbacks of inkjet bioprinting for hybrid biological-synthetic 3D printing, as it allows for a lower cell number requirement and wider material selection. It particularly excels in soft tissue recapitulation and organ printing because of the ease of incorporating multiple cell types and factors into the same construct simply by switching syringes. In addition, several printers that can readily leverage this technology are available in the open market, from open source solutions to proprietary commercial machines. Despite these advantages, bioplotting requires a liquid material to serve as bioink, limiting its efficacy in reconstructing tissues with high requirements for mechanical strength. In this author's opinion, bioplotting is best suited for scaffolds and tissues which are designed to evaluate co-cultured cell types, as well as for larger constructs that do not require high-resolution details.
Fused-deposition modeling
The oldest 3D additive manufacturing technology involves depositing a melted thermoplastic in thin layers at a level, flat surface and again building the model layer by layer. This is referred to as fused-deposition modeling (FDM) (Fig. 1). FDM is inexpensive, relatively fast, and a generally well-explored technology which can produce scaffolds that are suitable for musculoskeletal application. However, it also struggles to replicate geometries with sharp overhanging structures, or long, unsupported spans within the scaffold. These limitations come from the fabrication modality itself; a molten thermoplastic lacks the mechanical strength to support itself while it is slowly cooling and hardening. The resolution of FDM-generated constructs is limited by nozzle diameter, available materials, and mechanical positioning of the extrusion end of the printing nozzle. Nozzle size partially determines minimum feature size, but variance exists between when the plastic leaves the nozzle orifice and before it comes into contact with the substrate. These factors compound to result in an overall resolution of 50–762 μm in layer height (set by software) and an accuracy of±127 μm.40 In addition, the material choices for FDM are limited, as raw material needs to be worked into a hard filament. There are currently few commercially available 3D printer ready filaments.40
FDM is a relatively under-explored modality in tissue engineering, primarily because of the lack of biomaterials developed for use with the platform. However, FDM-printed constructs are strong and easy to store/handle/transport for the long term, unlike hydrogel-based modalities that dominate most other types of 3D printing for tissue engineering applications. FDM printers are also increasingly inexpensive due to a large influx of economical options from RepRep open source projects. In addition, many hobbyists, inventors, and scientists have worked together to not only decrease the cost of a typical FDM printer, but also increase the build area and print speed dramatically, without adding significant additional cost. The open source nature of many affordable FDM machines ensures frequent developments and improvements for multiple materials, and other technologies, when more biomimetic FDM compatible filament types are fabricated. The high mechanical strength exhibited by constructs printed via FDM are ideal for hard tissue regeneration, and the ease and speed of printing many models, or large-format objects also make FDM ideal for fluid flow testing, surgical planning, and demonstration pieces.
Selective laser sintering
A higher resolution alternative to FDM is a process known as selective laser sintering (SLS). This modality uses a long wavelength laser or high-energy light source to fuse beads of material together one layer at a time. Each layer of beads is deposited; the corresponding beads to the designed construct are heated by a laser and fuse with the solid structure beneath it. The process is repeated to replicate a 3D design. SLS touts high resolution, and a wide range of potential materials, but is slow and machines are costly.41–43 Industrial applications go so far as to use high-energy electron beams to melt beads of metal into a fully functioning prototype in a modified SLS process known as electron beam melting. SLS has been growing in popularity among tissue engineers looking to design rigid tissues, such as bone, but the biocompatibility of commercial materials needs improvement and it is difficult to remove nonsintered material from a completed construct.
Similar to FDM, SLS is limited to using stiff materials, but with one important difference. While a biomaterial should be processed into a filament to be used with FDM, a material only needs to be powdered/processed to a controlled size for implementation in SLS machines. In this way, SLS touts additional versatility and higher resolution when compared with FDM, and, as such, has seen use in the clinic.44,45 Unlike FDM, SLS is slower, and machines are both larger and orders of magnitude more expensive. As such, SLS is suitable for hard tissues of a single material type, such as bone scaffolds, or supporting structures for tissues within the body.44,45
Stereolithography
In terms of resolution, light is currently the most precise printing/material curing mechanism. Stereolithography (SL) is a 3D printing technique which uses light to cross link polymeric materials and create geometrically patterned layers that together form a 3D construct.46–48 Most SL systems use a laser and a directed mirror array to project patterned light onto the surface of a resin-containing vat. The resin is cured, a fresh layer of resin is added, and the process is repeated. These systems project a cross-section of the 3D structure and create an entire layer of the substrate at once, increasing the speed of manufacture over traditional point-by-point SL systems, but the chemical process is the same in both cases. SL is attractive, because it produces constructs that have high resolution, and the uncured resin is easily removed from the final product. However, the process is relatively slow, and commercial systems use proprietary, nonbiomimetic resins. Despite this, SL has been a hot topic in the consumer and research world, and biomaterials scientists are working on developing resins that are suitable for use in tissue engineering applications.
SL is versatile in material choice, but is limited to photocrosslinkable liquids. Its high resolution enables precise reconstructions of tissue morphology, and surrounding uncured resin automatically supports dramatic overhanging architecture or thin walled features that it would be impossible to create reliably with other printing modalities. Similar to SLS, SL can be slow, but the liquid base material enables the incorporation of various nanomaterials, growth factors, or other materials without any additional processing. These advantages lend SL to being ideal for various biomimetic and bioactive nanomaterial scaffold fabrication.
Integrating 3D Printing and Nanomaterials for Complex Tissue and Organ Regeneration
Three-dimensional printing bone
When engineering hard tissues such as bone, a high degree of porosity combined with high mechanical strength is extremely desirable but sometimes difficult to attain with traditional techniques.49–51 Similar to traditional bone tissue engineering, one of the most common cell lines used in 3D printing bone is bone marrow mesenchymal stem cells, with less emphasis on other stem cell types.21 Among the current available rapid prototyping modalities, FDM utilizes hard thermoplastic polymers with a relatively high mechanical strength that presents an opportunity for the use of this technology for bone tissue regeneration. Schantz et al. fabricated a biodegradable polymer-ceramic scaffold via FDM.52 Human mesenchymal stem cells (MSCs) within fibrin glue were put into the scaffolds and cultured in vitro for 8 weeks. It was reported that MSCs were able to attach, migrate, and osteogenic differentiate within the biomimetic bone scaffold.
Human bone ECM is a nanocomposite that consists of a protein-based soft hydrogel matrix, of collagen, osteopontin, and water, and inorganic components, primarily nHA (Ca10(PO4)6(OH)2),53 which constitutes 70% of the bone matrix.54 The incorporation of biomimetic calcium phosphate nanomaterials such as nHA55 TCP56 and calcium polyphosphate57 is at the forefront of 3D printing research.58,59 Due to its excellent cytocompatibility, osteoconductive and bioactive characteristics, nHA has been targeted as a promising bone nanomaterial to be included in 3D printing systems, and even used as the main constituent. Our lab has developed a table-top SL setup, including a UV laser, to cross-link a biocompatible poly(ethylene glycol) diacrylate (PEG-DA) hydrogel with nHA particles. A custom-designed nHA 3D hydrogel scaffold with varying pore sizes (15 mm in diameter and 400 μm in thickness) has been fabricated via the 3D bioprinter. Apart from nHA, TCP is utilized in 3D printing as well. It can be processed into a fine powder60 and applied for use in a novel 3D sintering method that rather utilizes microwaves, as heat was utilized for scaffold fabrication.60,61 Sintered TCP scaffolds exhibited an increase in compressive strength and more optimal microporosity: macroporosity ratio, which, in turn, increased the formation of new bone in vivo when compared with constructs fabricated through a conventional energy source.60
Recently, a company called Oxford Performance Materials used SLS and a proprietary poly(ether- keytone-ketone) (deemed OPEKK-IG) biomimetic polymer to create a bone substitute designed for use in craniofacial defects. They indicated that OPEKK-IG is osteoconductive, mechanically strong, and exhibits a highly textured surface while “maintaining capacity for cell proliferation without exhausting metabolic demands on the cells.62” The FDA approved OPEKK-IG for clinical use in March 2013, making it one of the first 3D printed polymer implants approved for human use.
Three-dimensional printing cartilage and osteochondral tissue
Degenerative and acute cartilage and osteochondral defects caused by a variety of maladies, including osteoarthritis, trauma, and sports injuries, present a common and serious clinical problem. More than 6 million people visit hospitals in the United States every year for various knee, wrist, and ankle issues63; however, articular cartilage and osteochondral repairs continue to be largely intractable due to the poor regenerative capacity and complex stratified structure.63 Even tiny defects can be permanent, and a largely avascular environment provides obstacles to efficient healing. Traditional fabrication techniques lack the ability to accurately mimic natural cartilage structures due to the limited porosity of available materials and the difficulty of recreating appropriate 3D architecture. Three-dimensional printing provides a promising technique for successful patient-specific cartilage and osteochondral tissue formation. However, 3D printing nanocomposite materials for cartilage and osteochondral tissue regeneration is a relatively unexplored field with limited available studies. Recently, Xu et al. combined aligned electrospun fiber scaffolds with inkjet bioprinting to create hybrid nanocomposite scaffolds. The results showed that the hybrid printed electrospun scaffolds can enhance cartilage formation, and have improved mechanical properties when compared with controls.64 This study shows a promising approach for cartilage regeneration. Moreover, great potential also lies with the use of nanomaterials combined with 3D printing techniques to improve the properties of the final construct.64 Bacterial nanocellulose is one such nanomaterial. This natural polymer has been used with a 3D printing system to produce patient-specific auricular facsimiles that closely match natural geometries.65 Furthermore, this material promotes adhesion of endothelial and NIH/3T3 cell lines66 and shows promise for an excellent biomaterial for 3D fabrication of chondrogenic scaffolds. Several other nanomaterials could be implemented to improve the efficacy of 3D fabricated constructs such as multi-walled carbon nanotubes and poly-L-Lysine,18 self-assembling DNA-based rosette nanotubes,29 or bioactive factor encapsulated nanospheres16 that have shown to be efficacious in cartilage and osteochondral regeneration.
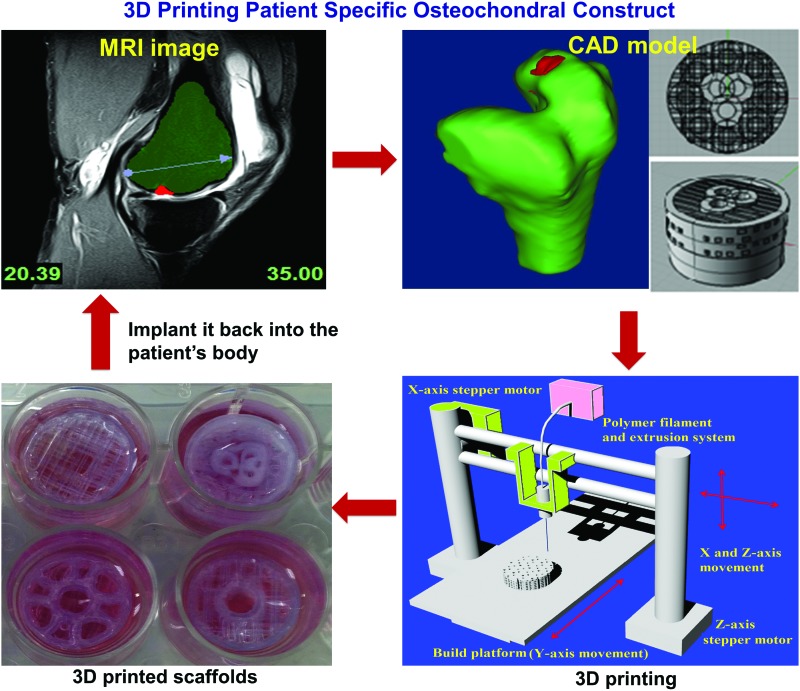
Osteochondral tissue, at the bone-cartilage interface, is complex and includes various chemical gradients, morphological gradients, and disparate mechanical properties. These characteristics present challenges to 3D printing, but exploration into the area is still highly warranted. Fedorovich et al. explored osteochondral tissue replication using bioplotting.67 Two types of osteogenic progenitor cells and chondrocytes were printed concurrently into an intricate alginate hydrogel scaffold with high cell viability. Both 1 cm thick layers adhered well to each other. The in vitro and in vivo results showed that distinctive ECM regions were formed in different parts of the construct, which made it promising for the repair of osteochondral defects. Due to the lack of mechanical strength of alginate hydrogel, another study used alginate in conjunction with a stronger, 3D printed poly caprolactone (PCL) supporting structure to create a more biomimetic osteochondral scaffold.67 Briefly, a PCL scaffold was extruded to take the role of the bone-forming scaffold, and osteoblast and chondrocyte laden hydrogel was deposited in layers to complete the scaffold. Good cellular proliferation was observed after 7 days. In addition, Cui et al. successfully inkjet bioprinted a poly(ethylene glycol) dimethacrylate solution containing chondrocytes into a defect formed in an osteochondral plug.38 They observed greater proteoglycan deposition in the interface of implant and native tissue. Recently, our lab created novel 3D printed osteochondral scaffolds for facilitating human bone marrow MSC functions. This work takes advantage of a poly-lactic acid filament with highly designed biphasic geometry (as shown in Fig. 2) to promote specific stem cell differentiation and improve mechanical strength and interfacial integration.68
FIG. 2.
Three-dimensional printing osteochondral scaffolds with designed internal structures for osteochondral defect treatment. The image is from Holmes et al.68 Color images available online at www.liebertpub.com/teb
Three-dimensional printing neural tissue
Another emerging and exciting 3D printing application is for neural tissue regeneration.69 It has been known that uniformity of spacing, in addition to proximity, of cells directly influences cell-to-cell communication, and morphological characteristics of neural tissue.70 Inkjet bioprinting enables precise placement of cells, which could allow for more efficient cross-talk to develop, an essential requirement for neural tissue regeneration.71 A variety of cells, proteins, and growth factors have been deposited using inkjet bioprinting in geometries that simulate what is observed in natural neural tissue.69,72,73 For instance, Xu et al.73 fabricated controlled patterns and structures of primary embryonic hippocampal and cortical neurons using inkjet bioprinting technology. Cellular properties and functional fidelity of neurons after being ejected through the nozzles of a thermal inkjet printer, including neuronal phenotypes and electrophysiology, were found to be retained after printing. In addition, Ferris and Cameron developed a gellan gum hydrogel and surfactant into a novel bioink that can mitigate some of the inherent limitations of modifying consumer printers for laboratory research. The bio-ink was shown to print reliably while containing several different cell types from two different commercially available drop-on-demand printing systems.74 The fluid properties of the bioink appeared to not only print well, but also inhibited cell aggregation in solution.
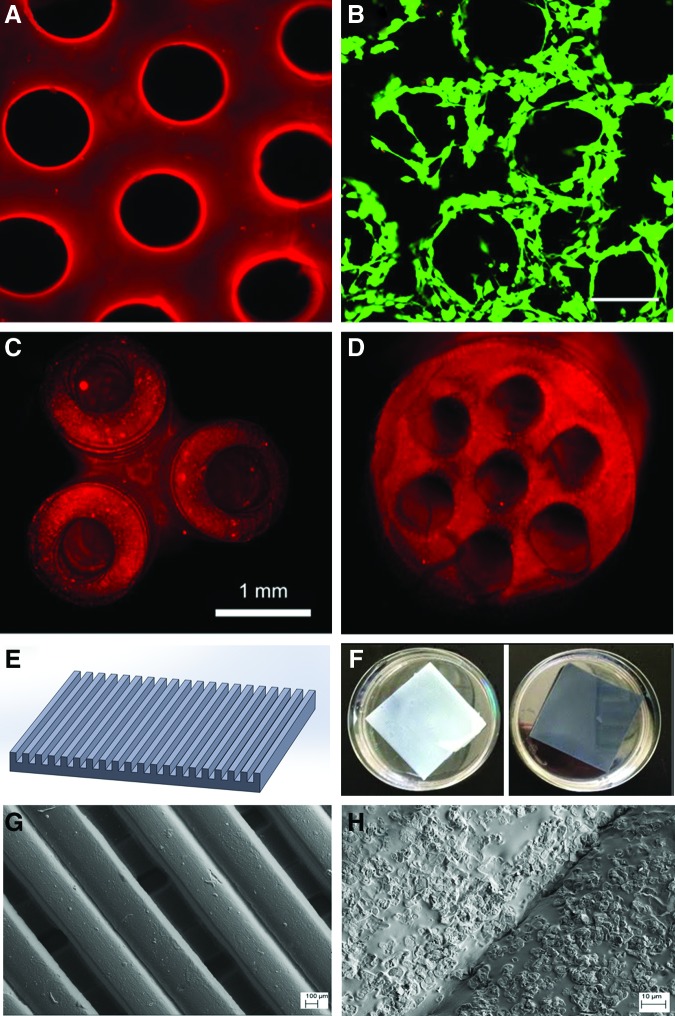
In addition to inkjet bioprinting, other 3D printing modalities are applicable for neural tissue engineering. One of the most promising methods is directed mirror device (DMD) SL. SL uses lasers or other light sources that have the ability to photocrosslink proteins or hydrogels and induce bond formation between specific side chains when proteins are suspended in a bulk, optically transparent hydrogel, After the complex structures are formed, other bioactive molecules can be coupled to the structures For example, Suri et al. also used DMD system and Schwann cells to create a neural scaffold of glycidyl methacrylated hyaluronic acid (Fig. 3A–D),75 a modified natural polymer commonly found in ECM of neural tissue.76,77 This fabrication technique allows for excellent resolution and for the creation of complex geometries that supported cell growth for 24 h and showcased the ability to incorporate gradients of nanoparticles within the construct to further augment differentiation. The ability to combine photocrosslinkable and nonphotocrosslinkable polymers enables DMD fabricated scaffolds to modulate porosity, as well as mechanical and chemical properties in this manner. Curley et al. used this approach to model an in vitro experiment of embryonic dorsal root ganglion neurite expansion.78 They used polyethylene glycol (PEG) and Puramatrix to create a constrained growth environment for the neurites in the form of a conduit. PEG provided mechanical structure, and Puramatrix lent itself to a suitable cellular environment for neurite outgrowth. The scaffold exhibited improved mechanical properties when compared with Puramatrix scaffolds alone, while capitalizing on an inherently favorable cellular environment. In a recent study performed in our lab, we 3D printed aligned nerve constructs with graphene nanoplatelets via a table-top SL (Fig. 3E–H). Our results have shown that the construct with graphene nanoplatelets has very good cytocompatibility properties. More importantly, the graphene nanoplatelets can greatly improve the conductivity of the scaffold, which makes the scaffold promising for neural regeneration.
FIG. 3.
(A–D) Fluorescence microscopy images of 3D printed nerve guidance scaffolds. (A) is a hyaluronic acid scaffold conjugated with laminin, and (B) shows the Schwann cells (green) that attached and grew well on the scaffold after 24 h of culture. Top views of (C) a 3D branched scaffold and (D) a multilumen nerve guidance scaffold. The images are from Suri et al.75 (E–H) are a 3D printed aligned PEG-DA neural construct sheet with highly conductive graphene nanoplatelets: (E) is a representative computer-aided design (CAD) model of an aligned neural construct sheet; (F) photo images of a 3D printed neural construct without (left) and with graphene nanoplatelets (right); (G, H) scanning electron microscopy images of the 3D printed scaffold with grapheme nanoplatelets at low and high magnifications. Color images available online at www.liebertpub.com/teb
Three-dimensional printing vascular tissue
One of the biggest current challenges when 3D printing tissues and organs is the need to create a highly efficient, perfusable 3D vascular network that can facilitate efficient nutrient transportation and waste removal. It is essential for cell survival in large tissue systems and successful integration of the native tissue and implant.79 Current available strategies for the fabrication of complex vascular networks are limited. In an effort to address these challenges, researchers have begun to explore 3D printed highly ordered microvascular networks to guide endothelial cells to form vessels in a predesigned pattern that shows great promise for tissue regeneration.80,81 For instance, Miller et al. first developed a blend of carbohydrates (glucose, sucrose, and dextran) to be a glass when cooled, and optically transparent such that it could be used in conjunction with photocrosslinkable materials. The carbohydrate glass was then extruded through a heated syringe into an interconnected microfluidic vascular network (Fig. 4A), allowed to cool, and a scaffold was cast around it in a mixture of 10T1/2 cells and photocrosslinkable ECM prepolymer. When immersed in water, the carbohydrate quickly dissolved, leaving a hollow network in its place. The cells adjacent to the perfused vascular network were observed to maintain phenotypic and proteomic expression at a high density, and human umbilical vein endothelial cells that perfused through the channels were observed to attach and form a biphasic, tissue-like structure (Fig. 4B).80 Lu and Chen also created a 3D printed interconnected network of tubes similar to natural vasculature.46
FIG. 4.
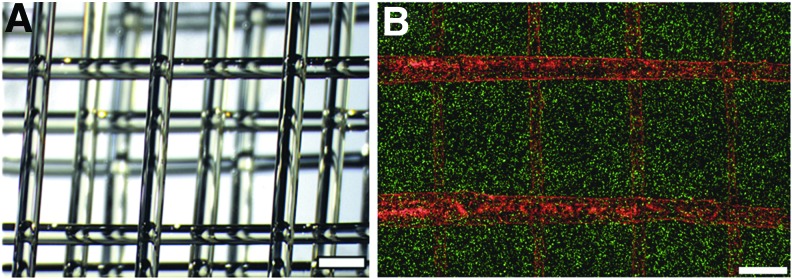
(A) A 3D printed carbohydrate-glass lattice and (B) image of human umbilical vein endothelial cells (red) residing in the vascular network of a fibrin gel with uniformly distributed 10T1/2 cells (green). Scale bar=1 mm. The images are from Miller et al.80 Color images available online at www.liebertpub.com/teb
In addition to structural considerations, appropriate materials should be chosen for vascular constructs.82 In response to a dearth of biomimetic materials available for vascular tissue engineering applications, a new composite biomaterial, hyaluronic acid methacrylate: gelatin methacrylate (HA-MA:GE-MA) was developed for use with an open-source hobbyist printer (Fab@Home). They were able to create tubular, cellularlized hydrogel constructs83 that resembled vascular channels. The biocompatibility of the novel hydrogel was characterized, and it was demonstrated that a cylindrical structure was printable through this printing system. This proof-of-concept print demonstrated the feasibility of economically printing simple, cellularized vascular constructs that could be a precursor to 3D printed organs. More immediately, this also shows the potential for fabrication of tissue engineering scaffolds that provide vascularized pathways to efficiently integrate with different native tissue types in vivo.83
Another substantial and exciting application is the possibility of 3D printing functional vascular tissue. In this vein, Duan et al.84 constructed a bioprinted aortic valve model using a 3D bioplotter loaded with alginate and gelatin hydrogel doped with two unique cell types. Aortic root sinus smooth muscle cells and aortic valve leaflet interstitial cells were extruded into a scaffold that mimicked the form of a porcine aortic valve and root. Cell-laden constructs were cultured for 7 days and evaluated for mechanical properties and cell viability via a live/dead assay. More than 80% of cells survived the printing process, and the Young's Modulus of the scaffolds decreased steadily from tissue formation and scaffold degradation, indicating that the compliance of the cellularized scaffold increased, resulting in a more biomimetic aortic valve model.
Three-dimensional printing organs
Although still a relatively untested treatment, 3D printing has already shown great promise in organ regeneration, and researchers have high hopes for its future in widespread clinical applications. For example, Dr. Atala and his research team at Wake Forest are at the forefront of 3D printing tissues and organs, especially when it comes to printing cellularized constructs. Based on their successful implantation of conventionally tissue engineered bladders85–88 into seven children and teenagers with terminal myelomeningocle, they designed a bladder that could be printed using modified inkjet bioprinting technology.89 In order to provide a suitable mechanically strong scaffold with nanofeatures suitable for stem cell performance, an inkjet bioprinter was used in conjunction with an electrospinning needle to simultaneously print scaffold and multiple cell populations into the three distinctive layers of the bladder. The cells were cultured after printing, were histologically examined, and were found to have maintained their relative positions. This study shows promise of providing an avenue to bioprint functional bladders with all proper cell types.89 Via inkjet bioprinting, scaffolds have been fabricated that can incorporate multiple tissue types simultaneously, as in an organ. Three separate types of stem cells were simultaneously printed into a construct and thoroughly evaluated for proliferation and differentiation. Scaffolds showcased not only excellent cell viability but also promising proliferation capacity. After in vivo analysis, scaffolds were adequately vascularized, and each cell type showed histological evidence of differentiation.90 This evidence shows not only the promise of inkjet bioprinting but also the feasibility of organ bioprinting as a whole.
Williams et al. have been studying the feasibility of using a different approach to print adipose-derived stromal vascular fraction stem cells91 in an organ-like fashion. The pluripotency of these cells allows the differentiation into smooth muscle cells, cardiac cells, and are plentiful in the human body, making them attractive for organ regeneration research. The researchers used a bioplotter, which forms spheres of alginate when plotted in calcium chloride solution. The spheres or “spheroids” were then monitored for differentiation and were shown to have good cell viability.91 This spheroid fabrication technique shows potential for future use in organ differentiation, and a similar technology is utilized in several bioprinting apparatus for organ regeneration.92 After being packed together closely in a 3D cell culture environment, the cell-laden alginate spheroids will fuse with the neighboring spheroids into the designed geometry, forming a composite structure of microtissue resembling vascular networks,91,93 cartilage,94 renal cell constructs,95 and several others.96
Many organ printing systems work as described earlier, but researchers are looking to improve the resolution of cell-based printing modalities so that individual cells or small groups of cells can be even more precisely placed within a construct. Xu et al. has created a printing system deemed “cell encapsulating droplet patterning.” This system precisely utilizes compressed nitrogen to deposit nanoliter-volume droplets. With this system, the group has been able to fabricate scaffolds with bladder cells,97 and develop a 3D ovarian cancer model with multiple cell types.98 In the bladder project, constructs were created that reached 5 mm by 5 mm and 20 μm in height, and after 51 days of culture appeared to form a well-integrated tissue network.97
One of the most advanced commercial bioprinters capable of organ printing was developed and operated by the company Organovo. It uses a hybrid bioplotting printer to deposit spherical drops of cell infused bioink that subsequently fuse together over time, organize, and self assemble into biologically similar constructs. The company's success derives from their fully functional blood vessel constructs which consist of fibroblasts and endothelial cells that migrate to the proper space in the lumen when printed with their method.99 They also claim to be developing a fully functioning artificial liver model that could potentially revolutionize pharmaceutical testing.
Conclusions and Future Directions
Three-dimensional printing complex tissue, or even organs, is a multifaceted and rapidly expanding field in science, engineering, and medicine. It has shown an advantage in being able to create constructs that are well defined on the micro or sub-micro scale and, thus, better suited to improve stem cell function and encourage tissue formation. Startling advancements have been made with regard to fabricating complex biomimetic cellular constructs with controlled microgeometries and advanced nano composition. The need to have mixed cell populations, biomimetic material properties, and chemical gradients propels researchers to continue to focus on furthering applications of 3D printing functional tissue/organ constructs, but more exploration of nanomaterials-based bioinks is needed. Incorporating nanomaterials can further improve on the geometric specificity of 3D printing and mitigate the disadvantages of some of the materials that are in use. In order to truly harness the potential of 3D printing, more research is necessary to develop novel nanomaterials that are suitable for all cell and tissue types to promote tissue regrowth and regeneration. Both cellular and acellullar printing have distinct advantages and disadvantages that need to be resolved before the best clinically viable option can be achieved. Concurrently with 3D printing research, advances by independent technically savvy groups have been driving down the cost and complexity of additive manufacturing modalities through collaborations such as the Fab@Home project and open source platforms developed through tight-knit communities such as RepRap.
Although it is in the early stage, the successful implantation of a functional 3D bioprinted organ into a live human being will someday mark the beginning of an exciting era in 3D printing, and it hints at clinical potential yet to be unlocked with further research in various advanced 3D printing modalities. They have great potential for integrating multiple disparate tissue types into an advanced biomimetic construct (such as organs, limbs, facial reconstruction, etc.) that is suitable for human use. From the current available technologies, bioplotting seems to be closer to creating a fully functioning, multicellular organ that can integrate into and thrive within a human body in the future. However, we believe that if more 3D printable nanomaterials with improved performance are created and advanced 3D printing modalities (such as nanoscale printing) can be explored, a more ideal biomimetic complex for tissues or organs can be created for human implantation in the near future. In summary, the ability for tissue engineers to design geometric features, tune material properties through nanomaterial integration, and incorporate drug delivery platforms through multimaterial selection indicates that an exciting revolution in tissue engineering is surely on us.
Acknowledgments
This work was supported by NIH Award Number UL1TR000075 from the NIH National Center for Advancing Translational Sciences, by the George Washington Institute for Biomedical Engineering (GWIBE), and by GW Institute for Nanotechnology (GWIN).
Disclosure Statement
No competing financial interests exist.
References
- 1.Berthiaume F., Maguire T.J., and Yarmush M.L.Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng 2,403, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Jones A.C., Arns C.H., Sheppard A.P., Hutmacher D.W., Milthorpe B.K., and Knackstedt M.A.Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials 28,2491, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Tel-Vered R., Yehezkeli O., and Willner I.Biomolecule/nanomaterial hybrid systems for nanobiotechnology. Adv Exp Med Biol 733,1, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L., Zhou Y., Zhu J., and Xu Q.An updated view on stem cell differentiation into smooth muscle cells. Vasc Pharmacol 56,280, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Gupta P.K., Das A.K., Chullikana A., and Majumdar A.S.Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther 3,25, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vacanti J.P., and Langer R.Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354Suppl 1,SI32, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Langer R., and Vacanti J.P.Tissue engineering. Science 260,920, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Samer S., Ben-David D., Lotan R., Livne E., Avrahami R., and Zussman E.Slow-release human recombinant bone morphogenetic protein-2 embedded within electrospun scaffolds for regeneration of bone defect: in vitro and in vivo evaluation. Tissue Eng Part A 17,269, 2011 [DOI] [PubMed] [Google Scholar]
- 9.de Valence S., Tille J.C., Mugnai D., Mrowczynski W., Gurny R., Moller M., and Walpoth B.H.Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 33,38, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Ahn S.H., Lee H.J., and Kim G.H.Polycaprolactone scaffolds fabricated with an advanced electrohydrodynamic direct-printing method for bone tissue regeneration. Biomacromolecules 12,4256, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Yoshimoto H., Shin Y.M., Terai H., and Vacanti J.P.A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 24,2077, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Zhao J., Han W., Chen H., Tu M., Huan S., Miao G., Zeng R., Wu H., Cha Z., and Zhou C.Fabrication and in vivo osteogenesis of biomimetic poly(propylene carbonate) scaffold with nanofibrous chitosan network in macropores for bone tissue engineering. J Mater Sci Mater Med 23,517, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Im O., Li J., Wang M., Zhang L.G., and Keidar M.Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int J Nanomedicine 7,2087, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M., Cheng X., Zhu W., Holmes B., Keidar M., and Zhang L.G.Design of biomimetic and bioactive cold plasma-modified nanostructured scaffolds for enhanced osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng Part A 20,1060, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Tran R.T., Naseri E., Kolasnikov A., Bai X., and Yang J.A new generation of sodium chloride porogen for tissue engineering. Biotechnol Appl Biochem 58,335, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Castro N.J., O'Brien C., and Zhang L.G.Biomimetic biphasic 3D nanocomposite scaffold for osteochondral regeneration. AICHE J 60,432, 2014 [Google Scholar]
- 17.Holmes B., Castro N.J., Zhang L.G., and Zussman E.Electrospun fibrous scaffolds for bone and cartilage tissue generation: recent progress and future developments. Tissue Eng Part B Rev 18,478, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Holmes B., Castro N.J., Li J., Keidar M., and Zhang L.G.Enhanced human bone marrow mesenchymal stem cell functions in novel 3D cartilage scaffolds with hydrogen treated multi-walled carbon nanotubes. Nanotechnology 24,365102, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Cui X., Boland T., D'Lima D.D., and Lotz M.K.Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul 6,149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., and Webster T.J.Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 4,66, 2009 [Google Scholar]
- 21.Zhao C., Tan A., Pastorin G., and Ho H.K.Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol Adv 31,654, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Kane R., and Ma P.X.Mimicking the nanostructure of bone matrix to regenerate bone. Mater Today 16,418, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J., Yang J., and Huang W.[Study on collagen membrane combinating with autogenous bone marrow stromal cells or platelet rich plasma in repairing alveolar bone defect in dogs]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 21,523, 2007 [PubMed] [Google Scholar]
- 24.Sun L., Zhang L., Hemraz U.D., Fenniri H., and Webster T.J.Bioactive rosette nanotube-hydroxyapatite nanocomposites improve osteoblast functions. Tissue Eng Part A 18,1741, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Wang M., Castro N.J., Li J., Keidar M., and Zhang L.G.Greater osteoblast and mesenchymal stem cell adhesion and proliferation on titanium with hydrothermally treated nanocrystalline hydroxyapatite/magnetically treated carbon nanotubes. J Nanosci Nanotechnol 12,7692, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Zhang L., Rodriguez J., Raez J., Myles A.J., Fenniri H., and Webster T.J.Biologically inspired rosette nanotubes and nanocrystalline hydroxyapatite hydrogel nanocomposites as improved bone substitutes. Nanotechnology 20,175101, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Todo M., and Arahira T.In vitro bone formation by mesenchymal stem cells with 3D collagen/beta-TCP composite scaffold. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference2013,409, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Castro N.J., Hacking S.A., and Zhang L.G.Recent progress in interfacial tissue engineering approaches for osteochondral defects. Ann Biomed Eng 40,1628, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Childs A., Hemraz U.D., Castro N.J., Fenniri H., and Zhang L.G.Novel biologically-inspired rosette nanotube PLLA scaffolds for improving human mesenchymal stem cell chondrogenic differentiation. Biomed Mater 8,065003, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Park S.Y., Kang B.S., and Hong S.Improved neural differentiation of human mesenchymal stem cells interfaced with carbon nanotube scaffolds. Nanomedicine 8,715, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Huang Y.J., Wu H.C., Tai N.H., and Wang T.W.Carbon nanotube rope with electrical stimulation promotes the differentiation and maturity of neural stem cells. Small 8,2869, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Tran P.A., Zhang L., and Webster T.J.Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv Drug Deliv Rev 61,1097, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Ryu S., and Kim B.S.Culture of neural cells and stem cells on graphene. Tissue Eng Regen Med 10,39, 2013 [Google Scholar]
- 34.Akhavan O., and Ghaderi E.Differentiation of human neural stem cells into neural networks on graphene nanogrids. J Mater Chem B 1,6291, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Zhang T., Yan K.C., Ouyang L., and Sun W.Mechanical characterization of bioprinted in vitro soft tissue models. Biofabrication 5,045010, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Pepper M.E., Parzel C.A., Burg T., Boland T., Burg K.J., and Groff R.E.Design and implementation of a two-dimensional inkjet bioprinter. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference2009,6001, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Godino N., Gorkin R., Bourke K., and Ducree J.Fabricating electrodes for amperometric detection in hybrid paper/polymer lab-on-a-chip devices. Lab Chip 12,3281, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Cui X., Breitenkamp K., Finn M.G., Lotz M., and D'Lima D.D.Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 18,1304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skardal A., Zhang J., and Prestwich G.D.Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 31,6173, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Pham D.T., and Gault R.S.A comparison of rapid prototyping technologies. Int J Mach Tool Manu 38,1257, 1998 [Google Scholar]
- 41.Kolan K.C., Leu M.C., Hilmas G.E., and Velez M.Effect of material, process parameters, and simulated body fluids on mechanical properties of 13–93 bioactive glass porous constructs made by selective laser sintering. J Mech Behav Biomed Mater 13C,14, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Kang H., Long J.P., Urbiel Goldner G.D., Goldstein S.A., and Hollister S.J.A paradigm for the development and evaluation of novel implant topologies for bone fixation: Implant design and fabrication. J Biomech 45,2241, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Kettner M., Schmidt P., Potente S., Ramsthaler F., and Schrodt M.Reverse engineering—rapid prototyping of the skull in forensic trauma analysis. J Forensic Sci 56,1015, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Zopf D.A., Flanagan C.L., Wheeler M., Hollister S.J., and Green G.E.Treatment of severe porcine tracheomalacia with a 3-dimensionally printed, bioresorbable, external airway splint. JAMA Otolaryngol 140,66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zopf D.A., Hollister S.J., Nelson M.E., Ohye R.G., and Green G.E.Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med 368,2043, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Lu Y., and Chen S.Projection printing of 3-dimensional tissue scaffolds. Methods Mol Biol 868,289, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Jolly S.W., He Z., McGuffey C., Schumaker W., Krushelnick K., and Thomas A.G.Stereolithography based method of creating custom gas density profile targets for high intensity laser-plasma experiments. Rev Sci Instrum 83,073503, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Zhang A.P., Qu X., Soman P., Hribar K.C., Lee J.W., Chen S., and He S.Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv Mater 24,4266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q., Mochalin V.N., Neitzel I., Knoke I.Y., Han J., Klug C.A., Zhou J.G., Lelkes P.I., and Gogotsi Y.Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials 32,87, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Yeo M., Lee H., and Kim G.Three-dimensional hierarchical composite scaffolds consisting of polycaprolactone, β-tricalcium phosphate, and collagen nanofibers: fabrication, physical properties, and in vitro cell activity for bone tissue regeneration. Biomacromolecules 12,502, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Wang Q., McGoron A.J., Pinchuk L., and Schoephoerster R.T.A novel small animal model for biocompatibility assessment of polymeric materials for use in prosthetic heart valves. J Biomed Mater Res A 93,442, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Schantz J.T., Brandwood A., Hutmacher D.W., Khor H.L., and Bittner K.Osteogenic differentiation of mesenchymal progenitor cells in computer designed fibrin-polymer-ceramic scaffolds manufactured by fused deposition modeling. J Mater Sci Mater Med 16,807, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Zhang L., Sirivisoot S., Balasundaram G., and Webster T.J.Nanomaterials for improved orthopedic and bone tissue engineering applications. In: Basu B., Katti D., and Kuma A., eds. Advanced Biomaterials: Fundamentals, Processing and Application. New Jersey: John Wiley & Sons, Inc., 2009, p. 205 [Google Scholar]
- 54.Kaplan F.S., Hayes W.C., Keaveny T.M., Boskey A., Einhorn T.A., and Iannotti J.P.Form and function of bone. In: Sinmon S.P., ed. Orthopedic Basic Science. Rosemont, IL: American Academy of Orthopaedic Surgeons, 1994, p. 127 [Google Scholar]
- 55.Bellucci D., Sola A., Gazzarri M., Chiellini F., and Cannillo V.A new hydroxyapatite-based biocomposite for bone replacement. Mater Sci Eng C Mater Biol Appl 33,1091, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Tarafder S., Balla V.K., Davies N.M., Bandyopadhyay A., and Bose S.Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J Tissue Eng Regen Med 7,631, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pilliar R.M., Kandel R.A., Grynpas M.D., and Hu Y.Porous calcium polyphosphate as load-bearing bone substitutes: in vivo study. J Biomed Mater Res Part B Appl Biomater 101,1, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Castilho M., Moseke C., Ewald A., Gbureck U., Groll J., Pires I., Tessmar J., and Vorndran E.Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 6,015006, 2014 [DOI] [PubMed] [Google Scholar]
- 59.Fedorovich N.E., Leeuwenburgh S.C., van der Helm Y.J., Alblas J., and Dhert W.J.The osteoinductive potential of printable, cell-laden hydrogel-ceramic composites. J Biomed Mater Res Part A 100,2412, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Tarafder S., Balla V.K., Davies N.M., Bandyopadhyay A., and Bose S.Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J Tissue Eng Regen Med 7,631, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner D.E., Jones A.D., Zhou H., and Bhaduri S.B.Cytocompatibility evaluation of microwave sintered biphasic calcium phosphate scaffolds synthesized using pH control. Mater Sci Eng C Mater Biol Appl 33,1710, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Ganey T.Cell proliferation and vitality determination of osteoblasts on different materials and surface characteristics; Interpretation of laboratory data. Available at www.oxfordpm.com/files/OXPEKK_OsteoFab_cell_proliferation___cell_vitality_report_-_Tim_Ganey_March_2011_-_CONFIDENTIAL.pdf, 2011
- 63.Zhang L., Hu J., and Athanasiou K.A.The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng 37,1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu T., Binder K.W., Albanna M.Z., Dice D., Zhao W., Yoo J.J., and Atala A.Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 5,015001, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Nimeskern L., Avila H.M., Sundberg J., Gatenholm P., Muller R., and Stok K.S.Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J Mech Behav Biomed 22,12, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Fu L.N., Zhou P., Zhang S.M., and Yang G.Evaluation of bacterial nanocellulose-based uniform wound dressing for large area skin transplantation. Mat Sci Eng C Mater 33,2995, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Fedorovich N.E., Schuurman W., Wijnberg H.M., Prins H.J., van Weeren P.R., Malda J., Alblas J., and Dhert W.J.Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods 18,33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmes B., Zhu W., Li J., Lee J.D., and Zhang L.G.Development of novel 3D printed scaffolds for osteochondral regeneration. Tissue Eng Part A 2014. [Epub ahead of print]; DOI: 10.1089/ten.TEA.2014.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu W., O'Brien C., O'Brien J.R., and Zhang L.G.3D nano/microfabrication techniques and nanobiomaterials for neural tissue regeneration. Nanomedicine, 9,859, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Moon S., Hasan S.K., Song Y.S., Xu F., Keles H.O., Manzur F., Mikkilineni S., Hong J.W., Nagatomi J., Haeggstrom E., Khademhosseini A., and Demirci U.Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng Part C Methods 16,157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rishal I., and Fainzilber M.Axon-soma communication in neuronal injury. Nat Rev Neurosci 15,32, 2014 [DOI] [PubMed] [Google Scholar]
- 72.Owens C.M., Marga F., Forgacs G., and Heesch C.M.Biofabrication and testing of a fully cellular nerve graft. Biofabrication 5,045007, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu T., Gregory C.A., Molnar P., Cui X., Jalota S., Bhaduri S.B., and Boland T.Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 27,3580, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Cameron J., and Ferris K.J.G.Bio-ink for on-demand printing of living cells. Biomater Sci 1,224, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Suri S., Han L.H., Zhang W., Singh A., Chen S., and Schmidt C.E.Solid freeform fabrication of designer scaffolds of hyaluronic acid for nerve tissue engineering. Biomed Microdevices 13,983, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Preston M., and Sherman L.S.Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front Biosci (Schol Ed) 3,1165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laurent T.C., Laurent U.B., and Fraser J.R.The structure and function of hyaluronan: an overview. Immunol Cell Biol 74,A1, 1996 [DOI] [PubMed] [Google Scholar]
- 78.Curley J.L., Jennings S.R., and Moore M.J.Fabrication of micropatterned hydrogels for neural culture systems using dynamic mask projection photolithography. J Vis Exp 2636, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Temple J.P., Hutton D.L., Hung B.P., Huri P.Y., Cook C.A., Kondragunta R., Jia X., and Grayson W.L.Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res Part A 2014. [Epub ahead of print]; DOI: 10.1002/jbm.a.35107 [DOI] [PubMed] [Google Scholar]
- 80.Miller J.S., Stevens K.R., Yang M.T., Baker B.M., Nguyen D.H., Cohen D.M., Toro E., Chen A.A., Galie P.A., Yu X., Chaturvedi R., Bhatia S.N., and Chen C.S.Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11,768, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu W., DeConinck A., and Lewis J.A.Omnidirectional printing of 3D microvascular networks. Adv Mater 23,H178, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Vatankhah E., Prabhakaran M.P., Semnani D., Razavi S., Zamani M., and Ramakrishna S.Phenotypic modulation of smooth muscle cells by chemical and mechanical cues of electrospun Tecophilic/gelatin nanofibers. ACS Appl Mater Interfaces 6,4089, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Skardal A., Zhang J., McCoard L., Xu X., Oottamasathien S., and Prestwich G.D.Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A 16,2675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duan B., Kapetanovic E., Hockaday L.A., and Butcher J.T.Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater 10,1836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horst M., Madduri S., Gobet R., Sulser T., Milleret V., Hall H., Atala A., and Eberli D.Engineering functional bladder tissues. J Tissue Eng Regen Med 7,515, 2013 [DOI] [PubMed] [Google Scholar]
- 86.Atala A.Re: innervation of reconstructed bladder above the level of spinal cord injury for inducing micturition by contractions of the abdomen-to-bladder reflex arc. J Urol 185,354, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Atala A.Tissue engineering of human bladder. Br Med Bull 97,81, 2011 [DOI] [PubMed] [Google Scholar]
- 88.Atala A., Bauer S.B., Soker S., Yoo J.J., and Retik A.B.Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367,1241, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Fullhase C., Soler R., Atala A., Andersson K.-E., and Yoo J.J.A novel hybrid printing system for the generation of organized bladder tissue. J Urol 181,282, 2009 [Google Scholar]
- 90.Xu T., Zhao W., Zhu J.M., Albanna M.Z., Yoo J.J., and Atala A.Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 34,130, 2013 [DOI] [PubMed] [Google Scholar]
- 91.Williams S.K., Touroo J.S., Church K.H., and Hoying J.B.Encapsulation of adipose stromal vascular fraction cells in alginate hydrogel spheroids using a direct-write three-dimensional printing system. Biores Open Access 2,448, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mironov V., Visconti R.P., Kasyanov V., Forgacs G., Drake C.J., and Markwald R.R.Organ printing: tissue spheroids as building blocks. Biomaterials 30,2164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alajati A., Laib A.M., Weber H., Boos A.M., Bartol A., Ikenberg K., Korff T., Zentgraf H., Obodozie C., Graeser R., Christian S., Finkenzeller G., Stark G.B., Heroult M., and Augustin H.G.Spheroid-based engineering of a human vasculature in mice. Nat Methods 5,439, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Cohen D.L., Malone E., Lipson H., and Bonassar L.J.Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng 12,1325, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Halberstadt C., Robbins N., McCoy D.W., Guthrie K.I., Bruce A.T., Knight T.A., and Payne R.G.Formulation of selected renal cells for implantation into a kidney. Methods Mol Biol 1001,279, 2013 [DOI] [PubMed] [Google Scholar]
- 96.Mironov V., Kasyanov V., and Markwald R.R.Organ printing: from bioprinter to organ biofabrication line. Curr Opin Biotechnol 22,667, 2011 [DOI] [PubMed] [Google Scholar]
- 97.Xu F., Moon S.J., Emre A.E., Turali E.S., Song Y.S., Hacking S.A., Nagatomi J., and Demirci U.A droplet-based building block approach for bladder smooth muscle cell (SMC) proliferation. Biofabrication 2,014105, 2010 [DOI] [PubMed] [Google Scholar]
- 98.Xu F., Celli J., Rizvi I., Moon S., Hasan T., and Demirci U.A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol J 6,204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marga F., Jakab K., Khatiwala C., Shepherd B., Dorfman S., Hubbard B., Colbert S., and Forgacs G.Toward engineering functional organ modules by additive manufacturing. Biofabrication 4,022001, 2012 [DOI] [PubMed] [Google Scholar]